Abstract
The aim of this study was to determine the long-term carriage rates and transmission dynamics of methicillin-resistant Staphylococcus aureus (MRSA) in pig farmers and their household members. During a 6-month period in 2009–2010, 4 pig farms in Denmark, Belgium, and the Netherlands, respectively, were studied for the presence of MRSA. The proportion of persistent carriers was significantly higher among farmers than among household members (87% vs. 11%) and significantly higher in household members from Belgium compared to those from Denmark and the Netherlands (29% vs. 0% vs. 6%). Determinant analysis of MRSA carriage revealed that pig contact was the most important determinant for MRSA carriage among household members and that the increased MRSA carriage rate observed among household members from Belgium is linked to country-specific differences in pig exposure.
These findings demonstrated that even in pig farms with very high carriage rates of MRSA both in livestock and farmers, the risk for household members to acquire MRSA is limited and still depends strongly on pig exposure. By restricting access to the stables and exposure to pigs, MRSA acquisition by household members could be greatly reduced.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) is a threat to public health worldwide. Next to the well-known hospital-associated and community-associated clones, another specific clone unrelated to the aforementioned has been discovered, which originates from an extensive reservoir in food-producing animals: livestock-associated (LA-) MRSA. This clone belongs typically to multi-locus sequence type (ST) 398 and closely related STs within clonal complex (CC) 398, lacks Panton-Valentine leukocidin (PVL), and is resistant to tetracycline. The presence of LA-MRSA in pigs and pig farmers was first described in France [1] and evidence of pig-to-farmer transmission of this clone was subsequently observed in the Netherlands [2]. Several surveys in livestock in Europe confirmed a high prevalence of LA-MRSA in pigs [3], [4] and in livestock farmers (up to 30%) [5], [6], [7], but lower carriage rates in people living on farms but with limited direct contact to food-producing animals (2% to 16%) [5], [8], [9].
Moreover, previous studies have shown that LA-MRSA carriage was directly related to the intensity of livestock exposure [7], [8], [9]. Nasal carriage seems to be persistent for farmers continuously exposed to colonized livestock [10] or transient for people with sporadic or indirect contact [11]. In addition, secondary transmission and spread amongst humans has so far been studied in healthcare settings only and seems to be limited compared to other MRSA clones [12], [13]. To date, little is known about the potential transmission routes amongst humans living in farm settings, the original environment of LA-MRSA. Some studies have suggested that carriage in household members could depend on the presence of positive livestock farmers [8] and that the contaminated farm environment could contribute to the transmission as well [10], [14]. However, these studies were limited to a short period of time and the in-house farm environment was not studied.
Therefore, the aim of this longitudinal epidemiological study was to determine the long-term carriage rates and transmission dynamics of LA-MRSA in pig farmers and their household members.
Materials and Methods
Study design
We conducted a 6-month longitudinal study (8 sampling moments) of MRSA carriage among farmers and their household members living on pig farms with LA-MRSA in Belgium, Denmark, and the Netherlands during 2009–2010. This study was developed as a tool for potential larger future investigations and therefore included only a small sample size per country. In each country, 4 pig farms were selected based on the following criteria: presence of LA-MRSA in the pig herd, which was detected in previous national screening studies in which ten randomly selected animals per age group were nasally swabbed to determine the presence of MRSA CC398; farrow-to-finish production system; and ≥3 members of the farmer's household. All household members were asked to participate in the study after receiving full information from the investigators. The study protocol was approved by the Ethics Committee of the Erasme Hospital-Université Libre de Bruxelles in Belgium (protocol no. P2009/203), the Danish National Committee on Biomedical Research Ethics (protocol no. H-4-2009-112) and the Danish Data Protection Agency (protocol no. 2009-54-0821), and the Medical Ethical Committee of the St. Elisabeth hospital in the Netherlands (protocol no. 0933). The volunteers signed an informed consent form and were asked to agree to nasal swabbing and to answer standard questionnaires at each sampling moment. In cases where children were under the age of 18 years, written consent was obtained from their parents. Swabs were taken before and during pig exposure by the investigators (during exposure: midday samples) and by the volunteers themselves (before exposure: morning samples) using the Venturi Transystem (Copan Innovation, Brescia, Italy). Samples from the house environment (farmer's favorite dog or cat, farmer's favorite chair, outside door handles, and TV remote control) were taken by the investigators using Sodibox wet wipe cloths (Sodibox, Nevez, France). The rate of sample collection was calculated considering available human and environmental samples at the time of the visit, some samples were missed for several reasons, among them: absence of households in the moment of the visit or impossibility to access into the farmer's house.
