Abstract
Mechanosensory hair cells (HCs) and surrounding supporting cells (SCs) in the mouse cochlea are important for hearing and are derived from the same prosensory progenitors. Notch1 signaling plays dual but contrasting and age-dependent roles in mouse cochlear development: early lateral induction and subsequent lateral inhibition. However, it has been difficult to directly visualize mouse cochlear cells experiencing various levels of Notch1 activity at single cell resolution. Here, we characterized two knock-in mouse lines, Notch1Cre (Low)/+ and Notch1Cre (High)/+, with different Cre recombinase activities, that can detect Notch1 receptor proteolysis or Notch1 activity at high and low thresholds, respectively. Using both lines together with a highly sensitive Cre reporter line, we showed that Notch1 activity is nearly undetectable during lateral induction but increases to medium and high levels during lateral inhibition. Furthermore, we found that within the neonatal organ of Corti, the vast majority of cells that experience Notch1 activity were SCs not HCs, suggesting that HCs kept undetectable Notch1 activity during the entire lineage development. Furthermore, among SC subtypes, ∼85–99% of Deiters’ and outer pillar cells but only ∼19–38% of inner pillar cells experience medium and high levels of Notch1 activity. Our results demonstrate that Notch1 activity is highly heterogeneous: 1) between lateral induction and inhibition; 2) between HC and SC lineages; 3) among different SC subtypes; 4) among different cells within each SC subtype. Such heterogeneity should elucidate how the development of the cochclear sensory epithelium is precisely controlled and how HC regeneration can be best achieved in postnatal cochleae.
Introduction
Sound detection in the mammalian inner ear is mediated via mechanosensory hair cells (HCs) in the sensory epithelium of the cochlea, also referred to as the organ of Corti [1]–[4]. The organ of Corti contains three rows of outer hair cells (OHCs) and one row of inner hair cells (IHCs), which are surrounded by different types of supporting cells (SCs): inner pillar cells (IPCs), outer pillar cells (OPCs) and Deiters’ cells (DCs) [1]. As demonstrated by linage tracing in the mouse cochlea [5]–[7], HCs and SCs are derived from the same prosensory progenitor cells. In mouse cochlear development, the period between embryonic day (E) 11 and E14 is defined as the early prosensory phase [8]–[10], when the lateral induction effects of Notch signaling specify prosensory progenitors [8], [11]–[17]. The period between E14 and perinatal ages is the lateral inhibition phase, when prosensory progenitors undergo differentiation and Notch signaling promotes SC’s, but antagonizes HC’s, fate commitment and differentiation [18], [19].
It remains unknown how Notch signaling evokes such dual but contrasting effects in the development of the inner ear and how cochlear cells sense and respond appropriately to Notch signaling at different developmental stages. Interestingly, Notch signaling also elicits similar contrasting responses in the development of the central nervous system [20] and the pancreas [21] and, in these tissues, Notch signaling influences cells in a level-dependent manner, where low levels of Notch promote cell proliferation and high levels induce quiescence and cell differentiation. Therefore, we hypothesize that Notch activity is relatively low during lateral induction and increases during lateral inhibition in the developing organ of Corti.
While Notch1 is the primary, active Notch receptor during mouse inner ear development [17], it has been challenging to visualize Notch1 activity levels at single cell resolution. Different levels of Notch1 activity have been inferred by the expression levels of downstream target genes (e.g., Jagged1 and Hes family genes) [22]–[24], or their recapitulation in reporter mice (Hes5-GFP) [25], but these methods have limitations [26]. For example, Jagged1 and Hey2 also respond to input from Wnt signaling [24], [27] and fibroblast growth factor (FGF) signaling [28]. To overcome such limitations and to visualize Notch1 signaling more directly, we utilized a genetic lineage tracing approach involving Notch1Cre (Low)/+ and Notch1Cre (High)/+ mouse strains [26], [29].
In both Notch1Cre (Low)/+ and Notch1Cre (High)/+ lines, Notch1 intracellular domain (NICD) was replaced by Cre recombinase with the nuclear localization signal (NLS), resulting in a null mutation of Notch1 (Fig. 1A). The differences between the two lines are mainly two-fold: first, Cre is tagged with 6×Myc in the Notch1Cre (Low)/+ line, while Cre is not tagged with 6×Myc in the Notch1Cre (High)/+ line; second, an extra copy of SV40 polyadenylation signal is added to the end of the construct in Notch1Cre (High)/+ line, which increase the Notch1/Cre mRNA level by two folds [29], [30]. These improvements make the Cre-mediated lineage tracing in Notch1Cre (High)/+ line much more sensitive (or of a lower detecting threshold) than in the Notch1Cre (Low)/+ line. For example, in lung tissue, only cells having high levels of Notch1 activity can be traced in the Notch1Cre (Low)/+ line, whereas in the Notch1Cre (High)/+ line, cells with both high and medium levels of Notch1 activity can be traced [31]. In addition, when combined with a floxed Notch1 allele (Notch1Cre (Low)/flox or Notch1Cre (High)/flox), Notch1Cre (High) achieves very high level of self-excision in embryonic endothelial cells, causing embryos to die at around E10.5; in contrast, the self-excision efficiency of Notch1Cre (Low) is so low that the embryos develop normally and the pups could be born at expected Mendelian ratios [29]. Last, heterozygous mice of both Notch1Cre (Low)/+ and Notch1Cre (High)/+ lines are fertile and viable whereas homozygous mice die at ∼E9.5, consistent with two independent Notch1−/− mouse lines previously characterized [32], [33].
