Abstract
Cluster of differentiation 14 (CD14) gene is an important component of the human innate immune system and its role in tuberculosis (TB) has been sparsely documented. The enhanced plasma CD14 levels in TB patients as compared to healthy controls are associated with CD14 gene promoter (C-159T) polymorphism. In the past few years, the relationship between CD14 −159 C>T (rs2569190) polymorphism and risk of TB has been reported in various ethnic populations; however, those studies have yielded contradictory results. In this study systemic assessment was done for the published studies based on the association between CD14 −159 C>T polymorphism and TB risk retrieved from PubMed (Medline) and EMBASE search. A total number of 1389 TB cases and 1421 controls were included in this study and meta-analysis was performed to elucidate the association between CD14 −159 C>T polymorphism and its susceptibility towards TB. Pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) were calculated for allele contrast, homozygous comparison, heterozygous comparison, dominant and recessive genetic model. It was found that T allele carrier was significantly associated with increased TB risk (T vs. C: p-value = 0.023; OR = 1.305, 95% CI = 1.038 to 1.640). Similarly, homozygous mutant TT genotype also revealed 1.6 fold increased risk of TB (TT vs. CC; p-value = 0.040; OR = 1.652, 95% CI = 1.023 to 2.667). Additionally, dominant genetic model demonstrated increased risk of developing TB (TT vs. CC+CT: p-value = 0.006; OR = 1.585, 95% CI = 1.142 to 2.201). The study demonstrates that CD14 gene (−159 C>T) polymorphism contributes increased susceptibility for TB. Moreover, this meta-analysis also suggests for future larger studies with stratified case control population and biological characterization for validation studies.
Introduction
Tuberculosis (TB) is an infectious disease, remains a major public health concern and leading cause of morbidity and mortality across the globe. According to the World Health Organization (WHO), approximately 2 million people die annually due to tuberculosis [1]. It is estimated that approximately one-third of the world’s population is infected with Mycobacterium tuberculosis (MTB), the causative agent of tuberculosis, among them only 10% develop clinical disease [2]. This has maintained interest in that there is an inter-individual heterogeneity in host susceptibility for TB. Increasing evidences suggested that genetic variants might contribute to the underlying pathophysiology of TB at the individual level [3], [4], although the exact mechanism is still unclear.
CD14 is a key pattern recognition receptor involved in the innate immune response and renowned co-receptor for numerous microbial products and other bacterial wall-derived components [5], [6]. CD14 has several other functions, including the clearance of apoptotic cells as it is constitutively expressed, primarily on the surface of monocytes, macrophages, and neutrophil [7], [8]. CD14 exists in both membrane-bound (mCD14) and soluble forms (sCD14). Soluble CD14 (sCD14) is present in the circulation and other body fluids, and its level increases in serum plasma during inflammation and other infectious diseases [9], [10]. Similar is the case of TB, where increased level of CD14 has been reported in TB patients [11].
A polymorphism C>T identified on the −159 position of promoter region of CD14 gene and found to be linked with increased transcriptional activity that affects expression level of CD14 [12]. Given the functional significance of this genetic variant, a number of case-control studies have been done in different populations to investigate its susceptibility towards TB, but the findings remain conflicting rather than conclusive [13]–[18]. Inconsistencies in results can be attributed in terms of sample size and ethnic diversity, and individual studies might lack sufficient strength and recognition to achieve a comprehensive and reliable conclusion. Meta-analysis is a significant tool for analyzing cumulative data from studies where individual sample sizes are small and represent lower statistical power [19], [20]. Hence, a quantitative synthesis may provide more clear evidence on the association of such genetic polymorphisms with TB. In view of above we conducted a meta-analysis study based on published research articles to make a more comprehensive and compelling evaluation of the connection between −159 C>T polymorphism and TB risk.
Materials and Methods
Identification and Eligibility of Relevant Studies
We performed a PubMed (Medline) and EMBASE search covering all research articles published with a combination of the following key words: ‘CD14 gene or CD14 polymorphisms’ and ‘Tuberculosis’ (last updated on February, 2013). We evaluated potentially relevant genetic association studies by examining their titles and abstracts, and all published studies matching with the eligible criteria were retrieved.
