Abstract
Despite the prominent roles played by R2R3-MYB transcription factors in the regulation of plant gene expression, little is known about the details of how these proteins interact with their DNA targets. For example, while Arabidopsis thaliana R2R3-MYB protein AtMYB61 is known to alter transcript abundance of a specific set of target genes, little is known about the specific DNA sequences to which AtMYB61 binds. To address this gap in knowledge, DNA sequences bound by AtMYB61 were identified using cyclic amplification and selection of targets (CASTing). The DNA targets identified using this approach corresponded to AC elements, sequences enriched in adenosine and cytosine nucleotides. The preferred target sequence that bound with the greatest affinity to AtMYB61 recombinant protein was ACCTAC, the AC-I element. Mutational analyses based on the AC-I element showed that ACC nucleotides in the AC-I element served as the core recognition motif, critical for AtMYB61 binding. Molecular modelling predicted interactions between AtMYB61 amino acid residues and corresponding nucleotides in the DNA targets. The affinity between AtMYB61 and specific target DNA sequences did not correlate with AtMYB61-driven transcriptional activation with each of the target sequences. CASTing-selected motifs were found in the regulatory regions of genes previously shown to be regulated by AtMYB61. Taken together, these findings are consistent with the hypothesis that AtMYB61 regulates transcription from specific cis-acting AC elements in vivo. The results shed light on the specifics of DNA binding by an important family of plant-specific transcriptional regulators.
Introduction
Much of plant growth and development is shaped by sequence-specific transcription factors, proteins that act in response to external and internal cues to modulate gene expression. The MYB family is the largest family of plant sequence-specific transcription factors, with greater than 100 family members in individual plant species [1], [2], [3], [4], [5], [6]. MYB transcription factors are recognised by the presence of the MYB domain, which comprises characteristic helix-helix-loop-helix repeats of approximately 50 amino acids. The MYB domain binds DNA in a sequence-specific manner and is highly conserved in yeast, vertebrates, and plants [7]. The MYB domain is normally found near the amino terminus of the protein, and generally contains either 1, 2, or 3 of the 50 amino-acid MYB repeat. R2R3-MYB proteins have two such repeats, and comprise the largest sub-family of the MYB family. Moreover, R2R3-MYB proteins are plant specific, regulating facets of plant growth, development and metabolism [3], [5], [8], [9], [10], [11], [12], [13], [14], [15].
While members of the R2R3-MYB family are being characterised in increasing numbers, these investigations largely focus on the involvement of a particular MYB in the manifestation of a specific plant phenotype. That is, most of these analyses do not extend to a more detailed examination of MYB function at the molecular level. Nevertheless, some general themes with respect to R2R3-MYB function at the molecular level are emerging [16]. For example, many R2R3-MYB transcription factors bind to DNA motifs that are enriched in adenosine (A) and cytosine (C) residues [8], [17], where guanine (G) residues are either absent or depleted [16], [18]. These motifs have been variously referred to as AC elements, H boxes, or PAL boxes [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. Some R2R3-MYB proteins function as transcriptional activators at these sites [15], [17], while others function as transcriptional repressors [28]. AC elements are relatively short, comprising 5 or 6 nucleotides, where 3 residues form a relatively invariant core [29], [30], [31]. R2R3-MYB proteins bind to AC elements in a manner that relies on specific amino acid residues in the R2R3-MYB domain [29], [30], [31], [32], [33]. To date, the details of such interactions have been relatively scant, aside from their putative involvement in the regulation of plant-specific gene expression.
AtMYB61, a member of the Arabidopsis thaliana R2R3-MYB family of transcription factors, illustrates the involvement of R2R3-MYB family members in the regulation of plant-specific processes. AtMYB61 is a pleiotropic regulator of three major facets of the plant transpiration system: xylem cell differentiation; lateral root outgrowth; and, stomatal aperture [13], [34]. AtMYB61 modifies gene expression in response to diurnal cues so as to appropriately modify the aperture of stomata [13], the pore-like structures on leaf surfaces that enable gas exchange. Thus, AtMYB61 plays a role in modifying the capacity to take up carbon dioxide for photosynthesis, while limiting the loss of water from the plant body. AtMYB61 also alters gene expression in response to sugars, resulting in modification of plant architecture and cell wall structure [14], [35], [36]. As is the case for most R2R3-MYB transcription factors, the precise mechanisms that enable AtMYB61 to bring about important changes in plant function are unknown. Furthermore, although AtMYB61 has been shown to bind to certain consensus motifs [34], the preferential binding of AtMYB61 has not yet been determined quantitatively.
Given that R2R3-MYB proteins are involved in a rich variety of plant-specific processes [2], it would be desirable to have a more detailed understanding of R2R3-MYB and DNA motif interactions. The work described herein focuses on the interplay between AtMYB61 and its DNA target sequences. Cyclic amplification and selection of targets (CASTing), which enables identification of a transcription factor’s DNA-binding sites from a pool of random oligonucleotides, was used to identify target DNA-binding sites for AtMYB61 [37]. The sequences identified served as a useful foundation to examine mechanisms responsible for AtMYB61 sequence-specific binding, and to hypotheses about the roles these may play in shaping AtMYB61 function in vivo.
Materials and Methods
Ethics Statement
Antibody generation was carried out in strict accordance with the Province of Ontario’s Animals for Research Act, and the requirements of the federal Canadian Council on Animal Care. The protocol was approved at the University of Toronto, which involved full committee review by the Local Animal Care Committee (LACC), followed by approval by the University of Toronto Office of Research Ethics, the University Veterinarian, and finally the University of Toronto Animal Care Committee (UACC) (Permit Number: 20007080, approved 14/01/08). All efforts were made to minimise suffering.
Expression of Recombinant Protein in Bacteria
Recombinant AtMYB61 protein was produced in E. coli using the coding sequence cloned in frame into the NdeI and BamHI sites of the pET15b vector (Novagen). Recombinant AtMYB61 protein was produced, extracted and affinity purified as described previously for pine MYB proteins [17].
Antibody Production and Western Blot Analysis
Anti-AtMYB61 polyclonal antibodies were produced against the recombinant fusion protein in rabbits as described previously [38]. Affinity-purified recombinant antigen was gel-purified on a 10% SDS-PAGE gel and shipped in phosphate buffered saline to University of Toronto BioScience Support Laboratories for antibody production. In brief, 2 rabbits were each injected a total of 4 times with 300 µg of antigen per injection over a 6 week period. Production bleeds were performed after nitrocellulose dot blot assays indicated acceptable titre.
For western blot analysis, total soluble protein extracts were separated by SDS-PAGE and transferred to Bio-Rad Laboratories Nitrocellulose Trans-Blot Transfer Medium (0.45 µm) by electrophoretic transfer (BioRad, Mississauga, ON, Canada). Chemiluminescent western blot analysis was performed on the filters with Invitrogen’s Western Breeze Chemiluminescent kit as described by the manufacturer (Invitrogen, Burlington, ON, Canada). Primary antibody dilutions were done at a final dilution of 1/20000.
Cyclic Amplification and Selection of Targets (CASTing)
The CASTing assay was completed according to Wright et al [37]. CASTing was completed by incubating 15 µg of double stranded random olionucleotides (27 mers) flanked in between two constant priming sequences with the AtMYB61 full length recombinant protein. This complex was added to a Protein G Dynabead (Invitrogen, Burlington, ON, Canada) plus post-injection AtMYB61 antibody complex, causing the complex to immunoprecipitate. The immunoprecipitated complex was then washed 3 times, resuspended in 100 µL PCR buffer, boiled and then PCR amplified for 30 cycles with 15 pmol of forward and reverse primers. 10 µl of the amplified selected targets were kept for analysis and 90 µL were used to continue with the next cycle. This cycle was repeated four more times to select for AtMYB61 consensus DNA target sequences. The selected targets were then cloned into Invitrogen’s pCR4 TOPO vector and sequenced (Invitrogen, Burlington, ON, Canada).