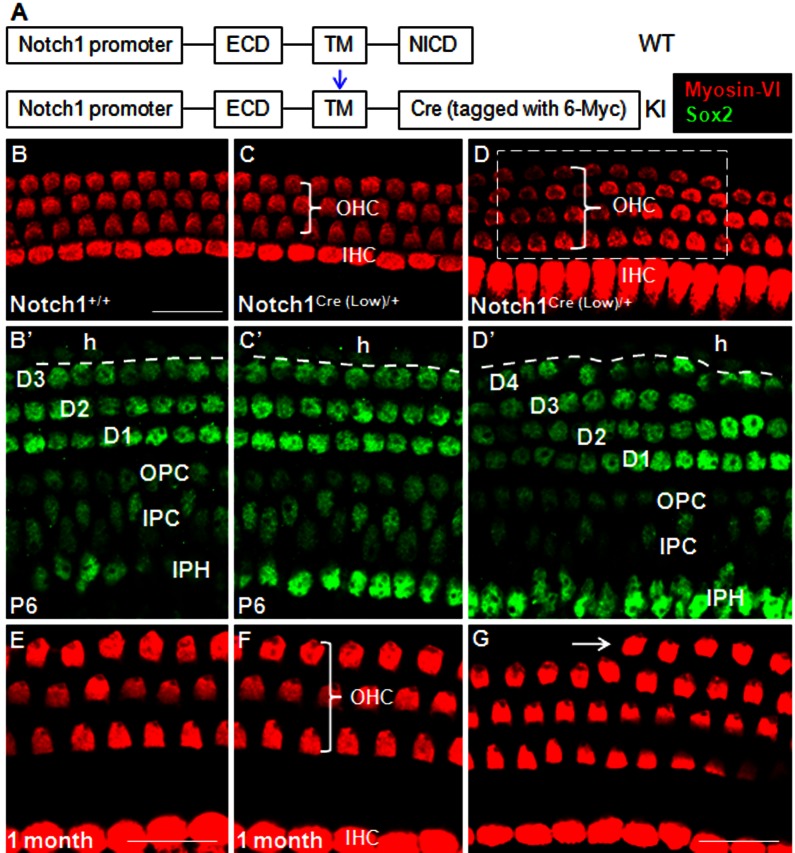
Figure 1. Characterization of the Notch1 Cre (Low)/+ mouse line.
(A) Schematic illustration of Notch1Cre/+ mice. The NICD was replaced by 6×Myc–tagged Cre recombinase. The blue arrow represents the cleavage site. (B–D’) Comparison between cochleae from Notch1Cre/+ mice and control mice (Notch1+/+). (B, B’) Myosin-VI+ OHCs (three rows; red) and IHCs (one row; red) sit above Sox2+ SCs (green) in a control (Notch1+/+) cochlea at P6. (C, C’) In most regions across the entire cochlea, Notch1Cre (Low)/+ mice is indistinguishable from controls. (D, D’) An extra row of DCs always appear underneath the fourth row of OHCs (white dotted rectangular area) in Notch1Cre (Low)/+ cochleae. Although extra DCs and OHCs are frequently observed, each of them spans only a short stretch. The Sox2+ cells outside the dotted line (B’, C’, and D’) are Hensen cells (h). (E–G) Morphology of HCs at P30 in control (E) and Notch1Cre (Low)/+ mice (F–G). The distance between OHCs and IHCs is extended. The extra row of OHCs (arrow in G) in Notch1Cre (Low)/+ mice survive and align well with surrounding HCs. D1–D4: three or four rows of Deiters’ cell; OPC: outer pillar cell; IPC: inner pillar cell; IPH: inner phalangeal cell; h: Hensen’s cell. ECD: extracellular domain; TM: transmembrane domain; NICD: Notch1 intracellular domain. Bars: 20 µm. Bar in (B) also applies to C–D’. Bar in (E) also applies to (F).
Both Notch1Cre (Low)/+ and Notch1Cre (High)/+-mediated lineage tracing recapitulates Notch1 proteolysis, but at different levels of sensitivity. When Notch1 activity is sufficiently low, Cre activity remains undetectable in either mouse line; when Notch1 activity is high, Cre activity in both lines are activated; and when Notch1 activity is at an intermediate level, the Notch1Cre (High)/+ line exhibits detectable Cre activity while the Notch1Cre (Low)/+ line does not. These Cre activities are readily visualized by crossing Notch1Cre/+ mice with floxed-stop reporter lines. Our lineage tracing results reported here support the hypothesis that Notch1 activity differs among different cochlear cell types, and between stages of lateral induction and lateral inhibition. Thus these Notch1Cre/+ lines can be widely used in other systems to ascertain variable levels of Notch1 activity in vivo.
