Abstract
AMP-activated protein kinase (AMPK) is a cellular and whole body energy sensor with manifold functions in regulating energy homeostasis, cell morphology and proliferation in health and disease. Here we apply multiple, complementary in vitro and in vivo interaction assays to identify several isoforms of glutathione S-transferase (GST) as direct AMPK binding partners: Pi-family member rat GSTP1 and Mu-family members rat GSTM1, as well as Schistosoma japonicum GST. GST/AMPK interaction is direct and involves the N-terminal domain of the AMPK β-subunit. Complex formation of the mammalian GSTP1 and -M1 with AMPK leads to their enzymatic activation and in turn facilitates glutathionylation and activation of AMPK in vitro. GST-facilitated S-glutathionylation of AMPK may be involved in rapid, full activation of the kinase under mildly oxidative physiological conditions.
Introduction
AMP-activated protein kinase (AMPK) is an evolutionary conserved heterotrimeric serine/threonine kinase that plays a central role in sensing and regulating energy homeostasis at the cellular, organ and whole-body level (recently reviewed in [1]–[7]). It exerts pleiotropic control of metabolic pathways and other physiological functions like cell growth, proliferation, motility or appetite control by affecting enzyme activities and transcription. This has made the kinase a prime pharmacological target for treating metabolic disorders or cancer [6], [8]. Activation of AMPK is triggered by a diverse array of external (e.g. hormones, cytokines, nutrients) and internal signals (e.g. AMP, ADP) linked to limited energy availability in physiological and pathological situations. Activation involves covalent phosphorylation of the α-subunit and allosteric binding of AMP or ADP to the γ-subunit. Covalent activation is complex, since it involves stimulated phosphorylation by upstream kinases (LKB1, CamKKβ) and inhibited dephosphorylation by phosphatases, both favored by binding of AMP and also ADP to different sites in the γ-subunit [9], [10] and myristoylation at the β-subunit [11].
Increasing evidence suggests that AMPK is also activated by reactive oxygen or nitrogen species (ROS, RNS), although the involved mechanisms are not entirely clear. The known inhibitory effect on mitochondrial ATP generation may simply increase cytosolic ADP/ATP and AMP/ATP ratios [12], but also other, non-canonical activation mechanisms are conceivable. We and others have reported for example that ROS/RNS, in particular peroxynitrite, may interfere with AMPK upstream signaling [13]–[15]. Vice versa, AMPK activation is involved in downstream redox regulation that can prolong cell survival [16] and induces expression of anti-oxidative proteins like superoxide dismutase (SOD), catalase or thioredoxin [17]–[19].
More recently, S-glutathionylation of Cys299 and Cys304 in the AMPK α-subunit via exposure to the strong oxidant H2O2 was reported to activate AMPK [20]. This reversible posttranslational protein modification can act as a functional switch like the well known protein phosphorylation and also protect thiol groups against further oxidation (reviewed in [21]–[23]). In case of AMPK, it probably causes activating conformational changes similar to those provoked by AMP-binding [24], [25]. Protein S-glutathionylation is often induced non-enzymatically upon expose to strong oxidants, in particular in vitro in combination with high glutathione levels [21]–[23], as shown for AMPK in presence of 200 μM H2O2 [20]. Intracellular levels of H2O2, e.g. in human fibroblasts, may at best reach the low nanomolar range [26], thus spontaneous S-glutathionylation in vivo would occur rather slowly and at low levels [21]. It may be more important in pathological, highly oxidative situations which change the cellular thiol redox state (ratio of reduced to oxidized glutathione, GSH/GSSG) and generate radical intermediates or oxidized cysteins. In vivo, protein glutathionylation is rather facilitated by specific enzymes that may constitute a dynamically regulated S-glutathionylation cycle [21], [22], [27]. Sulfiredoxins (SRx) and glutaredoxins (Grx) can act in protein deglutathionylation, and the latter enzyme also catalyzes the inverse reaction. More recently, isoforms of glutathione S-transferase (GST [28]), mainly GSTP1, were identified as catalysts of protein S-glutathionylation [28]–[32] confirming earlier models proposed by Townsend, Tew and colleagues (for recent reviews see [33]–[35]).