MEME (Multiple Em for Motif Elicitation)
MEME was conducted as described previously [39]. The MEME filter criteria was set for a min/max motif width of 6, any number of repetitions of a single motif distributed among the sequences, and no restrictions on the number of motifs identified. This allowed for the identification of all over-represented hexamer sequences in the recovered CASTing-enriched oligonucleotides. Moreover, it allowed for the identification of repeats of over-represented hexamer sequences in a given CASTing-enriched oligonucleotide.
Nitrocellulose Filter-binding Assay
The nitrocellulose filter-binding assay was conducted as described by Hall and Kranz [40]. The CASTing targets that were over-represented were ordered from Invitrogen and PCR amplified (Invitrogen, Burlington, ON, Canada). These PCR products were Qiagen nucleotide purified according to the Qiagen manufacturer (Qiagen, Toronto, ON, Canada). The cleaned up PCR products were then radioactively labelled with P-32 via primer extension and further Qiagen nucleotide purified according to the Qiagen manufacturer (Qiagen, Toronto, ON, Canada). The CPM levels were measured via a liquid scintillation counter to measure the incorporation of P-32 into the probe. The radioactively labelled probes were combined in a binding reaction with recombinant AtMYB61 protein and passed through BioRad nitrocellulose filters (0.2 µm) (BioRad, Mississauga, ON, Canada). The relative binding of recombinant AtMYB61 protein to the CASTing motifs and mutated AC-I sequences were recorded. The dissociation constants (Kd) of the CASTing targets to AtMYB61 were determined by GRAFIT program which linearised the nonlinear regression via scatchard plots to calculate the point at which half of the ligand was bound to AtMYB61.
Electrophoretic Mobility-Shift Assay (EMSA)
Recombinant AtMYB61 protein was produced, extracted and affinity purified as described previously for pine MYB proteins [17]. EMSA conditions were exactly as described previously [8], [17] but using recombinant AtMYB61 protein in place of pine MYB protein.
Molecular Modelling
The tertiary structure of AtMYB61 was predicted using the tool PHYRE [41]; www.sbg.bio.ic.ac.uk/phyre/html/index.html). PHYRE proposed that the resolved structure that shared the most homology to AtMYB61 was the animal c-MYB DNA-binding domain, which was resolved previously with its DNA consensus motif (AACNG) by heteronuclear multidimensional NMR [31]. This solution structure was used to predict a 3D protein model of AtMYB61 with an E-value of 3.8e-13 and an estimated precision of 100%. The two protein sequences were 44% alike using amino acid sequence alignment. The PBD (protein data bank) file recovered from the PHYRE analysis (PBD ID = c1msfC) was used to superimpose the predicted AtMYB61 crystal structure with the c-MYB crystal structure using DaliLite [42]. The c-MYB protein was resolved along with its DNA binding sequence allowing one to predict the binding domain of AtMYB61 using homology. The PBD files for the AC-I and NBS nucleotide motifs were created from the http://structure.usc.edu/make-na/server.html server. Using Pymol [43] the two structures were modelled and superimposed [44]. Polar interactions were determined using Pymol.
Transcriptional Activation Assay
Transcriptional activation assays using yeast were as described previously [17], but substituting the AtMYB61 coding sequence in place of pine MYB sequences. Transcriptional activation assays were conducted with three biologically independent replicates per condition.
Results and Discussion
AtMYB61 Bound a Discrete Subset of DNA Target Sequences
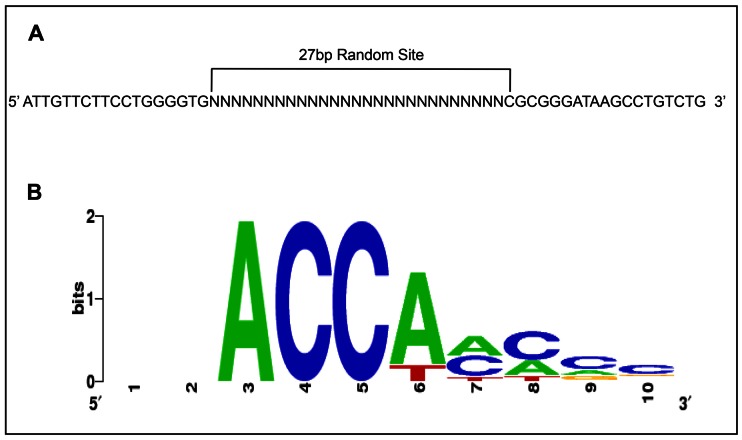
To generate an antibody of adequate specificity for the cyclic amplification and selection of targets (CASTing) assay, antibodies were raised against a non-conserved region in the AtMYB61 C-terminus (Figure S1). CASTing was initiated with a pool of 63-base-pair double-stranded oligonucleotides, where each oligonucleotide consisted of a segment of 27 random nucleotides flanked by designed sequences for PCR priming. A 15 µg (2.21×1014 DNA molecules) pool of “randomers” was incubated with AtMYB61 full-length recombinant protein (Figure 1A). Assuming the average protein-binding site is a hexamer, the 27-bp degenerate core of each double-stranded oligomer contained 21 possible positions. Therefore, in the initial round of CASTing, 21×1014 unique sites were available for binding, when using 15 µg of randomers.
Figure 1. Cylic amplification and selection of targets (CASTing) recovered a suite of hexamer target sequences that bound to AtMYB61.
(A) 27 bp random sequences flanked by two primer sites (63 bp in total) were used in the CASTing assay. (B) Sequence logo of CASTing targets discovered by MEME. The ACC motif was conserved among all target sequences. Two nucleotides upstream and downstream of the over-represented hexamer target sequences were included to analyse if the over-represented motifs could be extended beyond a hexameric sequence.
Five CASTing cycles were undertaken to enrich the pool of oligonucleotides in DNA binding-sites bound by AtMYB61. The enriched oligonucleotides were cloned into pCR4 TOPO (Invitrogen, Burlington, ON, Canada) and sequenced. Following enrichment, 89 CASTing-derived oligonucleotides were sequenced. Sequences were subjected to analysis to discover over-represented motifs using MEME (Multiple Em for Motif Elicitation) [39] (Table 1, Table 2, Figure 1B). MEME filtering criteria identified sequences with a min/max motif width of 6, any number of repetitions of a single motif distributed among the sequences, and no restrictions on the number of motifs identified. Following MEME analysis, all CASTing-enriched sequences contained over-represented motifs characterised by an abundance of adenosine and cytosine residues. These over-represented motifs had a conserved set of ACC nucleotides present at the beginning of the motifs, suggesting that these nucleotides may be essential for recognition and binding (Table 1, Table 2, Figure 1B). These motifs correspond to canonical AC elements, also known as H-boxes or PAL-boxes (Table 1, Table 2, Figure 1B). Notably, a subset of CASTing-enriched oligonucleotides had multiple AC elements present in individual target sequences (Table 1). That said, a CASTing assay is a method to identify novel DNA-binding targets of a transcription factor of interest, and further characterisations are required to determine preferred targets.