Results
Characterization of Both Notch1Cre (Low)/+ and Notch1Cre (High)/+ Alleles in the Mouse Cochlear Development
We first described and characterized the Notch1Cre (Low)/+ and Notch1Cre (High)/+ lines.
Heterozygous mice of both lines exhibited identical minor phenotypes of haploinsuffiency in the organs of Corti, thus we present here data only from the Notch1Cre (Low)/+ mice (Fig. 1A). Like the control wild-type littermates (Notch1+/+), Notch1Cre (Low)/+ mice had 3 predominant rows of OHCs and 1 row of IHCs at postnatal day (P) 6 (Fig. 1B and C). However, there were discontinuous patches distributed along the length of the cochlear duct in which a 4th row of OHC was observed (white rectangular area in Fig. 1D). Interestingly, extra Sox2+ SCs were also found in the same confocal scanning area where ectopic OHCs were present at P6 (Fig. 1B’–D’; n = 3). These extra HCs and SCs survived at adult ages (Fig. 1E–G). Furthermore, whole-mount analysis showed that there was no substantial difference in length of the entire cochlear duct between Notch1+/+ (6050 µm ±110 µm) and Notch1Cre (Low)/+ (6160 µm ±191 µm) mice (n = 3 in each group), which rules out the possibility that the increased density of HCs or SCs in Notch1Cre (Low)/+ mice are secondary phenotypes arising from a shortened cochlear duct. Such a phenotype is consistent with presence of supernumerary SCs in the Hes1/Hes5/Hey1 or Hes1/Hes5/Hey2 compound mutant mice [22], [23] and the Notch1 +/− mice [34].
Heterogeneity of Notch1 Activity Levels between Lateral Induction and Inhibition Stages of Cochlear Development
Notch1 is turned on at the onset of inner ear development, and the Jagged1 is the major Notch1 ligand in lateral induction stage [9], [11], [24], [27]. The strength of NICD immunostaining at lateral induction is much weaker than that of lateral inhibition stage [35], [36]. Because severe phenotypes were observed in cochleae where Notch1 activity is lost during lateral induction stage [12], [13], we asked whether an alternative way is available to better detect Notch activities in cochlear cells at lateral induction stage. We opted to use Cre-mediate lineage tracing which identifies all cells that have experienced Notch activity at single cell resolution, irrespective of their temporal and spatial characteristics.
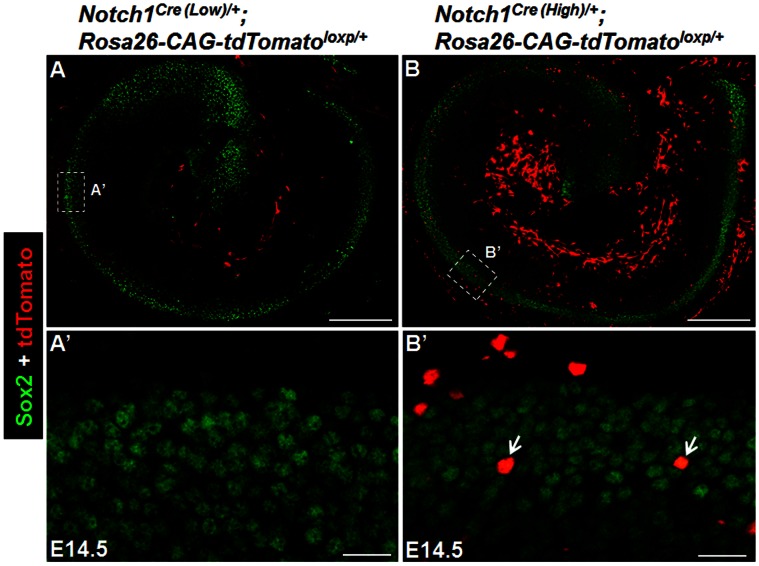
We crossed the Notch1Cre (Low)/+ and Notch1Cre (High)/+ lines with a highly sensitive Rosa26-CAG-tdTomato loxp/+ reporter line which would express tdTomato upon floxed STOP excision by Cre liberated from cell membrane after the mimics of Notch1 proteolysis [37]. Thus tdTomato labels cells that have experienced Notch activities at any point in their lineage. By E14.5, no tdTomato+ cell was observed inside the organ of Corti of Notch1Cre (Low)/+; Rosa26-CAG-tdTomato loxp/+ mice (Fig. 2A–A’). However, a small number (0.97% ±0.3%) of the Sox2+ cells in the cochlear prosensory regions were tdTomato+ in Notch1Cre (High)/+; Rosa26-CAG-tdTomato loxp/+ mice (Fig. 2B–B’). Together, consistent with the NICD immunostaining approach [36], these support that Notch1 activity is generally undetectable or very low but not completely absent in the lateral induction period.
Figure 2. Notch1Cre/+-mediated reporter expression is difficult to detect in the cochlear prosensory domain at embryonic day (E) 14.5.