Inclusion and Exclusion Criteria
In order to minimize heterogeneity and facilitate the appropriate interpretation and understanding of the findings, studies included in the current meta-analysis had to meet the following criteria: a) evaluation of CD14 −159 C>T polymorphism and TB risk, b) use of case-control design, c) recruitment of pathologically confirmed TB patients and TB free controls, d) have available genotype frequency in cases and controls, e) the articles must be published in English language. Among the retrieved articles, if any of the case-control study was included by more than one article using the same case series, we selected the study that included the largest number of individuals. The major reasons for exclusion of the studies were, [i] overlapping data, and [ii] case-reports and review articles.
Data Extraction and Quality Assessment
For each research publication, the methodological quality assessment and data extraction was independently abstracted in duplicate by two independent investigators using a standard protocol and data-collection form according to the inclusion criteria listed above to ensure the accuracy and consistency of the data. In case of disagreement on any item of the data, the problem was fully discussed in order to reach a mutual consensus. Characteristics abstracted from the studies included the first author’s name, year of publication, country of origin, sources of cases and controls, number of cases and controls, type of study, and genotype frequencies.
Statistical Analysis
Meta-analysis is a unique technique which merges outcomes of independent similar type of studies and derives a definitive conclusion for wider applications [19], [20]. The statistical analysis for the present study was done by using the (CMA) V2 meta-analysis comprehensive software (Biostat, USA). Meta-analysis program CMA V2 has some advantages over other programs currently available in a market for computing meta-analysis study (http://www.meta-analysis.com/pages/comparisons.html). We calculated the ORs and corresponding 95% CI values, to evaluate the association between CD14 −159 C>T polymorphism and TB risk. Basically, heterogeneity in meta-analysis refers to the variations in outcomes between different studies. Hence, heterogeneity assumption was checked by the chi-square based Q-test [21], p-value >0.05 for the Q-test indicated a lack of heterogeneity among the studies. The pooled ORs were calculated either by the fixed effects model (Mantel-Haenszel method) [22] or the random-effects model (Der Simonian and Laird) [23]. Additionally, I2 statistics was employed to quantify inter-study variability. It was in range of 0% to 100%, where a value of 0% indicated no observed heterogeneity, and higher values signified an increasing degree of heterogeneity [22]. Hardy-Weinberg equilibrium (HWE) in the control group was measured by chi-square test and significance level was maintained at p-value <0.05. Publication bias was examined through visual inspection of the funnel plots in which the standard error of log (OR) of each study had been plotted against its own log (OR), for i.e., an asymmetric plot indicated a possible publication bias [24], [25]. Funnel plot asymmetry was evaluated by Egger’s linear regression test, which is a linear regression approach for the measurement of funnel plot asymmetry on the natural logarithmic scale of the OR [20], [26]. The significance of the intercept was determined by the t-test (p-value <0.05 was considered as a representation of statistically significant publication bias) [19].
Results
Characteristics of Published Studies
A total number of forty (N = 40) research articles were retrieved through literature search from the PubMed (Medline) and EMBASE web portals/databases (Figure S1). All retrieved articles were examined by reading the titles and abstracts, and full texts for the potentially relevant publications were further checked for their suitability of incorporation in this meta-analysis. Besides the database search, the references listed in the retrieved articles were also screened for other potential articles. Studies either using CD14 polymorphism to predict survival in TB patients or considering CD14 variants as indicators for response to therapy were excluded. Moreover, studies based on investigation of the levels of CD14 mRNA or protein expression or review articles were also excluded. We included only case-control or cohort design studies showing frequency of all three genotypes. After careful screening and following the inclusion and exclusion criteria, we found seven (N = 7) eligible original published studies and included in this meta-analysis (Table 1). The distribution of genotypes in the controls did not deviate from HWE (Table 2).