Microbiological analysis
MRSA from individual samples was detected using standard methods [6]. Briefly, swabs were inoculated in a brain heart infusion enrichment broth containing 7.5% NaCl and incubated for 24 h at 35°C. Subsequently, 10 µl of a 0.5× McFarland suspension was inoculated onto chromID MRSA agar plates (bioMérieux, Marcy l'Etoile, France) and incubated for 24 h at 35°C. One suspected staphylococcal colony was selected from each plate and purified twice on Columbia agar with 5% sheep blood (Bio-Rad, Belgium) for further identification.
Species identification, presence of the lukF-lukS genes encoding PVL, and resistance to methicillin were confirmed by a triplex PCR assay [15]. Isolates (N = 100) from the first and last sampling moment at which MRSA was isolated from each volunteer/environmental site, respectively, was characterized by spa typing using the Ridom Staph Type standard protocol (http://www.ridom.com) and the Ridom SpaServer (http://spa.ridom.de/index.shtml), SCCmec typing [16], and antimicrobial susceptibility testing (spectinomycin, gentamicin, kanamycin, tobramycin, rifampin, trimethoprim-sulfamethoxazole, clindamycin, erythromycin, linezolid, chloramphenicol, mupirocin, ciprofloxacin, minocycline, tetracycline, and fusidic acid) using Neo-Sensitabs (Rosco, Taastrup, Denmark) in accordance with the Clinical Laboratory Standards Institute guidelines (CLSI 2011), as described elsewhere [17]. Multidrug resistance (MDR) was defined as resistance to ≥4 non-β-lactam antimicrobial classes. MLST [18] was performed on MRSA isolates representing each spa type.
The genetic relatedness of a subset of these isolates (N = 36) representing one farm per country was further assessed by pulsed-field gel electrophoresis (PFGE) using Cfr9I (Fermentas Gmbh, Germany) as previously described [19]. PFGE patterns were analysed using Bionumerics version 6.5 (Applied Maths, Kortrijk, Belgium) according to previously described criteria [20].
Epidemiological data
Demographic data (gender, age, occupation, status in the family), farm- and animal-related variables (exposure to pigs, cattle, poultry, horses, and pets, handling antimicrobial drugs to pigs, use of hygiene/protective measures, and occupational activities), life style determinants (eating preferences, exposure to raw meat, smoking, contact sports, travel), and medical history (exposure to health care facilities, antibiotic usage) were collected for each volunteer at each sampling moment.
Definitions
Volunteers were categorized into individuals exposed to pigs >30 hours per week and individuals exposed to pigs ≤30 hours per week, on average (termed farmers and household members, respectively), and were assigned to 1 of 3 groups with regard to MRSA carriage: persistent carriers (100% of the cultures were positive for MRSA), non-carriers (100% of the cultures were negative for MRSA), and intermittent carriers (all other volunteers).
Statistical analysis
The data were analyzed using SAS software version 9.2 (SAS Institute Inc., Cary, North Carolina, USA). Comparison of proportions was done with Chi-square tests, or Fisher's exact tests when expected cell counts were below 5. Determinants for MRSA carriage in household members were stratified per country. All tests were 2-sided, and the significance level was set at P = 0.05.
Results
MRSA carriage in the study population
A total of 60 persons (20 per country) participated in the study, including 15 farmers and 45 household members (Table 1). Altogether, 453 midday samples (both farmers and household members, sample collection rate 95% [453/480]), 69 morning samples (farmers only, sample collection rate 71% [69/96]), and 357 environmental samples (sample collection rate 93% [357/384]) were analyzed. The proportion of persistent carriers was significantly higher among farmers than among household members (87% vs. 11%; Fisher's exact P<0.0001, Table 2) and did not vary between countries (farmers from Belgium, 100%; Denmark, 80%; the Netherlands, 75%; Fisher's exact P = 0.49). The majority (87% [60/69]) of morning samples from farmers were positive for MRSA.