(A–A’) A single slice of confocal image demonstrating that tdTomato reporter expression (red) was undetectable in Sox2 positive (green) sensory precursor cells in cochleae of Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ mice at E14.5. (A’) is the high magnification image of the rectangular region in (A) taken in the organ of Corti region. (B–B’) A single slice of confocal image taken in cochleae of Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+mice. (B’) is the high magnification image of the rectangular region in (B) taken in the organ of Corti region, showing that a few cells were Sox2+/tdTomato+ (arrows), whereas the majority were Sox2+ only. Scale bar is 200 µm (A, B), 20 µm (A’, B’).
Heterogeneity of Notch1 Activities Across Cell Types during the Lateral Inhibition Stage of Cochlear Development
We next determined cochlear cell types experiencing Notch1 activity during lateral inhibition stage in two genetic models: Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ and Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+. We analyzed the reporter tdTomato expression at P6 when cochlear cell fate commitment should be completed and Notch1 activity should be diminished, as evidenced by decreased NICD expression during the first postnatal week [36] and the fact that the cochlear SCs become much less responsive to Notch1 inactivation as they age [38]. Furthermore, in the cochleae of Notch1Cre (Low)/+;Rosa26-EYFPloxp/+ at E14.5, E16.5, E18.5, P2, and P6, very few EYFP+ SCs began to appear at E18.5 and the number of EYFP+ SCs continuously increased between E18.5 and P6, but stopped further increase after P6 (data not shown). Thus, by P6, all cells experiencing different levels of Notch1 activity during both lateral induction and inhibition in development should be labeled.
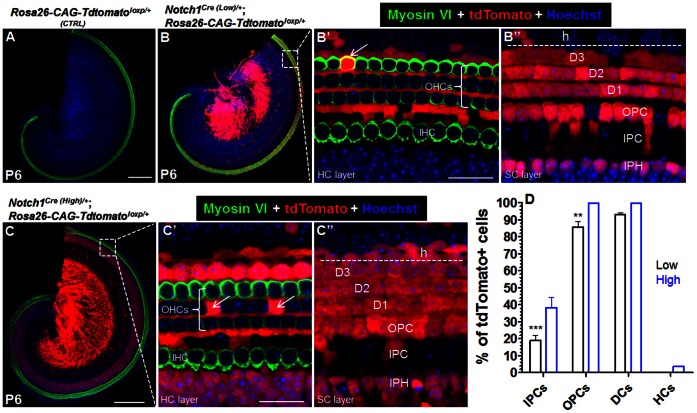
In control Rosa26-CAG-tdTomatoloxp/+ mice (n = 3), tdTomato expression was never observed (Fig. 3A). In Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+mice at P6 (n = 4), inside the organ of Corti, many tdTomato+ cells were observed (Fig. 3B–B” and D). For each SC subtype, 19.0% ±3.1% of IPCs, 85.7% ±3.4% of OPCs and 93.0% ±1.0% of DCs were tdTomato+. In contrast, only 0.13% ±0.06% of HCs were tdTomato+ (Fig. 3B’ and D).
Figure 3. Heterogeneity of Notch activities among different cells.
(A) Tdtomato expression was absent in control Rosa26-CAG-tdTomatoloxp/+ mice. (B–B”) Cre-mediated Tdtomato expression in Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ mice. Arrow pointed to a tdTomato+ HC (B’). (C–C”) Broader Cre-mediated Tdtomato expression in Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+mice. Two arrows were two tdTomato+ HCs. (D) Quantification of tdTomato+cells. The percentage of tdTomato+ HCs in “Low” model was too small to see in the graph. The SEM bar (OPC and DC) was also too small to see in the “High” model. “Low” represents Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+, while “High” does Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+mice. ***p<0.001, **p<0.01. D1-D3: three rows of Deiters’ cell; OPC: outer pillar cell; IPC: inner pillar cell; IPH: inner phalangeal cell; h: Hensen’s cell. Bars: 200 µm (A, C); 20 µm (B’, C’).
In Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ at P6 (n = 4), similar observations with higher percentages were found (Fig. 3C–C”). Approximately 38.4% ±5.8% of IPCs, 99.4% ±0.4% of OPCs, and 99.8% ±0.2% of DCs were tdTomato+. Again, only 3.5% ±0.9% of total HCs were tdTomato+ (Fig. 3C’ and D). Thus, the absolute percentage differences of tdTomato+cells between Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ and Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+ line are 19.4%, 13.7%, 6.8% and 3.37% for IPCs, OPCs, DCs and HCs, respectively. To highlight the difference, we normalized the percentage to each other and found that the percentages of tdTomato+cells in Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+ are 49.5%, 86.2%, 93.0% and 3.7% of those of Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ for IPCs, OPCs, DCs and HCs, respectively. Taken together, the data strongly suggest heterogeneous Notch1 activity among various cell types within the organ of Corti at lateral inhibition stage and a distinct difference in the sensitivity of the two Notch1Cre/+ mouse lines to different levels of Notch activity.
In Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ and Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+ mice, the percentages of tdTomato+ cells at P6 were similar to those analyzed at P21 (data not shown) and tdTomato expression was also found in cells in the spiral ganglion region, greater epithelial ridge (GER) cells, inner phalangeal cells (IPhs), Hensen’s cells, Claudius cells and the vessel endothelial cells underneath the basilar membrane (Fig. 3B and C); however these are beyond the focus of our current study.