Table 1. Alignment of AtMYB61 binding sites obtained from CASTing Assay.
| MEME (Multiple EM for Motif Elicitation) identified 7 over-represented hexamer motifs. | |||
| Group | AtMYB61 Site | ||
| ACCACC | |||
| 1 | ACCCCAGAGTCCC | ACCACC | CGACCCCC |
| 2 | ACCCAAACACCACGCCCTAG | ACCACC | C |
| 3 | GCTAAACGTTCATTCCCCT | ACCACC | CC |
| 4 | A | ACCACC | TCAACAAACCCCGGCCGCCC |
| 5 | ACCAC | ACCACC | ACCCACCCCCCCCCCC |
| 6 | G | ACCACC | CTCCAACCTATACCGGCCCC |
| 7 | CCAAACTCGACCGTTCCCGC | ACCACC | C |
| 8 | GCACCCC | ACCACC | ACCATACCTACCCC |
| 9 | ACCCGATCAGGCCCTCC | ACCACC | CCCC |
| 10 | CCACACCCCACCCCGAACG | ACCACC | GC |
| 11 | ACCAACGGACTAGCTCCCAC | ACCACC | C |
| 12 | C | ACCACC | CCACCATACAATCCCTAGGC |
| 13 | ACCAC | ACCACC | ACCCCACCCTAGGACC |
| 14 | ACCACC | ACTACCCGGACCCGGCCCCCC | |
| 15 | ACACGAGATAACGACCCG | ACCACC | CCC |
| ACCTAC | |||
| 16 | GACACAAGACAC | ACCTAC | ACCCCCCCC |
| 17 | GCAGCCC | ACCTAC | ACTCCCGCTCCCCC |
| 18 | GCACCCCACCACCACCAT | ACCTAC | CCC |
| 19 | ACCCCCCCTAATTG | ACCTAC | GGCAGGC |
| 20 | CAG | ACCTAC | CCCCGCCCCCAACCCGCC |
| 21 | CACCCACCGTCCAACG | ACCTAC | ACCCC |
| 22 | GCGCACCCCACCCCCC | ACCTAC | GGCCC |
| ACCACA | |||
| 23 | ACCACA | ATGCAGCCGTACTTCGACCCC | |
| 24 | ACCACA | CCACCACCCACCCCCCCCCCC | |
| 25 | A | ACCACA | TCAACAAACCCCGGCCGCCC |
| 26 | CAACCCCTCCA | ACCACA | CCTCCCCGCC |
| 27 | CC | ACCACA | CTCTGCATTCTTGACCGCC |
| ACCATA | |||
| 28 | GGGTAATGTC | ACCATA | GCCCCCCCCCC |
| 29 | GCACCCCACCACC | ACCATA | CCTACCCC |
| 30 | CA | ACCATA | CACAACGCCCCGACCCCCC |
| 31 | CACCACCCC | ACCATA | CAATCCCTAGGC |
| 32 | CAGGCACCCCCAACCCCCC | ACCATA | CC |
| ACCAAT | |||
| 33 | AAAGGGTATACACAGGT | ACCAAT | GGCC |
| 34 | AACCTTAGGG | ACCAAT | CAATAAGGGAC |
| 35 | ACCAAT | GAAGAGACCCCTAACCATTAC | |
| 36 | ATGTGTAG | ACCAAT | GGCATAATCTGCA |
| 37 | GTCGAGTCG | ACCAAT | GCAGCACGCAGC |
| ACCAAC | |||
| 38 | CAG | ACCAAC | CTCATACCCCCCCCTGCC |
| 39 | CC | ACCAAC | CCTCCCTCCCAATGCCCGC |
| 40 | ACCAAC | GGACTAGCTCCCACACCACCC | |
| 41 | AACATGCTGTGCAACCAA | ACCAAC | ACC |
| ACCAAA | |||
| 42 | ACCAAA | AGATCAACCCCCCCCCGTACC | |
| 43 | AACATGCTGTGCA | ACCAAA | CCAACGCC |
| 44 | ACACATAAACAGCA | ACCAAA | CCAGCCC |
| 45 | AACATGCTGTGCA | ACCAAA | CCAACACC |
MEME (Multiple EM for Motif Elicitation) identified 7 over-represented hexamer motifs.
Table 2. AtMYB61 consensus sequence was derived from a comparison 89 sequences recovered from 5 cycles of CASTing.
| −2 | −1 | A | C | C | W | H | H | +1 | +2 | |
| G | 3 | 11 | – | – | – | – | – | – | 9 | 7 |
| A | 10 | 8 | 45 | – | – | 38 | 20 | 14 | 10 | 4 |
| T | 2 | 3 | – | – | – | 7 | 5 | 5 | 2 | 4 |
| C | 20 | 17 | – | 45 | 45 | – | 20 | 26 | 24 | 27 |
| Total | 45 | 45 | 45 | 45 | 45 | 45 |
The composition of each base at each position of the hexameric sequence is provided. −/+ indicate the bases 5′ or 3′ of hexameric consensus sequence. The bases 5′ or 3′ of sequences does not add up to 45 in certain circumstances because primer sites were negated from the analysis. W corresponds to A/T, H corresponds to A/T/C, – corresponds to a zero value.
AC elements, also known as PAL boxes or H-boxes, play key roles in regulating transcription for a variety of genes, particularly those encoding enzymes implicated in phenylpropanoid metabolism [18], [19], [20], [21], [22], [23], [24], [25], [26], [27]. R2R3-MYB proteins are known to bind AC elements and activate transcription from these motifs in yeast and in planta [16]. For example, pine (Pinus taeda) MYB1 [15] and MYB4 [17] and eucalyptus (Eucalyptus grandis) MYB2 [45], were all able to bind to AC elements present in the promoters of lignin biosynthetic genes. Similarly, pine (Pinus taeda) MYB1 and MYB4 bound AC elements present in the gene regulatory sequences of a pine gene encoding glutamate synthetase (GS1b) [8]. R2R3-MYB binding to AC elements is predicted to play a role in dictating xylem-localised expression of the aforementioned genes [8], [15], [17], [45]. Given the xylem-localised expression of AtMYB61 [34], it is likely that it functions in an equivalent manner to drive AC-element-mediated expression in Arabidopsis thaliana.
AtMYB61 Bound to DNA Target Sequences with Varying Degrees of Affinity
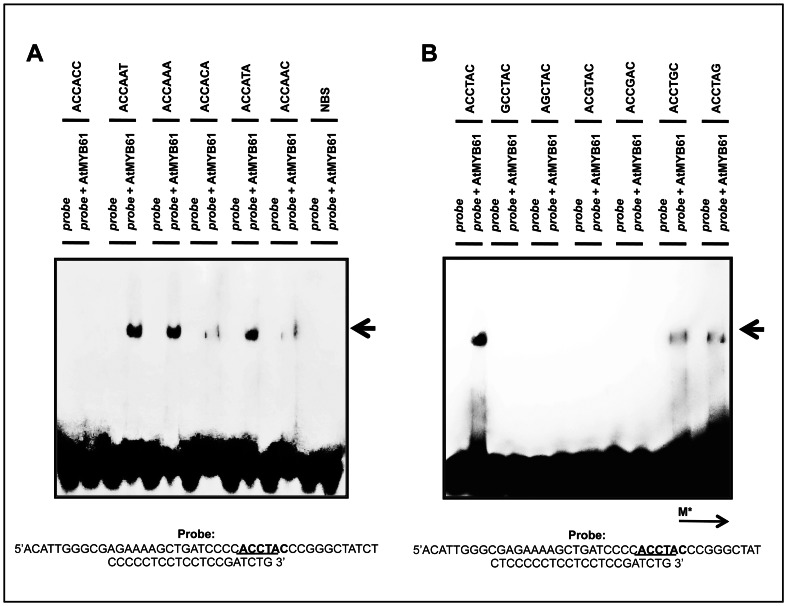
The relative binding affinities of recombinant AtMYB61 protein to the CASTing-derived sequences were determined (Table S1). Dissociation constants for each CASTing target were calculated by GRAFIT software program by using Scatchard plots (Table 3). The CASTing target that bound with the highest affinity (9.12E−09 M) was ACCTAC (AC-I) (Table 3). Since the AC-I motif was the preferred target of AtMYB61, a mutational assay was conducted on this motif to examine which nucleotides were essential for binding (Table 4). A guanine nucleotide was substituted one nucleotide at a time and shifted along the motif. A nitrocellulose filter-binding assay was used to calculate the Kds of the mutated AC-I motifs (Table 4). Binding diminished when a mutation was present in the first three nucleotides of the AC-I motif (Kd>5.00E−06 M); however, when a mutation is present in the last three nucleotides of the AC-I motif, the binding is reduced but not completely abolished (Table 4). The relative binding affinities of recombinant AtMYB61 protein to CASTing targets and mutated motifs were validated by EMSAs (Figure 2AB). EMSAs were conducted at a protein concentration of 5×10−08 M because this was the protein concentration at which not all the targets reached their binding max as determined by nitrocellulose filter-binding assay (Figure 2AB, Table S1). This enabled detection of differential binding via EMSAs.