Table 1. Methicillin-resistant Staphylococcus aureus (MRSA) carriage among 15 farmers and 45 household members.
| Category, country | No. of non-carriers (%) | No. of intermittent MRSA carriers (%) | No. of persistent MRSA carriers (%) |
| Farmers | |||
| Belgium | 0 (0) | 0 (0) | 6 (100) |
| Denmark | 0 (0) | 1 (20) | 4 (80) |
| The Netherlands | 0 (0) | 1 (25) | 3 (75) |
| Total | 0 (0) | 2 (13) | 13 (87) |
| Household members | |||
| Belgium | 2 (14) | 8 (57) | 4 (29) |
| Denmark | 14 (93) | 1 (7) | 0 (0) |
| The Netherlands | 13 (81) | 2 (13) | 1 (6) |
| Total | 29 (64) | 11 (24) | 5 (11) |
Table 2. Isolation rates of methicillin-resistant Staphylococcus aureus in 357 environmental samples.
| Origin | No. of positive samples (%) | |||
| Belgium | Denmark | The Netherlands | Total | |
| Dog or cat | 11 (48) | 9 (29) | 2 (6) | 22 (26) |
| Chair | 9 (29) | 10 (31) | 5 (16) | 24 (25) |
| Outside door handle | 11 (46) | 2 (6) | 0 (0) | 13 (15) |
| Television remote control | 12 (50) | 7 (22) | 0 (0) | 19 (22) |
| Total | 43 (42) | 28 (22) | 7 (5) | 78 (22) |
The proportions of both intermittent and persistent MRSA carriers were significantly higher in household members from Belgium compared to Denmark and the Netherlands (intermittent: 57% vs.7% vs. 13% [Fisher's exact P = 0.004] and persistent: 29% vs. 0% vs. 6% [Fishers's exact P = 0.03]).
Environmental samples
The isolation rates of MRSA in the environmental samples are shown in Table 2. The overall isolation rate from environmental samples was 22% (78/357), with important geographic variations (Belgium 42%; Denmark 22%; the Netherlands, 5%; BE vs DK P = 0.0004; BE vs NL P<0.0001; NL vs DK P = 0.0001).
Our study did not show a positive association between environmental samples and MRSA carriage in household members (100% (9/9) of Belgian household members with MRSA in environmental samples were MRSA positive during the study, compared to 60% (3/5) of Belgian household members from MRSA-negative environments, P = 0.11; for Danish household members these numbers were 10% (1/10), 0% (0/5), P = 1.00; and for Dutch household members 23% (3/13), 0% (0/3), P = 1.00).
Molecular and phenotypic characterization
All 100 isolates subjected to molecular genotyping and antimicrobial susceptibility testing had characteristics that were compatible with LA-MRSA CC398: they displayed closely related spa types t011, t034, t0108, t1451, t2370, and t6017 and belonged to ST398 within CC398; they lacked the lukF-lukS genes encoding PVL; they carried SCCmec type V (92%) or IV (8%); and they were resistant to tetracycline (100%). In addition, 19% were MDR. Isolates recovered from farmers, household members, and environmental samples from each farm were highly homogeneous in terms of spa typing, SCCmec typing, and antimicrobial susceptibility patterns (data not shown). Furthermore, isolates originating from the same farm had indistinguishable PFGE patterns (Figure 1).
Figure 1. PFGE patterns of MRSA isolates (N = 36) from a single farm per country (Belgium n = 16, Denmark n = 14, Netherlands n = 6).
Determinants of MRSA carriage among household members
Pig contact rate (hours per week), exposure to pigs within the last 7 days, contact to sows, and handling antimicrobial drugs to pigs were significantly associated with MRSA carriage among household members (Table 3), whereas no associations were found for gender, age, status in the family, other occupations, eating preferences, exposure to raw meat, smoking, contact sports, travel, medical history, exposure to other animals (cattle, poultry, horses, and pets), use of hygiene/protective measures, presence of a farmer with MRSA in the household, and presence of MRSA in the household environment.