Discussion
Our in vivo lineage tracing results reported here demonstrate that, during mouse cochlear development, Notch1 activity is heterogeneous in four aspects: 1) between lateral induction and inhibition stages; 2) between HC and SC lineages; 3) among different SC subtypes; and 4) among different cells within each SC subtype.
The genetic approach of Notch1Cre/+ -mediated lineage tracing is reliable to reflect the Notch activity that cells experienced during development. In support, in a previous study, retinoic acid (RA) response-element (RARE)-driven Cre mice (RARE-Cre+) are used to trace cells experiencing different levels of RA activity. In RARE-Cre+; Rosa26-lacZloxp/+ mice, the posterior but not anterior otocyst cells are X-gal+ [39]. These findings are consistent with the fact that a lower level and brief RA signaling activity is present at the anterior side of the otocyst, while a higher and longer-lasting RA activity at the posterior end [40].
In our two Notch1Cre/+ models (Notch1Cre (High)/+ and Notch1Cre (Low)/+), the readout of tdTomato reporter expression is primarily dependent on the dosage of Cre activity within each individual cell which is proportional to the level of Notch1 signaling each cell is experiencing. The recombinase Cre level/activity in Notch1Cre (High)/+ was reported to be much higher than in Notch1Cre (Low)/+ [29], [31]. We therefore defined that: 1) those cells without tdTomato expression in either Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ or Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ were cells with low to undetectable Notch1 activities; 2) those cells with tdTomato expression in Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ but not in Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ were those with medium Notch1 activities; and 3) those cells with tdTomato expression in both Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ and Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ experienced high Notch1 activities. In support, our results demonstrated that Notch1 activity is generally low except a few cells during lateral induction (by E14.5); but it dramatically increases to medium and high levels in many cells by P6 during lateral inhibition. These results are consistent with NICD immunostaining and other loss-of-function genetic studies of Notch1 signaling [36], [41], and further validate our Notch1Cre/+ lineage tracing approach. Finally, we found that Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ and Notch1Cre (low)/+; Rosa26-CAG-tdTomatoloxp/+ cochleae at P21 exhibited similar reporter expression patterns as those at P6; these results are consistent with previous results that Notch1 levels decrease with age, such as the down-regulation of Hes5 expression in Hes5-EGFP+ transgenic mice [25], the decrease in NICD staining in older SCs [36], and the declining responsiveness of SCs to modulations of Notch1 activity [38].
Using this in vivo lineage tracing strategy, we observed several interesting findings at P6 cochleae during lateral inhibition. First, 96.5% of HCs had low, while only 3.37% had medium and 0.13% had high levels of Notch1 activities. These HCs with detectable levels of Notch1 activity might have been, in part, newly converted from SCs at late embryonic ages, because of the haploinsufficiency of Notch1 heterozygous alleles (Fig. 1). Alternatively, they may be original HCs that somehow experienced medium or high Notch1 activities and yet still maintained a HC fate. However, we cannot yet distinguish between these two explanations and both scenarios may contribute to the detected Notch1 activity in HCs. Nonetheless, the Notch1 signaling in neonatal HCs might not necessarily affect their development, as shown in our recent study where ectopic expression of NICD increased Sox2 and Prox1 expression in endogenous HCs without detectable hearing abnormalities [42].
Second, in contrast to HCs, the SC lineage significantly increases Notch1 activity during lateral inhibition stage. In cochleae of Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+, very few (∼ 0.97%) progenitor cells were tdTomato+ by E14.5 (Fig. 2B), and only ∼ 3.5% of total HCs were tdTomato+ by P6 (Fig. 3C’ and D). These results support that the common progenitor cells during lateral induction stage must experience low levels of Notch1 activity, otherwise many HCs would be tdTomato +. Recently, two reports have shown that Notch1 is not required to specify or maintain the properties of progenitor cells in the cochlea [35], [43]. One simple explanation might be that the loss of low level of Notch1 during lateral induction is compensated by other signaling pathways such as Wnt and Fgf [24], [44]–[46]. However, during lateral inhibition, medium to high levels of Notch1 activity cannot be simply compensated, a conjecture that is consistent with loss-of-function study of Notch activity during late embryonic or neonatal cochlear development [12], [18], [28], [41].