Table 3. Dissociation constants (Kd) in mol/L and associated errors of CASTing targets.
| Kd | Error | |
| ACCTAC | 9.12E-09 | 3.11E-09 |
| ACCAAT | 1.21E-08 | 3.42E-09 |
| ACCAAA | 1.68E-08 | 4.07E-09 |
| ACCATA | 1.83E-08 | 5.06E-09 |
| ACCAAC | 7.37E-08 | 1.53E-08 |
| ACCACA | 8.08E-08 | 6.93E-09 |
| ACCACC | 6.90E-07 | 2.27E-08 |
| NBS | >5.00E-06 |
Relative binding affinities of the CASTing targets to AtMYB61 were determined by a nitrocellulose filter-binding assay. The relative binding affinities were used to determine the dissociation constants of the CASTing targets by GRAFIT program which linearised the nonlinear regression via scatchard plots to calculate the point at which half of the ligand was bound to AtMYB61. ACCTAC bound with the greatest affinity to AtMYB61. NBS or non-binding site did not bind to recombinant AtMYB61.
Table 4. Dissociation constants (Kd) in mol/L and associated errors of mutated ACCTAC (AC1 element) sequences.
| Kd | Error | |
| ACCTAC | 9.12E-09 | 3.11E-09 |
| GCCTAC | >5.00E-06 | |
| AGCTAC | >5.00E-06 | |
| ACGTAC | >5.00E-06 | |
| ACCGAC | 7.19E-07 | 2.12E-07 |
| ACCTGC | 7.97E-08 | 1.83E-08 |
| ACCTAG | 5.60E-08 | 5.09E-09 |
A guanine nucleotide was inserted one nucleotide at a time and shifted along the AC1 motif. Relative binding affinities of the mutated AC1 elements to AtMYB61 were determined by a nitrocellulose filter-binding assay. The relative binding affinities were used to determine the dissociation constants of the CASTing targets by GRAFIT program which linearised the nonlinear regression via scatchard plots to calculate the point at which half of the ligand was bound to AtMYB61. Underlined bases correspond to a substituted guanine.
Figure 2. Relative binding affinities of AtMYB61 to CASTing targets and to mutated ACCTAC motif determined by nitrocellulose filter-binding assays are confirmed by electrophoretic mobility-shift assays (EMSAs).
(A) EMSA of recombinant AtMYB61 protein binding to 6 labelled CASTing target sequences. The protein concentration used was 5×10–08M. Protein concentrations were conducted at 5×10–08M because this was the protein concentration at which targets had not all reached their binding max as determined by nitrocellulose filter-binding assay, allowing one to observe differential binding. (B) EMSA validating relative binding affinities of AtMYB61 to mutated ACCTAC motif. The protein concentration used was 5×10–08M. Mutations were conducted by substituting a single guanine nucleotide along the AC1 element. Black arrow indicates gel shift by the probe. Non-binding site (NBS) is a sequence that does not bind AtMYB61, acting as a negative control. Probes were engineered for the EMSA reaction by inserting the hexamer CASTing sequence or mutated AC1 element sequence into the underlined area.
AtMYB61 bound its preferred target AC-I (ACCTAC) with a binding constant of 9.12E−09 M (Table 3), which is similar to the tight binding of the vertebrate c-MYB R2R3 domain to the MYB binding site ((T/C)AAC(G/T)G(A/C/T)(A/C/T)) (binding constant = 1.5E−09 M±28% ) [46], [47]. Tanikawa et al. found that AACG nucleotides in the MYBSI binding site were critical for binding [47]. The second adenine, fourth cytosine, and sixth guanine were particularly important in determining binding specificity. If any of these core nucleotides were mutated, binding affinity decreased by greater than 500 fold. The third adenine was not as crucial - if it was mutated, the binding affinity would be decreased up to 15 fold. Consistent with this, AtMYB61 had a set of core recognition nucleotides – ACC – that could not be mutated without abolishing binding (Figure 2b, Table 4). Moreover, mutation of the latter half of the binding site, occurring at residues TAC, reduced binding but did not abolish it completely.
The Affinity of AtMYB61 to Specific Target DNA Sequences was Predicted by Molecular Interactions Determined in Silico
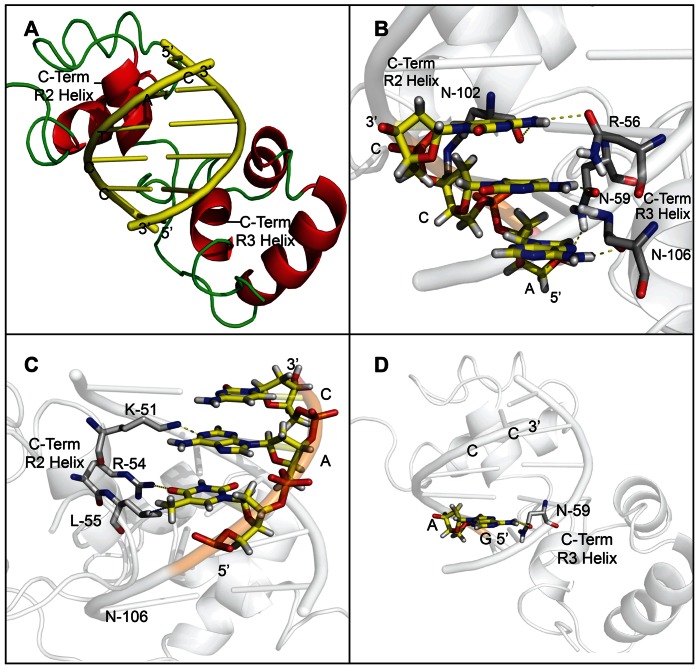
Computational analysis of the 3-dimensional structure of the N-terminal DNA-binding region of AtMYB61 was conducted in order to validate the role of this domain in sequence-specific binding. Previously, the structure of the N-terminal DNA-binding domain of animal c-MYB bound to its DNA consensus motif (AACNG) was solved by heteronuclear multidimensional NMR [31]. Animal c-MYB DNA-binding region contains a conserved R2R3-MYB domain that exhibits high similarity to plant R2R3-MYB DNA binding domains. This NMR structure was used as a template to model the structure of AtMYB61. The AC-I (ACCTAC) and NBS (GAGACC) nucleotide models were then docked into the predicted binding sites of the AtMYB61 model (Figure 3).