Table 3. Determinants for persistent and intermittent methicillin-resistant Staphylococcus aureus (MRSA) carriage among household members.
| Determinant | Value | Belgium | Denmark | Netherlands | ||||||
| Total no. | No. of carriers (%) | P | Total no. | No. of carriers (%) | P | Total no. | No. of carriers (%) | P | ||
| Total | 14 | 12 (86) | 15 | 1 (7) | 16 | 3 (19) | ||||
| Pig exposure time (hours per week) | 10–30 | 4 | 4 (100) | 1,00 | 1 | 0 (0) | 1,00 | 2 | 2 (100) | 0,03 |
| <10 | 10 | 8 (80) | 14 | 1 (7) | 14 | 1 (7) | ||||
| Exposure to pigs within last 7 days | Yes | 11 | 11 (100) | 0,03 | 3 | 0 (0) | 1,00 | 6 | 3 (50) | 0,04 |
| No | 3 | 1 (33) | 12 | 1 (8) | 10 | 0 (0) | ||||
| Contact to sows | Yes | 9 | 9 (100) | 0,11 | 3 | 0 (0) | 1,00 | 6 | 3 (50) | 0,04 |
| No | 5 | 3 (60) | 12 | 1 (8) | 10 | 0 (0) | ||||
| Handling antimicrobial drugs to pigs | Yes | 1 | 1 (100) | 1,00 | 1 | 0 (0) | 1,00 | 2 | 2 (100) | 0,03 |
| No | 13 | 11 (85) | 13 | 1 (8) | 14 | 1 (7) | ||||
Notes: P, Fisher's Exact P value. P values in bold indicate significant differences.
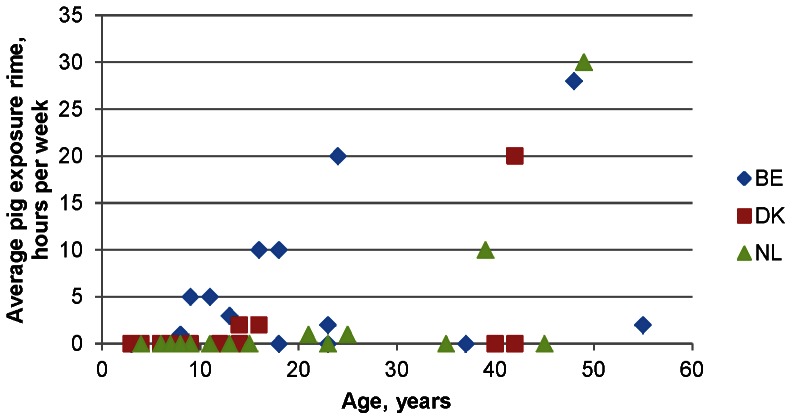
The association between country, age, and average pig exposure time among household members in each country is illustrated in Figure 2. In general, household members from Belgium were more exposed to pigs and at an earlier age compared to household members from the Netherlands and Denmark. These findings suggest that the increased MRSA carriage rate observed among household members from Belgium is linked to country-specific differences in pig exposure.
Figure 2. Association between country, age, and average pig exposure time among household members in each country.
BE, Belgium; DK, Denmark; NL, the Netherlands.
Discussion
In this study, we found that 87% of pig farmers were persistent LA-MRSA nasal carriers for a period of at least 6 months. Moreover, the majority of the pig farmers tested MRSA positive before exposure to pigs, which is consistent with persistent carriage rather than re-acquisition and loss on a daily basis. This finding supports that farmers can be a source of household transmission. However, presence of a farmer with MRSA could not be associated with household transmission, since all farmers were MRSA positive during the study.