Third, there appears to be significant heterogeneity of Notch1 activity levels among SCs and even within each of the three subtypes in the organ of Corti. Among DCs, 0.2%, 6.8%, and 93.0% of the cells experienced low, medium, and high levels of Notch1 activity, respectively. Similarly, 0.6%, 13.7%, and 85.7% of OPCs experienced low, medium, and high levels of Notch activity, respectively. Most surprisingly, for IPCs, 61.6%, 19.4%, and 19.0% experienced low, medium, and high levels of Notch1 activity, respectively. Consistently, using NICD antibody, its staining strength in SCs (including IPCs) near the IHCs is much weaker than that in SCs near the OHCs (i.e. DCs) [36]. Such extensive heterogeneity has significant implications for our understanding of sensory epithelium development and regeneration. It may account for the heterogeneous responses of different SCs to ectopic Atoh1 expression where only ∼10% neonatal PCs and DCs were converted to immature HCs upon ectopic Atoh1 expression [47]. It is possible that SCs with high levels of Notch1 activity would inhibit Atoh1-mediated conversion into HCs; that is, given our observed Notch1 heterogeneity among SCs, 93% of DCs, 85.7% of OPCs and 19% of IPCs with high Notch1 activities could not be converted into HCs. Because loss of Notch1 activity in damaged cochleae promoted conversion of SCs into HCs [48], simultaneous inactivation of Notch1 and ectopic Atoh1 overexpression might be synergistic in vivo. Because of low levels of Notch1 activity in 61.6% of IPCs and the proximity of IPCs to Fgf8-expressing IHCs, we speculate that Fgf8/Fgfr3-mediated Fgfr signaling is also critically important and may collaborate with low levels of Notch1 to control IPC development [28], [49]. Such interactions might also explain the fact that only IPCs, but not OPCs and DCs, proliferate when the Sox2 gene is conditionally deleted at neonatal ages [50]. As Notch1 is also important in keeping SCs quiescent at perinatal ages [41], we conjecture that medium or high levels of Notch1 activity can compensate for Sox2 deletion in 99.4% OPCs and 99.8% DCs (and only 38.4% IPCs), preventing their proliferation.
Finally, these two new Notch1Cre/+ lines exhibit Cre activities at various levels, a valuable tool not only for discriminating levels of Notch1 activity across cell types, tissues, and developmental stages, but also for lineage tracing and genetic manipulation of various genes specifically in cells that experience different levels of Notch1 signaling. In the cochlea, these mouse lines would thus be invaluable for such manipulations in SCs after E16. In summary, our results revealed significant heterogeneity of Notch1 signaling during cochlear development and will have significant implications in our understanding of the development of the organ of Corti and potentially for HC regeneration in mammalian cochleae.
Materials and Methods
Mice Strains and Embryonic Age Designation
Notch1Cre (Low)/+ (stock number: 006953) [26] and Rosa26-CAG-tdTomatoloxp/+ (stock number: 007908) [51] mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Notch1Cre (High)/+ mice were described in [29]–[31]. Mice were crossed at 5 pm, and checked for presence of the vaginal plug at 7 am the next day. If plugs were present, the morning was designated as E0.5. Notch1Cre (Low)/+; Rosa26-CAG-tdTomatoloxp/+ mice were bred at St. Jude Children’s Research Hospital (St. Jude). Notch1Cre (High)/+; Rosa26-CAG-tdTomatoloxp/+ mice were maintained in the animal facility at Washington University, and inner ear samples fixed in 4% paraformaldehyde (PFA) were shipped to and analyzed at St. Jude. All animal work conducted during the course of this study was approved by the Institutional Animal Care and Use Committee at St. Jude and Washington University and performed according to NIH guidelines.
Histology and Immunofluorescence
Preparation of embryonic, neonatal, and adult-age inner ear samples have been described previously [52]. All samples were examined by using a Zeiss LSM 700 confocal microscope. The following primary antibodies were used: anti-Myosin-VI (rabbit, 1∶200, 25–6791, Proteus Bioscience, Ramona, CA), anti-Sox2 (goat, 1∶1000, sc-17320, Santa Cruz Biotechnology, Santa Cruz, CA). The following secondary antibodies were used: goat anti rabbit Alexa Fluor 568 (1∶1000, A11036, Invitrogen), donkey anti goat Alexa Fluor 568 (1∶1000, A11057, Invitrogen), donkey anti rabbit Alexa Fluor 647 (1∶1000, A31573, Invitrogen).
Cell Counting
Embryonic and neonatal cochlear samples were divided into two parts, whereas adult samples were divided into three parts. We purposely left a tiny cut in spiral ganglion areas of each turn to help distinguish the two ends under the confocal microscope. With the preliminary low-magnification image, we first measured the length of each turn by drawing a curved line in the middle of OHCs and IHCs and then added up the length of the three turns. Confocal Z stac (40× oiled lens) scanning was performed at 1 µm intervals to tdTomato or Myosin-VI or Sox2-expressing cells, with Hoechst33342 being used to label cell nuclei. This approach was used to reduce the counting variations among different samples. For each SC subtype, the percentage of SCs traced by tdTomato was calculated by normalizing the number of tdTomato+cells with respect to the total number of SCs (using Sox2 as a marker) in the same confocal Z stack scanning area.
Statistical Analysis
All data were expressed as mean ± S.E.M. Each cell type counting between 2 different genetic models at P6 was compared using a one-way ANOVA followed by a Student’s t test with a Bonferroni correction. Statistical analysis was conducted using GraphPad Prism 5.0 Software.
Acknowledgments
We thank J. Woods, G. Redd, C. Davis, and D. Wash for determining the embryonic ages and S. Connell, J. Peters, and Y. Ouyang for help in confocal imaging.