Table 1. Main characteristics of all seven studies included in a meta-analysis.
| Authors | Year | Country of origin | Study design | Genotyping method | Cases | Controls |
| Alavi-Naini et al., 2012 [13] | 2012 | Iran | HB | ARMS PCR | 120 | 131 |
| Ayaslioglu et al., 2012 [14] | 2012 | Turkey | HB | PCR-RFLP | 88 | 116 |
| Zhao et al., 2012 [15] | 2012 | China | HB | PCR-Sequencing | 410 | 404 |
| Kang et al., 2009 [16] | 2009 | Korea | HB | PCR-RFLP | 274 | 422 |
| Rosas-Taraco et al., 2007 [11] | 2007 | Mexico | HB | PCR-RFLP | 104 | 114 |
| Druszczyńska et al., 2006 [17] | 2006 | Poland | HB | PCR-RFLP | 126 | 122 |
| Pacheco et al., 2004 [18] | 2004 | USA | HB | PCR-RFLP | 267 | 112 |
Note: HB = Hospital based.
Table 2. Distribution of gene polymorphism of studies included in a meta-analysis.
| Authors, year and reference | Control | Case | TB risk | |||||||
| Genotype | Minor allele | Genotype | Minor allele | HWE (p-value) | ||||||
| CC | CT | TT | MAF | CC | CT | TT | MAF | |||
| Alavi-Naini et al., 2012 [13] | 38 | 71 | 22 | 0.43 | 18 | 66 | 36 | 0.57 | 0.25 | Significant association |
| Ayaslioglu et al., 2012 [14] | 15 | 59 | 42 | 0.61 | 16 | 43 | 29 | 0.57 | 0.41 | No association |
| Zhao et al., 2012 [15] | 76 | 208 | 120 | 0.55 | 75 | 149 | 186 | 0.63 | 0.39 | Significant association |
| Kang et al., 2009 [16] | 72 | 215 | 135 | 0.57 | 39 | 118 | 117 | 0.64 | 0.38 | Significant association |
| Rosas-Taraco et al., 2007 [11] | 37 | 63 | 14 | 0.39 | 16 | 51 | 37 | 0.60 | 0.10 | Significant association |
| Druszczyńska et al., 2006 [17] | 48 | 59 | 19 | 0.38 | 38 | 59 | 25 | 0.44 | 0.90 | No association |
| Pacheco et al., 2004 [18] | 31 | 54 | 27 | 0.48 | 92 | 119 | 56 | 0.43 | 0.71 | No association |
Publication Bias
Begg’s funnel plot and Egger’s linear regression test were performed to assess the publication bias in the reports included for meta-analysis [20], [26]. The shape of funnel plots and Egger’s test did not show any evidence of publication bias (Table 3).
Table 3. Statistics to test publication bias and heterogeneity in a meta-analysis.
| Comparisons | Egger’s regression analysis | Heterogeneity analysis | Model used for meta-analysis | ||||
| Intercept | 95% Confidence Interval | p-value | Q-value | Pheterogeneity | I2 (%) | ||
| T vs. C | −0.41 | −8.38 to 7.55 | 0.89 | 24.13 | <0.0001 | 75.14 | Random |
| TT vs. CC | 1.19 | −6.07 to 8.47 | 0.68 | 24.73 | <0.0001 | 75.44 | Random |
| CT vs. CC | 3.00 | −2.59 to 8.59 | 0.22 | 12.72 | 0.04 | 52.86 | Random |
| TT+CT vs. CC | 2.03 | −4.85 to 8.91 | 0.48 | 16.92 | 0.01 | 64.54 | Random |
| TT vs. CC+CT | −0.60 | −6.22 to 5.00 | 0.79 | 19.59 | 0.003 | 69.38 | Random |
Test of Heterogeneity
Heterogeneity among selected published studies was examined by Q-test and I2 statistics and their outcomes have been shown in Table 3. Heterogeneity was observed in all the models, thus random effect model was used for calculation of combined OR and 95% CI [23].