The carriage rate found in this study is much higher than previously reported on positive pig farms (49% of farmers and 6% of household members) [5], veal farms (positive and negative farms combined: 38% of farmers and 16% of household members) [8], and in field workers visiting MRSA positive pig and veal farms (48% of field workers) [11] in the Netherlands. In addition, lower rates were found in Belgium as well (37.8% of farmers, co-workers and households in positive and negative farms combined) [6]. This could be the result either of a rising MRSA prevalence in people over time, or, more likely, due to the limited number of farms per country included in this study. A very remarkable finding was the large difference in LA-MRSA carriage rate among household members of the different countries. In Denmark and the Netherlands, the carriage rate, defined as intermittent and persistent carriers together, ranged between 7–19%, which is comparable to the MRSA nasal carriage rates found in family members of Dutch veal calf farmers (16%) [8], but much higher than the 0.2% reported in the Netherlands in people without any livestock contact [21]. In Belgium, a dramatically high carriage rate was found among household members (86%), which was comparable to that of the farmers. This can be explained by our finding that household members in Belgium were more exposed to pigs and at an earlier age compared to household members from the Netherlands and Denmark where exposure to pigs was largely restricted to farmers. As expected, all MRSA isolates shared typical characteristics of LA-MRSA in terms of spa typing and MLST, lack of the lukF-lukS genes encoding PVL, SCCmec typing, and antimicrobial susceptibility patterns as previously reported [6], [8], [9], [17] A novel finding was the frequent isolation of LA-MRSA in Belgian farm house environments (42%), which can be a reflection of the higher LA-MRSA carriage rate among Belgian household members. Although it has been suggested that the environment might play a role in LA-MRSA transmission amongst family members, our study did not show a positive association between environmental samples and MRSA carriage in household members (100% (9/9) of Belgian household members with MRSA in environmental samples were MRSA positive during the study, compared to 60% (3/5) of Belgian household members from MRSA-negative environments, Fisher's exact P = 0.11; for Danish household members these numbers were 10% (1/10), 0% (0/5), P = 1.00; and for Dutch household members 23% (3/13), 0% (0/3), P = 1.00). However, the high rates found in companion animals, particularly in Belgium, have to be interpreted with caution since the role of pets as potential vectors and/or reservoirs of LA-MRSA is still not clear and needs future research. Notably, the finding that exposure to a persistent carrier (farmer) did not imply a risk for spread to household members confirms that human-to-human transmission of this clone seems to be very limited, as previously reported [12], [13], [22].
Our results are of interest when developing strategies for preventing spread of LA-MRSA to household members of pig farmers. By restricting access to the stables and exposure to pigs, the risk of LA-MRSA acquisition by household members could be greatly reduced. However, this needs further investigation and confirmation by future studies.
In conclusion, we have demonstrated that even in pig farms with a very high carriage rates of MRSA in both livestock and pig farmers, the risk for household members to acquire MRSA is limited and depends strongly on pig exposure.
Acknowledgments
The authors would like to thank Raf De Ryck for performing PFGE analysis.
Funding Statement
This study was supported by the EU-HEALTH project PILGRIM of the 7th Framework Programme (www.fp7-pilgrim.eu). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Armand-Lefèvre L, Ruimy R, Andremont A (2005) Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg Infect Dis 11: 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Voss A, Loeffen F, Bakker J, Klassen C, Wulf M (2005) Methicillin-resistant Staphylococcus aureus in pig farming. Emerg Infect Dis 11: 1965–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. European Food Safety Authority (EFSA) (2009) Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs in the EU, 2008- Part A: MRSA prevalence estimates. Eur Food Safety Authority J 7: 1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. European Food Safety Authority (EFSA) (2010) Analysis of the baseline survey on the prevalence of methicillin-resistant Staphylococcus aureus (MRSA) in holdings with breeding pigs, in the EU, 2008 - Part B: factors associated with MRSA contamination of holdings. Eur Food Safety Authority J 8: 1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Van den Broek IV, Van Cleef BA, Haenen A, Broens EM, Van der Wolf PJ (2009) Methicillin-resistant Staphylococcus aureus in people living and working in pig farms. Epidemiol Infec 137: 700–708. [DOI] [PubMed] [Google Scholar]