Funding Statement
This work was supported by the National Institutes of Health (NIH): DC06471, CA21765 and DK066408; Office of Naval Research: N000140911014, N000141210191, and N000141210775; the American Lebanese Syrian Associated Charities (ALSAC) of St. Jude Children’s Research Hospital; The Hartwell Individual Biomedical Research Award (JZ); Travel Awards from University of Tennessee Health Science Center, Academic Programs of St. Jude Children’s Research Hospital, and Society of Developmental Biology (Zhiyong Liu); HHF and NOHR (BW). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kelley MW (2006) Regulation of cell fate in the sensory epithelia of the inner ear. Nat Rev Neurosci 7: 837–849. [DOI] [PubMed] [Google Scholar]
- 2. Bok J, Chang W, Wu DK (2007) Patterning and morphogenesis of the vertebrate inner ear. Int J Dev Biol 51: 521–533. [DOI] [PubMed] [Google Scholar]
- 3. Kelly MC, Chen P (2009) Development of form and function in the mammalian cochlea. Curr Opin Neurobiol 19: 395–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kwan T, White PM, Segil N (2009) Development and regeneration of the inner ear. Ann N Y Acad Sci 1170: 28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carroll Driver E, Sillers L, Coate TM, Rose MF, Kelley MW (2013) The Atoh1-lineage gives rise to hair cells and supporting cells within the mammalian cochlea. Dev Biol. [DOI] [PMC free article] [PubMed]
- 6. Matei V, Pauley S, Kaing S, Rowitch D, Beisel KW, et al. (2005) Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev Dyn 234: 633–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yang H, Xie X, Deng M, Chen X, Gan L (2010) Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis 48: 407–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hayashi T, Kokubo H, Hartman BH, Ray CA, Reh TA, et al. (2008) Hesr1 and Hesr2 may act as early effectors of Notch signaling in the developing cochlea. Dev Biol 316: 87–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eddison M, Le Roux I, Lewis J (2000) Notch signaling in the development of the inner ear: lessons from Drosophila. Proc Natl Acad Sci U S A 97: 11692–11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Daudet N, Lewis J (2005) Two contrasting roles for Notch activity in chick inner ear development: specification of prosensory patches and lateral inhibition of hair-cell differentiation. Development 132: 541–551. [DOI] [PubMed] [Google Scholar]
- 11. Kiernan AE, Ahituv N, Fuchs H, Balling R, Avraham KB, et al. (2001) The Notch ligand Jagged1 is required for inner ear sensory development. Proc Natl Acad Sci U S A 98: 3873–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brooker R, Hozumi K, Lewis J (2006) Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 133: 1277–1286. [DOI] [PubMed] [Google Scholar]
- 13. Kiernan AE, Xu J, Gridley T (2006) The Notch ligand JAG1 is required for sensory progenitor development in the mammalian inner ear. PLoS Genet 2: e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kokubo H, Tomita-Miyagawa S, Hamada Y, Saga Y (2007) Hesr1 and Hesr2 regulate atrioventricular boundary formation in the developing heart through the repression of Tbx2. Development 134: 747–755. [DOI] [PubMed] [Google Scholar]
- 15. Hartman BH, Reh TA, Bermingham-McDonogh O (2010) Notch signaling specifies prosensory domains via lateral induction in the developing mammalian inner ear. Proc Natl Acad Sci U S A 107: 15792–15797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pan W, Jin Y, Stanger B, Kiernan AE (2010) Notch signaling is required for the generation of hair cells and supporting cells in the mammalian inner ear. Proc Natl Acad Sci U S A 107: 15798–15803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Liu Z, Owen T, Fang J, Zuo J (2012) Overactivation of notch1 signaling induces ectopic hair cells in the mouse inner ear in an age-dependent manner. PLoS One 7: e34123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lanford PJ, Lan Y, Jiang R, Lindsell C, Weinmaster G, et al. (1999) Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet 21: 289–292. [DOI] [PubMed] [Google Scholar]
- 19. Dabdoub A, Puligilla C, Jones JM, Fritzsch B, Cheah KS, et al. (2008) Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci U S A 105: 18396–18401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guentchev M, McKay RD (2006) Notch controls proliferation and differentiation of stem cells in a dose-dependent manner. Eur J Neurosci 23: 2289–2296. [DOI] [PubMed] [Google Scholar]
- 21. Ninov N, Borius M, Stainier DY (2012) Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development 139: 1557–1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tateya T, Imayoshi I, Tateya I, Ito J, Kageyama R (2011) Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev Biol. [DOI] [PubMed]
- 23. Li S, Mark S, Radde-Gallwitz K, Schlisner R, Chin MT, et al. (2008) Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev Biol 8: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jayasena CS, Ohyama T, Segil N, Groves AK (2008) Notch signaling augments the canonical Wnt pathway to specify the size of the otic placode. Development 135: 2251–2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hartman BH, Basak O, Nelson BR, Taylor V, Bermingham-McDonogh O, et al. (2009) Hes5 expression in the postnatal and adult mouse inner ear and the drug-damaged cochlea. J Assoc Res Otolaryngol 10: 321–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vooijs M, Ong CT, Hadland B, Huppert S, Liu Z, et al. (2007) Mapping the consequence of Notch1 proteolysis in vivo with NIP-CRE. Development 134: 535–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ohyama T, Mohamed OA, Taketo MM, Dufort D, Groves AK (2006) Wnt signals mediate a fate decision between otic placode and epidermis. Development 133: 865–875. [DOI] [PubMed] [Google Scholar]