Figure 3. Molecular modelling of AtMYB61 with target sequences confirm binding preferences determined by nitrocellulose filter-binding assays and EMSAs.
(A) Pymol models of ACCTAC motif docked into the binding site of AtMYB61. Molecular modelling was completed by using the online program PHYRE (Protein Homology/analogY Recognition Engine) to predict a crystal structure of AtMYB61 using homology to the c-MYB DNA binding domain. The PBD (protein data bank) file recovered from the PHYRE analysis was used to superimpose the predicted AtMYB61 crystal structure with the c-MYB crystal structure using DaliLite. Using Pymol the 3D sequence model – ACCTAC – was docked into the predicted binding sites of AtMYB61. The AC1 element model is displayed in yellow, the loop secondary structure of AtMYB61 inferred model is displayed in green, and the helix secondary structure of AtMYB61 inferred model is displayed in red. (B) Model of AtMYB61 binding site with the first three ACC nucleotides in the ACCTAC sequence determines that these nucleotides are essential for binding. The AC1 (ACCTAC) nucleotide model was docked into the predicted binding site of AtMYB61. The specific hydrogen bonding between the amino acids of AtMYB61 binding site to the ACC nucleotides of AC1 were predicted by Pymol and listed as follows: asparagine-59 (R3 helix) hydrogen to adenine-1 nitrogen; asparagine-106 (R3 helix) oxygen to adenine-1 hydrogen; asparagine-59 (R3 helix) oxygen to cytosine-2 hydrogen; asparagine-102 (R3 helix) oxygen to cytosine-3 hydrogen; and arginine-56 (R2 helix) oxygen to cytosine-3 hydrogen. This confirms binding data determined by the nitrocellulose filter-binding assay and EMSAs, iterating that the ACC motif is the core recognition motif of AtMYB61. (C) Model of AtMYB61 binding site with the TAC nucleotides in the ACCTAC sequence determine that these nucleotides are less essential for binding. The AC1 (ACCTAC) nucleotide models were docked into the predicted binding sites of AtMYB61. The molecular interactions between the amino acids of AtMYB61 binding site and the TAC nucleotides of AC1 were analyzed by Pymol and are listed as follows: leucine-55 (R2 helix) methyl group was predicted to form a non-polar bond with thymidine-4 methyl group; Arginine-54 (R2 helix) hydrogen was predicted to form a hydrogen bound with thymidine-4 oxygen; lysine-51 (R2 helix) hydrogen was predicted to form a hydrogen bound with adenine-5 nitrogen; and cytosine-6 remained unbound in the model. (D) Model of AtMYB61 binding site with non-binding site (GAGACC) predicts that this motif is not recognised by AtMYB61. The non binding site model was docked into AtMYB61 binding site via Pymol and hydrogen bonding was analyzed. Only one hydrogen bond was predicted between AtMYB61 asparagine-59 (R3 helix) oxygen and the non-binding site adenine-2 hydrogen. Yellow dashed lines indicate hydrogen bonding established by Pymol program, and blue dashed lines indicate non-polar interactions.
Based on the model of AtMYB61, the molecular interactions shared between the binding sites of AtMYB61 to its targets supported in vitro binding data (Figure 3BCD). For example, there were more hydrogen bonds shared between AtMYB61 DNA-binding domain and AC-I compared to NBS (Figure 3BCD). Based on the model of AtMYB61 bound to AC-I, several specific intermolecular interactions are predicted to create binding specificity. These include hydrogen bonds between the following residues: asparagine-59 (R3 helix) of AtMYB61 with adenine-1 nitrogen of AC-I; asparagine-106 (R3 helix) oxygen of AtMYB61 with adenine-1 hydrogen of AC-I; asparagine-59 (R3 helix) oxygen of AtMYB61 with cytosine-2 hydrogen of AC-I; asparagine-102 (R3 helix) oxygen of AtMYB61 with cytosine-3 hydrogen of AC-I; arginine-56 (R2 helix) oxygen of AtMYB61 with cytosine-3 hydrogen of AC-I; arginine-54 (R2 helix) hydrogen of AtMYB61 with thymidine-4 oxygen of AC-I; and, lysine-51 (R2 helix) of AtMYB61 with adenine-5 nitrogen of AC-I. The leucine-55 (R2 helix) methyl group of AtMYB61 is predicted to form a non-polar bond with thymidine-4 methyl group of AC-I. Cytosine-6 remained unbound in the model. In comparison, the NBS model had only one hydrogen bond present, involving asparagine-59 (R3 helix) oxygen of AtMYB61 with adenine-2 hydrogen of AC-I.
The Affinity of AtMYB61 to Specific Target DNA Sequences did not Correlate with AtMYB61-driven Transcriptional Activation with each of the Target Sequences
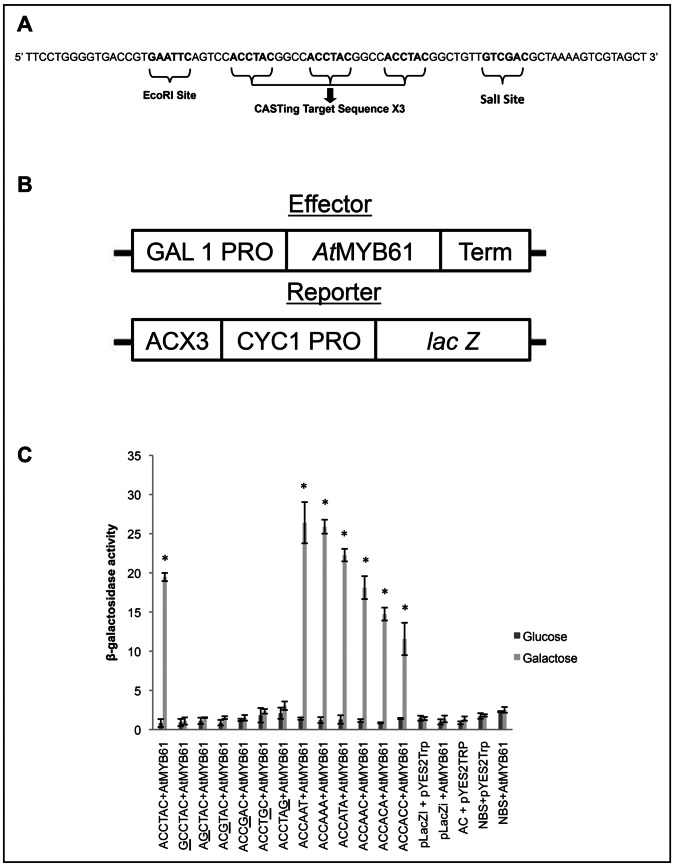
Previous studies have shown that AtMYB61 protein is sufficient to drive transcription in yeast from promoter sequences that contain AC elements [34]. Consequently, yeast transcriptional activation assays were used to determine the relationship between AtMYB61 affinity to specific DNA sequences and its capacity to drive transcription (Figure 4). Reporter constructs comprised the coding sequence for β-galactosidase under the control of the yeast minimal CYC1 promoter fused to triple repeats of a given CASTing target or a mutated AC-I motif (Figure 4). The minimal CYC1 promoter is unable to support transcription, so reporter expression would be contingent on the capacity of AtMYB61 to bind to the fused motifs, which would function as gene regulatory sequences. The expression of AtMYB61 was under the control of the galactose-inducible GAL1 promoter. As determined by the quantification of β-galactosidase activity, when AtMYB61 protein was induced by galactose, the protein was able to activate transcription from the CASTing target sequences but not from the mutated AC-I elements (Figure 4). The extent of transcriptional activation varied for each CASTing target (Figure 4C). Notably, CASTing target sequences ACCATA, ACCAAT, and ACCAAA supported greater amounts of β-galactosidase induction relative to the AC-I element, which bound with the greatest affinity to AtMYB61 (Figure 4C).