- 6. Denis O, Suetens C, Hallin M, Catry B, Ramboer I, et al. (2009) Methicillin-resistant Staphylococcus aureus ST398 in swine farm personnel, Belgium. Emerg Infect Dis 15: 1098–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Graveland H, Wagenaar JA, Heesterbeek H, Mevius D, van Duijkeren E, et al. (2010) Methicillin resistant Staphylococcus aureus ST398 in veal calf farming: human MRSA carriage related with animal antimicrobial usage and farm hygiene. PloS One 5: e10990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Graveland H, Wagenaar JA, Bergs K, Heesterbeek H, Heederik D (2011) Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PloS One 6: e16830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cuny C, Nathaus R, Layer F, Strommenger B, Altmann D, et al. (2009) Nasal colonization of humans with methicillin-resistant Staphylococcus aureus (MRSA) CC398 with and without exposure to pigs. PloS One 8: e6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Köck R, Loth B, Köksal M, Schulte-Wülwer J, Harlizius J, et al. (2012) Persistence of nasal colonization with livestock-associated methicillin-resistant Staphylococcus aureus in pig farmers after holidays from pig exposure. Appl Environ Microbiol 78: 4046–4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Cleef BA, Graveland H, Haenen AP, van de Giessen AW, Heederik D, et al. (2011) Persistence of livestock-associated methicillin-resistant Staphylococcus aureus in field workers after short-term occupational exposure to pigs and veal calves. J Clin Microbiol 49: 1030–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bootsma MC, Wassenberg MW, Trapman P, Bonten MJ (2011) The nosocomial transmission rate of animal-associated ST398 methicillin-resistant Staphylococcus aureus . J R Soc Interface 8: 578–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wassenberg MW, Bootsma MC, Troelstra A, Kluytmans JA, Bonten MJ (2011) Transmissibility of livestock-associated methicillin resistant Staphylococcus aureus (ST398) in Dutch hospitals. Clin Microbiol Infect 17: 316–319. [DOI] [PubMed] [Google Scholar]
- 14. van de Giessen AW, van Santen-Verheuvel MG, Hengeveld PD, Bosch T, Broens EM, et al. (2009) Occurrence of methicillin-resistant Staphylococcus aureus in rats living on pig farms. Prev Vet Med 91: 270–273. [DOI] [PubMed] [Google Scholar]
- 15. Larsen AR, Stegger M, Sorum M (2008) spa typing directly from a mecA, spa and pvl multiplex PCR assay-a cost-effective improvement for methicillin-resistant Staphylococcus aureus surveillance. Clin Microbiol Infect 14: 611–614. [DOI] [PubMed] [Google Scholar]
- 16. Kondo Y, Ito T, Ma XX, Watanabe S, Kreiswirth BN, et al. (2007) Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob Agents Chemother 51: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Garcia-Graells C, Antoine J, Larsen J, Catry B, Skov R, et al. (2012) Livestock veterinarians at high risk of acquiring methicillin-resistant Staphylococcus aureus ST398. Epidemiol Infect 104: 383–389. [DOI] [PubMed] [Google Scholar]
- 18. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG (2000) Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus . J Clin Microbiol 38: 1008–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bosch T, de Neeling AJ, Schouls LM, van der Zwaluw KW, Kluytmans JA, et al. (2010) PFGE diversity within the methicillin-resistant Staphylococcus aureus clonal lineage ST398. BMC Microbiol 9: 10: 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Denis O, Deplano A, De Ryck R, Nonhoff C, Struelens MJ (2003) Emergence and spread of gentamicin-susceptible strains of methicillin-resistant Staphylococcus aureus in Belgian hospitals. Microb Drug Resist 9: 61–71. [DOI] [PubMed] [Google Scholar]
- 21. van Cleef BA, Verkade EJ, Wulf MW, Buiting AG, Voss A, et al. (2010) Prevalence of livestock-associated MRSA in communities with high pig-densities in The Netherlands. PloS One 5: e9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hallin M, De Mendonça R, Denis O, Lefort A, El Garch F, et al. (2011) Diversity of accessory genome of human and livestock-associated ST398 methicillin resistant Staphylococcus aureus strains. Infect Genet Evol 11: 290–299. [DOI] [PubMed] [Google Scholar]