Meta-analysis Results
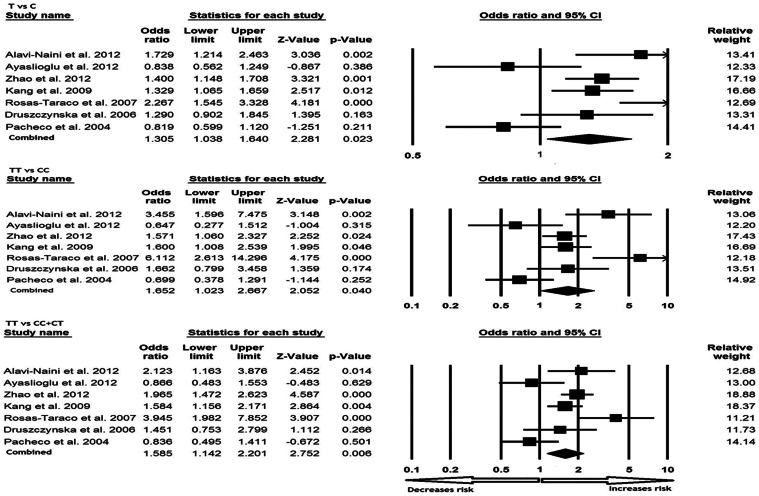
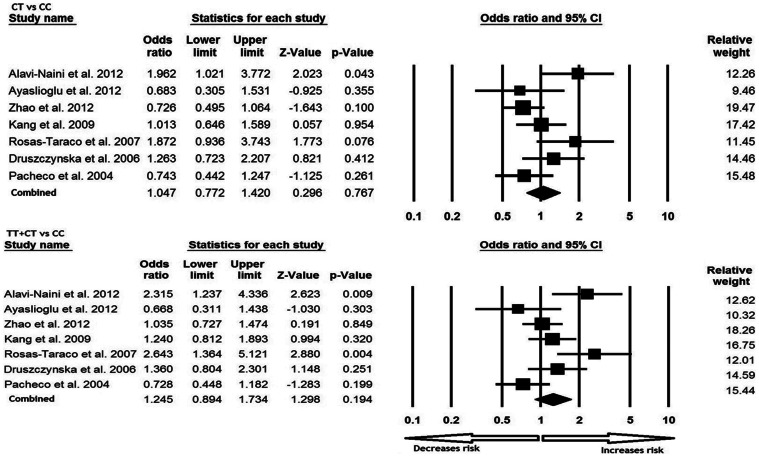
All the seven studies were pooled together which resulted into 1389 TB cases and 1421 controls, and meta-analysis was performed using random effects model (based on heterogeneity test) to assess the overall association of CD14 −159 C>T polymorphism with TB risk. The variant T allele demonstrated significant association with the risk of developing TB in terms of frequency in comparison with wild allele (T vs. C: p-value = 0.023; OR = 1.305, 95% CI = 1.038 to 1.640). Similarly, homozygous mutant genotype TT also showed increased risk of TB as compared with the wild type homozygous CC genotype (TT vs. CC; p-value = 0.040; OR = 1.652, 95% CI = 1.023 to 2.667). In addition, analysis of the dominant genetic model revealed 1.5 fold increased risk of developing TB (TT vs. CC+CT: p-value = 0.006; OR = 1.585, 95% CI = 1.142 to 2.201) (Figure 1). Whereas, heterozygous genotype CT (CT vs. CC: p-value = 0.767; OR = 1.047, 95% CI = 0.772 to 1.420) and recessive model (TT+CT vs. CC: p-value = 0.194; OR = 1.245, 95% CI = 0.894 to 1.734) failed to demonstrate significant increase in risk of developing TB as compared to CC genotype (Figure 2).
Figure 1. Forest plots (C vs. T; TT vs. CC; TT vs. CC+CT) of CD14 −159 C>T polymorphism in association with TB risk.
A meta-analysis was performed including previous reports by comprehensive meta-analysis program. Random model of meta-analysis was employed for calculation of the combined odds ratios and p-values.
Figure 2. Forest plots (CT vs. CC; TT+CT vs. CC) of CD14 −159 C>T polymorphism in association with TB risk.
A meta-analysis was performed including previous reports by comprehensive meta-analysis program. Random model of meta-analysis was employed for calculation of the combined odds ratios and p-values.