Figure 4. AtMYB61-mediated activation of promoter activity in Saccharomyces cerevisiae in an AC dependent fashion.
(A) The sequence of the oligonucleotides cloned into the reporter vector using EcoRI and SalI sites. Each AC element or mutated ACI element is triplicated within the segment. (B) Schematic representations of the Effector (pYES2TRP::AtMYB61) and Reporter (pLacZi::AC) constructs used in this assay (CYC1: minimal yeast promoter). (C) Quantitative analysis of β-galactosidase activity in yeast after induction. The measurements in liquid assay were made from three biological independent replicates. Activation of artificial genes comprising a minimal CYC1 promoter fused to a tandem AC element or mutated ACI element upstream of the lacZ gene by AtMYB61 protein, upon growth of the yeast in galactose (light grey bars), gave rise to β-galactosidase activity that was significantly greater than the controls, as determined by analysis of variance (P<0.005); including each vector alone, or both together after growth on non-inducing glucose (dark grey bars). Error bars represent standard deviations. *indicates statistically significant, P<0.005, determined by t-test. Underlined bases corresponds to a substituted guanine.
Previously, R2R3-MYB proteins have been shown to bind to AC elements and activate transcription in yeast and in planta; however, these studies did not correlate binding affinity with ability to activate transcription [8], [15], [17], [28]. Yeast activation assays determined that the affinity of AtMYB61 to specific target DNA sequences did not correlate with AtMYB61-driven transcriptional activation with each of the target sequences. This is not surprising given the multitude of studies that have observed that in vitro binding and endogenous transcriptional regulation frequently disagree [48], [49], [50], [51], [52]. Consistent with this are results obtained using the glucocorticoid receptor (GR), where no correlation between in vitro binding affinities and in vivo transcriptional activities was observed [53]. GR target sequences, differing by as little as a single nucleotide, differentially affected GR DNA binding and transcriptional activity, with no correlation between these parameters. Similarly, binding affinity of AtMYB61 to specific target DNA sequences did not correlate with AtMYB61-driven transcriptional activation with each of the target sequences. It may be that conformation of AtMYB61 changes when binding to a specific DNA sequence, altering its ability to activate transcription.
CASTing Target Sequences were Found in the Promoter Regions of Three Putative Direct Downstream Targets of AtMYB61
Previous experiments predicted three putative direct downstream target genes of AtMYB61 [34]. The predicted targets of AtMYB61 were determined by a two-stage comparative transcriptome analysis. This transcriptome analysis entailed identification and comparison of genes whose transcript abundance was modulated by differences in AtMYB61 activity, relative to those genes whose transcript abundance profiles paralleled AtMYB61 across development and in different organs. In the first stage of the analysis, publicly available, complete Arabidopsis transcriptome microarray data were used to identify those genes sharing the same transcript abundance profile as AtMYB61 across multiple stages of development. The second stage of transcriptome analysis identified genes whose transcript abundance was influenced by the presence or absence of AtMYB61. A complete transcriptome microarray dataset was generated using WT, myb61 loss-of-function mutants and 35S::MYB61 gain-of-function transgenic plants, enabling comparison of the impact of AtMYB61 on transcriptome activity. Genes were identified in this dataset whose transcript abundance was contingent on the relative abundance of AtMYB61. Both gene lists were then compared to identify genes that were most likely direct targets of AtMYB61. Three genes were identified in the intersection set. They encode the following gene products: a KNOTTED1-like transcription factor (KNAT7, At1g62990); a Caffeoyl-CoA 3-O-methyltransferase (CCoAOMT7, At4g26220); and a pectin-methylesterase (PME, At2g45220). In keeping with their role as putative AtMYB61 targets, loss-of-function mutants corresponding to each of the genes phenocopy aspects of the myb61 loss-of-function mutant phenotype.
The CASTing targets were identified in the 1000 bp 5′ non-coding regions of the three putative direct target genes and were determined by algorithm-based screening to be statistically over-represented (Figure 5). AtMYB61 bound to the 5′ gene regulatory sequences of all three putative direct target genes in an AC dependent manner [34]. These data support the hypothesis that AtMYB61 binds to AC elements in a distinct set of target genes to modify gene expression.
Figure 5. Sequences recovered from the CASTing assay were over-represented in all three promoter regions of predicted direct downstream targets of AtMYB61.
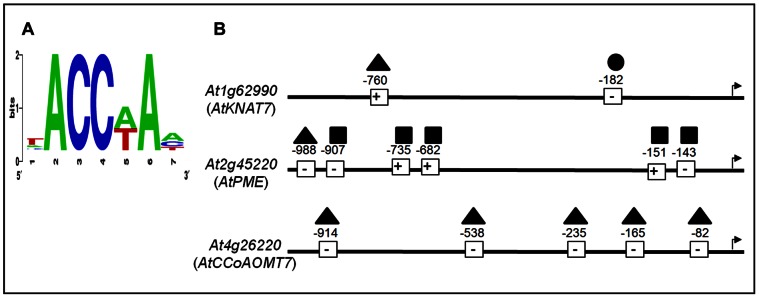
(A) Sequence logo of the over-represented motifs in the promoter sequences of the three target genes of AtMYB61, as determined by the Promomer algorithm [54] (average = 2.9; Z-score = 13; significance = 0.001). Right: Schematic representation of the promoter regions of the three putative AtMYB61 downstream target genes. (B) The three putative AtMYB61 direct downstream target genes,, KNOTTED1-like transcription factor (KNAT7, At1g62990); Caffeoyl-CoA 3-O-methyltransferase (CCoAOMT7, At4g26220), and pectin-methylesterase (PME, At2g45220), were identified by Romano et al (33). 1000 bp upstream regulatory regions were examined of the three genes. +/− indicate the orientation of CASTing target sequences relative to the sense coding strand; whereas, numbers indicate the position of these motifs relative to the putative transcriptional start (indicated by an arrow). Triangle represents ACCAAA, square represents ACCAAT, and circle represents ACCATA.
Conclusion
Despite the size and importance of the plant R2R3-MYB family of transcriptional regulators, little is known about the molecular functioning of given family members. The work described herein casts greater light on the interaction between an R2R3-MYB family member and its cognate DNA targets. The findings support the hypothesis that AtMYB61 is recruited to target genes via its interactions with a set of unique sequences, and thereby modifies gene expression. Surprisingly, the affinity of AtMYB61 to specific target DNA sequences did not correlate with AtMYB61-driven transcriptional activation with each of the target sequences, suggesting that the conformation of AtMYB61 may be altered allosterically when bound to specific target sequences. These findings point to additional complexities in the regulation of plant gene expression, and argue for the need for greater exploration of the molecular intricacies involved in the interactions between plant transcription factors and their DNA targets.