- 28. Doetzlhofer A, Basch ML, Ohyama T, Gessler M, Groves AK, et al. (2009) Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev Cell 16: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu Z, Turkoz A, Jackson EN, Corbo JC, Engelbach JA, et al. (2011) Notch1 loss of heterozygosity causes vascular tumors and lethal hemorrhage in mice. J Clin Invest 121: 800–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Z, Obenauf AC, Speicher MR, Kopan R (2009) Rapid identification of homologous recombinants and determination of gene copy number with reference/query pyrosequencing (RQPS). Genome Res 19: 2081–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Morimoto M, Liu Z, Cheng HT, Winters N, Bader D, et al. (2010) Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J Cell Sci 123: 213–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T (1994) Notch1 is essential for postimplantation development in mice. Genes Dev 8: 707–719. [DOI] [PubMed] [Google Scholar]
- 33. Conlon RA, Reaume AG, Rossant J (1995) Notch1 is required for the coordinate segmentation of somites. Development 121: 1533–1545. [DOI] [PubMed] [Google Scholar]
- 34. Zhang N, Martin GV, Kelley MW, Gridley T (2000) A mutation in the Lunatic fringe gene suppresses the effects of a Jagged2 mutation on inner hair cell development in the cochlea. Curr Biol 10: 659–662. [DOI] [PubMed] [Google Scholar]
- 35. Basch ML, Ohyama T, Segil N, Groves AK (2011) Canonical Notch signaling is not necessary for prosensory induction in the mouse cochlea: insights from a conditional mutant of RBPjkappa. J Neurosci 31: 8046–8058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Murata J, Tokunaga A, Okano H, Kubo T (2006) Mapping of notch activation during cochlear development in mice: implications for determination of prosensory domain and cell fate diversification. J Comp Neurol 497: 502–518. [DOI] [PubMed] [Google Scholar]
- 37. Madisen L, Zwingman TA, Sunkin SM, Oh SW, Zariwala HA, et al. (2010) A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci 13: 133–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Groves AK (2010) The challenge of hair cell regeneration. Exp Biol Med (Maywood) 235: 434–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dolle P, Fraulob V, Gallego-Llamas J, Vermot J, Niederreither K (2010) Fate of retinoic acid-activated embryonic cell lineages. Dev Dyn 239: 3260–3274. [DOI] [PubMed] [Google Scholar]
- 40. Bok J, Raft S, Kong KA, Koo SK, Drager UC, et al. (2011) Transient retinoic acid signaling confers anterior-posterior polarity to the inner ear. Proc Natl Acad Sci U S A 108: 161–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kiernan AE, Cordes R, Kopan R, Gossler A, Gridley T (2005) The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 132: 4353–4362. [DOI] [PubMed] [Google Scholar]
- 42. Liu Z, Owen T, Fang J, Srinivasan RS, Zuo J (2012) In vivo Notch reactivation in differentiating cochlear hair cells induces Sox2 and Prox1 expression but does not disrupt hair cell maturation. Dev Dyn 241: 684–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yamamoto N, Chang W, Kelley MW (2011) Rbpj regulates development of prosensory cells in the mammalian inner ear. Dev Biol 353: 367–379. [DOI] [PubMed] [Google Scholar]
- 44. Hayashi T, Ray CA, Bermingham-McDonogh O (2008) Fgf20 is required for sensory epithelial specification in the developing cochlea. J Neurosci 28: 5991–5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pirvola U, Ylikoski J, Trokovic R, Hebert JM, McConnell SK, et al. (2002) FGFR1 is required for the development of the auditory sensory epithelium. Neuron 35: 671–680. [DOI] [PubMed] [Google Scholar]
- 46. Huh SH, Jones J, Warchol ME, Ornitz DM (2012) Differentiation of the lateral compartment of the cochlea requires a temporally restricted FGF20 signal. PLoS Biol 10: e1001231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu Z, Dearman JA, Cox BC, Walters BJ, Zhang L, et al. (2012) Age-dependent in vivo conversion of mouse cochlear pillar and deiters’ cells to immature hair cells by atoh1 ectopic expression. J Neurosci 32: 6600–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Mizutari K, Fujioka M, Hosoya M, Bramhall N, Okano HJ, et al. (2013) Notch inhibition induces cochlear hair cell regeneration and recovery of hearing after acoustic trauma. Neuron 77: 58–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jacques BE, Montcouquiol ME, Layman EM, Lewandoski M, Kelley MW (2007) Fgf8 induces pillar cell fate and regulates cellular patterning in the mammalian cochlea. Development 134: 3021–3029. [DOI] [PubMed] [Google Scholar]
- 50. Liu Z, Walters BJ, Owen T, Brimble MA, Steigelman KA, et al. (2012) Regulation of p27Kip1 by Sox2 maintains quiescence of inner pillar cells in the murine auditory sensory epithelium. J Neurosci 32: 10530–10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, et al. (2001) Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Liu Z, Owen T, Zhang L, Zuo J (2010) Dynamic expression pattern of Sonic hedgehog in developing cochlear spiral ganglion neurons. Dev Dyn 239: 1674–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]