Discussion
It is well established that TB susceptibility is determined not only by the infectious agent and environmental factors but also by the host genetic factors [27]. Therefore, a number of candidate genes have been investigated to assess the possible association between modulations of TB risk across different population. In the present study, we performed a meta-analysis to examine the relationship between the −159 C>T variant in the promoter region of the CD14 gene with the risk of TB. The purpose of this study was to summarize the collected data from seven research publications included in this study and assess, whether any association exists between CD14 −159 C>T polymorphism and TB risk or not.
CD14 signaling contributes a part of host response to intracellular bacterial pathogens, such as MTB; and increased sCD14 levels in serum and bronchoalveolar lavage fluid of patients with active TB have been well documented [28], [29], and this enhanced sCD14 level mediates the pathogen-induced cell activation of cells lacking membranous CD14 (mCD14) [30]. In view of the functional relevance of CD14 signaling in MTB, it is conceivable that functional variants in the promoter region of CD14 may influence the pathogenesis of TB. The cellular and subcellular level functional studies have also revealed that CD14 −159 C>T promoter polymorphism influences the binding of the Specificity protein (Sp1 and Sp3) transcription factors that regulate the surface expression of CD14 [12]. Here in this study, individuals with CD14 −159 TT genotype had increased serum sCD14 levels, which increases the inflammatory response [31], [32]. Hence, we can speculate that CD14 −159 C>T polymorphism could therefore be a genetic factor for inter-individual variations in susceptibilities towards TB.
In the present analysis, we found an overall increase in TB risk for the carriers of one or two allele variants as compared to wild allele C and homozygous CC genotype. After stratification into dominant and recessive genetic models, the dominant model (TT vs. CC+CT) showed increased (1.5 fold) risk of TB. However, the influence and exact mechanism of CD14 expression levels on the incidence of TB is still unclear. But it can be predicted that an up-regulation of CD14 expression through MTB might help in pathogenesis of TB by easing immune interactions with mannosylated lipoarabinomannan, which subsequently enhances the production of transforming growth factor (TGF)-β and suppresses the immune response [33]. Furthermore, earlier studies have indicated that CD14 play a critical role in regulation of IgE responses via promotion of Th1 differentiation and suppression of Th2-dependent IgE responses [34], [35]. Pacheco et al. (2004) pioneered to investigate the association between the TB risk and CD14 −159 C>T polymorphism [18], thereafter, many studies have been performed to further evaluate the association in different ethnic groups; however the findings were varying and contradictory. Our meta-analysis suggests significant association of CD14 −159 C>T polymorphism with the increased risk of developing TB. Nevertheless, the etiology of TB is polygenic in nature and a single genetic variant is typically inadequate to forecast the risk of this infectious disease. Also, the major characteristic of CD14 gene −159 C>T polymorphism is that its incidence is varying substantially between different races or ethnicities. In this meta-analysis we found inter-study heterogeneity for CD14 −159 C>T polymorphism, which was probably due to various factors, such as, ethnicity, and allele and genotype distributions for −159 C>T locus. This study has several limitations which need to be addressed in future studies, for e.g., our assessment was based on relatively fewer studies possibly due to the fact that the papers included in our meta-analysis were limited to those published in English only. Further, due to limited number of reports we failed to re-analyze the association of CD14 −159 C>T polymorphism with increase TB risk in different ethnic populations.
Conclusion
In conclusion, our meta-analysis results suggest that −159 C>T polymorphism in the CD14 promoter could be employed as new risk factor for TB. Future well designed large studies incorporated with environmental factors might be necessary to validate this association in different populations in order to deduce the susceptibility of TB. Such studies might eventually lead to a better and more comprehensive understanding of the association between the CD14 −159 C>T polymorphism and TB risk.
Supporting Information
PRISMA 2009 Flow Diagram.
(DOC)
PRISMA 2009 Checklist.
(DOC)
Acknowledgments
We thank Jazan University (Jazan, Saudi Arabia) and Institute of Life Sciences (Bhubaneswar, India) for providing the software related help in data analysis.