Supporting Information
At MYB61 antibody generation and validation. (A) Amino acid sequence similarity of AtMYB61 along with its closest family member AtMYB50. The two proteins have conserved N-terminal amino acid sequences but unique C-terminal domains, which was the domain selected to generate AtMYB61 antibodies against (highlighted region). (B) A chemiluminescent western blot validate anti-AtMYB61 antibody specificity. Lanes correspond to full-length recombinant AtMYB61 protein (Lane 1), of antibody alone (Lane 2), and AtMYB61 recombinant protein immunoprecipitated with pre-immune serum (Lane 3) and with AtMYB61-specific antiserum (Lane 4). Western blot was done with 1∶20000 dilution of immune serum. Western blot shows greater quantities of AtMYB61 protein eluted from the Magnetic Dynabeads Protein G antibody complex compared to the Magnetic Dynabeads Protein G pre-immune serum complex, showing that the immunoprecipitation was successful.
(TIF)
Relative binding of CASTing targets and mutated AC-I sequences to At MYB61.
(DOC)
Acknowledgments
We are very grateful to Ms. Joan Ouellette for technical assistance, Mr. Ke Wu for assistance on the CASTing assay and to Ms. Stephanie Tung, Ms. Kate Lee and Ms. Trisha Min for assistance with the yeast experiments.
Funding Statement
This work was generously supported by a Natural Science and Engineering Research Council of Canada (NSERC) Canadian Graduate Scholarship (CGSD) awarded to MBP, and funding from NSERC to MMC. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Arabidopsis Genome I (2000) Analysis of the genome sequence of the flowering plant Arabidopsis thaliana. Nature 408: 796–815. [DOI] [PubMed] [Google Scholar]
- 2. Dubos C, Stracke R, Grotewold E, Weisshaar B, Martin C, et al. (2010) MYB transcription factors in Arabidopsis. Trends in Plant Science 15: 573–581. [DOI] [PubMed] [Google Scholar]
- 3. Martin C, PazAres J (1997) MYB transcription factors in plants. Trends in Genetics 13: 67–73. [DOI] [PubMed] [Google Scholar]
- 4. Riechmann JL, Heard J, Martin G, Reuber L, Jiang CZ, et al. (2000) Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 290: 2105–2110. [DOI] [PubMed] [Google Scholar]
- 5. Stracke R, Werber M, Weisshaar B (2001) The R2R3-MYB gene family in Arabidopsis thaliana. Current Opinion in Plant Biology 4: 447–456. [DOI] [PubMed] [Google Scholar]
- 6. Tombuloglu H, Kekec G, Sakcali MS, Unver T (2013) Transcriptome-wide identification of R2R3-MYB transcription factors in barley with their boron responsive expression analysis. Mol Genet Genomics 288: 141–155. [DOI] [PubMed] [Google Scholar]
- 7. Rosinski JA, Atchley WR (1998) Molecular evolution of the Myb family of transcription factors: Evidence for polyphyletic origin. Journal of Molecular Evolution 46: 74–83. [DOI] [PubMed] [Google Scholar]
- 8. Gomez-Maldonado J, Avila C, de la Torre F, Canas R, Canovas FM, et al. (2004) Functional interactions between a glutamine synthetase promoter and MYB proteins. Plant Journal 39: 513–526. [DOI] [PubMed] [Google Scholar]
- 9. Glover BJ, Perez-Rodriguez M, Martin C (1998) Development of several epidermal cell types can be specified by the same MYB-related plant transcription factor. Development 125: 3497–3508. [DOI] [PubMed] [Google Scholar]
- 10. Jin HL, Martin C (1999) Multifunctionality and diversity within the plant MYB-gene family. Plant Molecular Biology 41: 577–585. [DOI] [PubMed] [Google Scholar]
- 11. Lipsick JS (1996) One billion years of Myb. Oncogene 13: 223–235. [PubMed] [Google Scholar]
- 12. Martin C, Bhatt K, Baumann K, Jin H, Zachgo S, et al. (2002) The mechanics of cell fate determination in petals. Philosophical Transactions of the Royal Society of London Series B-Biological Sciences 357: 809–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liang YK, Dubos C, Dodd IC, Holroyd GH, Hetherington AM, et al. (2005) AtMYB61, an R2R3-MYB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Current Biology 15: 1201–1206. [DOI] [PubMed] [Google Scholar]
- 14. Newman LJ, Perazza DE, Juda L, Campbell MM (2004) Involvement of the R2R3-MYB, AtMYB61, in the ectopic lignification and dark-photomorphogenic components of the det3 mutant phenotype. Plant Journal 37: 239–250. [DOI] [PubMed] [Google Scholar]
- 15. Patzlaff A, Newman LJ, Dubos C, Whetten R, Smith C, et al. (2003) Characterisation of PtMYB1, an R2R3-MYB from pine xylem. Plant Molecular Biology 53: 597–608. [DOI] [PubMed] [Google Scholar]
- 16. Prouse MB, Campbell MM (2012) The interaction between MYB proteins and their target DNA binding sites. Biochimica Et Biophysica Acta-Gene Regulatory Mechanisms 1819: 67–77. [DOI] [PubMed] [Google Scholar]
- 17. Patzlaff A, McInnis S, Courtenay A, Surman C, Newman LJ, et al. (2003) Characterisation of a pine MYB that regulates lignification. Plant Journal 36: 743–754. [DOI] [PubMed] [Google Scholar]
- 18. Hatton D, Sablowski R, Yung MH, Smith C, Schuch W, et al. (1995) 2 Classes of cis sequences contribute to tissue-specific expression of a PAL2 promtoer in transgenic tobacco. Plant Journal 7: 859–876. [DOI] [PubMed] [Google Scholar]
- 19. BellLelong DA, Cusumano JC, Meyer K, Chapple C (1997) Cinnamate-4-hydroxylase expression in Arabidopsis - Regulation in response to development and the environment. Plant Physiology 113: 729–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hauffe KD, Lee SP, Subramaniam R, Douglas CJ (1993) Combinatorial interactions between positive and negative cis-acting elements control spatial patterns of 4CL-1 expression in transgenic tobacco Plant Journal. 4: 235–253. [DOI] [PubMed] [Google Scholar]
- 21. Joos HJ, Hahlbrock K (1992) Phenylalanine ammonia-lyase in potato (Solanum-tuberosum L) - genomic complexity, structural comparison of 2 selected genes and modes of expression European Journal of Biochemistry. 204: 621–629. [DOI] [PubMed] [Google Scholar]
- 22. Lacombe E, Van Doorsselaere J, Boerjan W, Boudet AM, Grima-Pettenati J (2000) Characterization of cis-elements required for vascular expression of the Cinnamoyl CoA Reductase gene and for protein-DNA complex formation. Plant Journal 23: 663–676. [DOI] [PubMed] [Google Scholar]
- 23. Lauvergeat V, Rech P, Jauneau A, Guez C, Coutos-Thevenot P, et al. (2002) The vascular expression pattern directed by the Eucalyptus gunnii cinnamyl alcohol dehydrogenase EgCAD2 promoter is conserved among woody and herbaceous plant species. Plant Molecular Biology 50: 497–509. [DOI] [PubMed] [Google Scholar]
- 24. Leyva A, Liang XW, Pintortoro JA, Dixon RA, Lamb CJ (1992) Cis-element combinations determine phenylalanine ammonia-lyase gene tissue-specific expression patterns. Plant Cell 4: 263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Logemann E, Parniske M, Hahlbrock K (1995) Modes of expression and common structural features of the complete phenylalanine ammonia-lyase gene family in parsley. Proceedings of the National Academy of Sciences of the United States of America 92: 5905–5909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lois R, Dietrich A, Hahlbrock K, Schulz W (1989) A phenylalanine ammonia-lyase gene from parsley - structure, regulation and identification of elicitor and light responsive cis-acting elements. Embo Journal 8: 1641–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seguin A, Laible G, Leyva A, Dixon RA, Lamb CJ (1997) Characterization of a gene encoding a DNA-binding protein that interacts in vitro with vascular specific cis elements of the phenylalanine ammonia-lyase promoter. Plant Molecular Biology 35: 281–291. [DOI] [PubMed] [Google Scholar]