Funding Statement
Annual research grant from Jazan University, KSA. Fund allocated from Dean, College of Nursing & Allied Health Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. King KY, Lew JD, Ha NP, Lin JS, Ma X, et al. (2011) Polymorphic allele of human IRGM1 is associated with susceptibility to tuberculosis in African Americans. PLoS One 6: e16317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stein CM (2011) Genetic epidemiology of tuberculosis susceptibility: impact of study design. PLoS Pathog 7: e1001189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cooke GS, Hill AV (2001) Genetics of susceptibility to human infectious disease. Nat Rev Genet 2: 967–977. [DOI] [PubMed] [Google Scholar]
- 4. Thye T, Vannberg FO, Wong SH, Owusu-Dabo E, Osei I, et al. (2010) Genome-wide association analyses identifies a susceptibility locus for tuberculosis on chromosome 18q11.2. Nat Genet 42: 739–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Triantafilou M, Triantafilou K (2002) Lipopolysaccharide recognition: CD14, TLRs and the LPS-activation cluster. Trends Immunol 23: 301–304. [DOI] [PubMed] [Google Scholar]
- 6. Wright SD, Ramos RA, Tobias PS, Ulevitch RJ, Mathison JC (1990) CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science 249: 1431–1433. [DOI] [PubMed] [Google Scholar]
- 7. Devitt A, Moffatt OD, Raykundalia C, Capra JD, Simmons DL (1998) Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392: 505–509. [DOI] [PubMed] [Google Scholar]
- 8. Haziot A, Chen S, Ferrero E, Low MG, Silber R, et al. (1988) The monocyte differentiation antigen, CD14, is anchored to the cell membrane by a phosphatidylinositol linkage. J Immunol 141: 547–552. [PubMed] [Google Scholar]
- 9. Ayaslioglu E, Tekeli E, Birengel S (2005) Significant elevation of serum soluble CD14 levels in patients with brucellosis. Jpn J Infect Dis 58: 11–14. [PubMed] [Google Scholar]
- 10. Juffermans NP, Verbon A, van Deventer SJ, Buurman WA, van Deutekom H, et al. (1998) Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J Infect Dis 178: 1839–1842. [DOI] [PubMed] [Google Scholar]
- 11. Rosas-Taraco AG, Revol A, Salinas-Carmona MC, Rendon A, Caballero-Olin G, et al. (2007) CD14 C(−159)T polymorphism is a risk factor for development of pulmonary tuberculosis. J Infect Dis 196: 1698–1706. [DOI] [PubMed] [Google Scholar]
- 12. LeVan TD, Bloom JW, Bailey TJ, Karp CL, Halonen M, et al. (2001) A common single nucleotide polymorphism in the CD14 promoter decreases the affinity of Sp protein binding and enhances transcriptional activity. J Immunol 167: 5838–5844. [DOI] [PubMed] [Google Scholar]
- 13. Alavi-Naini R, Salimi S, Sharifi-Mood B, Davoodikia AA, Moody B, et al. (2012) Association between the CD14 gene C-159T polymorphism and serum soluble CD14 with pulmonary tuberculosis. Int J Tuberc Lung Dis 16: 1383–1387. [DOI] [PubMed] [Google Scholar]
- 14.Ayaslioglu E, Kalpaklioglu F, Kavut AB, Erturk A, Capan N, et al.. (2012) The role of CD14 gene promoter polymorphism in tuberculosis susceptibility. J Microbiol Immunol Infect doi:pii: S1684-1182(12)00109-0. [DOI] [PubMed]
- 15. Zhao MY, Xue Y, Zhao ZQ, Li FJ, Fan DP, et al. (2012) Association of CD14 G(−1145)A and C(−159)T polymorphisms with reduced risk for tuberculosis in a Chinese Han population. Genet Mol Res 11: 3425–3431. [DOI] [PubMed] [Google Scholar]
- 16. Kang YA, Lee HW, Kim YW, Han SK, Shim YS, et al. (2009) Association between the −159C/T CD14 gene polymorphism and tuberculosis in a Korean population. FEMS Immunol Med Microbiol 57: 229–235. [DOI] [PubMed] [Google Scholar]