- 28. Jin HL, Cominelli E, Bailey P, Parr A, Mehrtens F, et al. (2000) Transcriptional repression by AtMYB4 controls production of UV-protecting sunscreens in Arabidopsis. Embo Journal 19: 6150–6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ogata K, Kanai H, Inoue T, Sekikawa A, Sasaki M, et al. (1993) Solution structures of Myb DNA-binding domain and its complex with DNA. Nucleic acids symposium series: 201–202. [PubMed]
- 30. Ogata K, Morikawa S, Nakamura H, Hojo H, Yoshimura S, et al. (1995) Comparison of the free and DNA-complexed forms of the DNA-binding domain from c-Myb. Nature Structural Biology 2: 309–320. [DOI] [PubMed] [Google Scholar]
- 31. Ogata K, Morikawa S, Nakamura H, Sekikawa A, Inoue T, et al. (1994) Solution structure of a specific DNA complex of the Myb DNA-binding domain with cooperative recognition helices. Cell 79: 639–648. [DOI] [PubMed] [Google Scholar]
- 32. Tahirov TH, Sasaki M, Inoue-Bungo T, Fujikawa A, Sato K, et al. (2001) Crystals of ternary protein-DNA complexes composed of DNA-binding domains of c-Myb or v-Myb, C/EBP alpha or C/EBP beta and tom-1A promoter fragment. Acta Crystallographica Section D-Biological Crystallography 57: 1655–1658. [DOI] [PubMed] [Google Scholar]
- 33. Tahirov TH, Sato K, Ichikawa-Iwata E, Sasaki M, Inoue-Bungo T, et al. (2002) Mechanism of c-Myb-C/EBP beta cooperation from separated sites on a promoter. Cell 108: 57–70. [DOI] [PubMed] [Google Scholar]
- 34. Romano JM, Dubos C, Prouse MB, Wilkins O, Hong H, et al. (2012) AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytologist 195: 774–786. [DOI] [PubMed] [Google Scholar]
- 35. Dubos C, Willment J, Huggins D, Grant GH, Campbell MM (2005) Kanamycin reveals the role played by glutamate receptors in shaping plant resource allocation. Plant Journal 43: 348–355. [DOI] [PubMed] [Google Scholar]
- 36. Penfield S, Meissner RC, Shoue DA, Carpita NC, Bevan MW (2001) MYB61 is required for mucilage deposition and extrusion in the Arabidopsis seed coat. Plant Cell 13: 2777–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wright WE, Binder M, Funk W (1991) Cyclic amplification and selection of targets (CASTing) for the myogenin consensus binding site. Molecular and Cellular Biology 11: 4104–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harlow E, and Lane D. (1988) Antibodies: A Laboratory Manual. Cold Spring Harbor NY. Cold Spring Harbor Laboratory Press.
- 39. Bailey TL, Williams N, Misleh C, Li WW (2006) MEME: discovering and analyzing DNA and protein sequence motifs. Nucleic Acids Research 34: W369–W373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall KB, and Kranz J.K. (2008) Nitrocellulose Filter Binding for Determination of Dissociation Constants. In RNA Protein Interaction Protocols Humana Press: 105–114. [DOI] [PubMed]
- 41. McDonnell AV, Jiang T, Keating AE, Berger B (2006) Paircoil2: improved prediction of coiled coils from sequence. Bioinformatics 22: 356–358. [DOI] [PubMed] [Google Scholar]
- 42. Holm L, Park J (2000) DaliLite workbench for protein structure comparison. Bioinformatics 16: 566–567. [DOI] [PubMed] [Google Scholar]
- 43. Seeliger D, de Groot BL (2010) Ligand docking and binding site analysis with PyMOL and Autodock/Vina. Journal of Computer-Aided Molecular Design 24: 417–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.DeLano WL (2002) The PyMOL Molecular Graphics System DeLano Scientific. http://wwwpymolorg.
- 45. Goicoechea M, Lacombe E, Legay S, Mihaljevic S, Rech P, et al. (2005) EgMYB2, a new transcriptional activator from Eucalyptus xylem, regulates secondary cell wall formation and lignin biosynthesis. Plant Journal 43: 553–567. [DOI] [PubMed] [Google Scholar]
- 46. Ebneth A, Schweers O, Thole H, Fagin U, Urbanke C, et al. (1994) Biophysical characterization of the c-Myb DNA-binding domain. Biochemistry 33: 14586–14593. [DOI] [PubMed] [Google Scholar]
- 47. Tanikawa J, Yasukawa T, Enari M, Ogata K, Nishimura Y, et al. (1993) Recognition of specific DNA sequences by the c-myb protooncogene product: role of three repeat units in the DNA-binding domain. Proceedings of the National Academy of Sciences of the United States of America 90: 9320–9324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fuxreiter M, Simon I, Bondos S (2011) Dynamic protein-DNA recognition: beyond what can be seen. Trends in Biochemical Sciences 36: 415–423. [DOI] [PubMed] [Google Scholar]
- 49. Lefstin JA, Yamamoto KR (1998) Allosteric effects of DNA on transcriptional regulators. Nature 392: 885–888. [DOI] [PubMed] [Google Scholar]
- 50. Massie CE, Mills IG (2008) ChIPping away at gene regulation. Embo Reports 9: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rohs R, Jin XS, West SM, Joshi R, Honig B, et al. (2010) Origins of Specificity in Protein-DNA Recognition. In: Kornberg RD, Raetz CRH, Rothman JE, Thorner JW, editors. Annual Review of Biochemistry, Vol 79. Palo Alto: Annual Reviews. 233–269. [DOI] [PMC free article] [PubMed]
- 52. Wang J, Lu J, Gu G, Liu Y (2011) In vitro DNA-binding profile of transcription factors: methods and new insights. Journal of Endocrinology 210: 15–27. [DOI] [PubMed] [Google Scholar]
- 53. Meijsing SH, Pufall MA, So AY, Bates DL, Chen L, et al. (2009) DNA Binding Site Sequence Directs Glucocorticoid Receptor Structure and Activity. Science 324: 407–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Toufighi K, Brady SM, Austin R, Ly E, Provart NJ (2005) The Botany Array Resource: e-Northerns, Expression Angling, and Promoter analyses. Plant Journal 43: 153–163. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
At MYB61 antibody generation and validation. (A) Amino acid sequence similarity of AtMYB61 along with its closest family member AtMYB50. The two proteins have conserved N-terminal amino acid sequences but unique C-terminal domains, which was the domain selected to generate AtMYB61 antibodies against (highlighted region). (B) A chemiluminescent western blot validate anti-AtMYB61 antibody specificity. Lanes correspond to full-length recombinant AtMYB61 protein (Lane 1), of antibody alone (Lane 2), and AtMYB61 recombinant protein immunoprecipitated with pre-immune serum (Lane 3) and with AtMYB61-specific antiserum (Lane 4). Western blot was done with 1∶20000 dilution of immune serum. Western blot shows greater quantities of AtMYB61 protein eluted from the Magnetic Dynabeads Protein G antibody complex compared to the Magnetic Dynabeads Protein G pre-immune serum complex, showing that the immunoprecipitation was successful.
(TIF)
Relative binding of CASTing targets and mutated AC-I sequences to At MYB61.
(DOC)