- 17. Druszczyńska M, Strapagiel D, Kwiatkowska S, Kowalewicz-Kulbat M, Rózalska B, et al. (2006) Tuberculosis bacilli still posing a threat. Polymorphism of genes regulating anti-mycobacterial properties of macrophages. Pol J Microbiol 55: 7–12. [PubMed] [Google Scholar]
- 18. Pacheco E, Fonseca C, Montes C, Zabaleta J, García LF, et al. (2004) CD14 gene promoter polymorphism in different clinical forms of tuberculosis. FEMS Immunol Med Microbiol 40: 207–213. [DOI] [PubMed] [Google Scholar]
- 19. Wu R, Li B (1999) A multiplicative-epistatic model for analyzing interspecific differences in outcrossing species. Biometrics 55: 355–365. [DOI] [PubMed] [Google Scholar]
- 20. Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22: 719–748. [PubMed] [Google Scholar]
- 21. DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7: 177–188. [DOI] [PubMed] [Google Scholar]
- 22. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315: 629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bellamy R (2003) Susceptibility to mycobacterial infections: the importance of host genetics. Genes Immun 4: 4–11. [DOI] [PubMed] [Google Scholar]
- 25. Hoheisel G, Zheng L, Teschler H, Striz I, Costabel U (1995) Increased soluble CD14 levels in BAL fluid in pulmonary tuberculosis. Chest 108: 1614–1616. [DOI] [PubMed] [Google Scholar]
- 26. Juffermans NP, Verbon A, van Deventer SJ, Buurman WA, van Deutekom H, et al. (1998) Serum concentrations of lipopolysaccharide activity-modulating proteins during tuberculosis. J Infect Dis 178: 1839–1842. [DOI] [PubMed] [Google Scholar]
- 27. Baldini M, Lohman IC, Halonen M, Erickson RP, Holt PG, et al. (1999) A Polymorphism in the 5′ flanking region of the CD14 gene is associated with circulating soluble CD14 levels and with total serum immunoglobulin E. Am J Respir Cell Mol Biol. 20: 976–983. [DOI] [PubMed] [Google Scholar]
- 28. Egger M, Smith GD, Phillips AN (1997) Meta-analysis: principles and procedures. BMJ 315: 1533–1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50: 1088–1101. [PubMed] [Google Scholar]
- 30. Chao YC, Chu HC, Chang WK, Huang HH, Hsieh TY (2005) CD14 promoter polymorphism in Chinese alcoholic patients with cirrhosis of liver and acute pancreatitis. World J Gastroenterol 11: 6043–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pugin J, Schurer-Maly CC, Leturcq D, Moriarty A, Ulevitch RJ, et al. (1993) Lipopolysaccharide activation of human endothelial and epithelial cells is mediated by lipopolysaccharide-binding protein and soluble CD14. P Natl Acad Sci USA 90: 2744–2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Peterson PK, Gekker G, Hu S, Sheng WS, Anderson WR, et al. (1995) CD14 receptor-mediated uptake of non-opsonized Mycobacterium tuberculosis by human microglia. Infect Immun 63: 1598–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shams H, Wizel B, Lakey DL, Samten B, Vankayalapati R, et al. (2003) The CD14 receptor does not mediate entry of Mycobacterium tuberculosis into human mononuclear phagocytes. FEMS Immunol Med Microbiol 36: 63–69. [DOI] [PubMed] [Google Scholar]
- 34. Vercelli D, Baldini M, Martinez F (2001) The monocyte/IgE connection: may polymorphisms in the CD14 gene teach us about IgE regulation? Int Arch Allergy Immunol 124: 20–24. [DOI] [PubMed] [Google Scholar]
- 35. Kang HJ, Choi YM, Chae SW, Woo JS, Hwang SJ, et al. (2006) Polymorphism of the CD14 gene in perennial allergic rhinitis. Int J Pediatr Otorhinolaryngol 70: 2081–2085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
PRISMA 2009 Flow Diagram.
(DOC)
PRISMA 2009 Checklist.
(DOC)