GSTs occur as a large superfamily of mitochondrial and cytosolic proteins. In mammals, there are seven classes of cytosolic GSTs, including the Alpha-, Mu-, and Pi-families [36], and lower eukaryotes express orthologs of these. A GST of the unicellular parasite Schistosoma japonicum belonging to the Mu-family is well known as the GST-tag used in fusion proteins to favor solubility and purification of proteins [37]. Historically, GSTs were characterized as class II detoxification enzymes that react glutathione with electrophilic compounds like by-products of oxidative stress and xenobiotics, thus facilitating their elimination from the cell (reviewed in [36], [38], [39]). However, some GSTs are now also emerging as ligands or modulators of signaling kinases like JNK, ASK1, PKC, PKA or EGFR, where either the interacting kinase or the GST is modified, functionally altered or relocated within the cell [40]–[44]. In particular GSTP1 was proposed to initiate a coordinated redox regulation of stress kinases to reduce cell death [40], [44].
While this kind of redox regulation relies exclusively on GST-protein interactions, the catalytic activity of GST is required for its role in protein glutathionylation. By hydrogen bonding of glutathione to their active site tyrosine, GST-Alpha, -Mu and -Pi enzymes decrease the pKa of the glutathione thiol group (R-SH) and thus favor thiol deprotonation to form the highly nucleophilic thiolate anion (R-S−) [22], [45], [46]. Such activated glutathione is used in various detoxification reactions, but also allows for S-glutathionylation of sulfenic acids (-SOH) on proteins with low pKa cysteines [28], [29], [31]. Inversely, it has been speculated that low GST peroxidatic activity in presence of peroxides could generate glutathione sulfenic acid intermediates that would react with protein cysteine thiolates [22]. The exact biochemical determinants of GST-catalyzed S-glutathionylation remain to be fully established. In particular, most studies so far were dedicated to the role of GSTP1 following expose to high concentrations of ROS or RNS, while few is known on its role under more physiological conditions and other GST isoforms [21].
Here we describe an in vitro glutathionylation and activation of AMPK that is catalyzed by two mammalian GST isoforms, GSTM1 and -P1, and relies on close and direct interaction of these GSTs with the AMPK β-subunit as evidenced by multiple assays. Such AMPK/GST complexes may amplify kinase activation under mildly oxidative, physiological conditions.
Materials and Methods
Cloning and protein production
Cloning, expression and purification of GSTM1, GSTP1, GST-Sj, CamKKβ, AMPK α2β2γ1 (221WT) and AMPK α2T172Dβ2γ1 mutant (221TD) is described in [7], [47] and Methods S1. For phosphorylation assays, the N-terminal Strep-tag in GSTM1 and P1 was removed, since mass spectrometry identified a serine being phosphorylated by AMPK within this tag (peptide ASWpSHPQFEK, see Methods S1, Fig. S1).
Yeast two-hybrid assays
Cytosolic yeast two-hybrid (Y2H) systems, Cyto-Y2H [48] and Split-Trp-Y2H [49], both as variants developed by Dualsystems Biotech (Schlieren, Switzerland), are described in Methods S1 and [48]. In short, GST and AMPK subunits were expressed as fusion proteins, in Cyto-Y2H with a membrane anchor and the C-terminal end of ubiquitin conjugated to a transcription factor (bait) or with the N-terminal end of ubiquitin (prey), and in Split-Trp-Y2H with the C-terminal (bait) or the N-terminal (prey) portion of Trp1p. Selective media to control the presence of bait and prey plasmid lacked tryptophan and leucine (SD-WL, Cyto-Y2H) or uracil and leucine (SD-UL, Split-Trp-Y2H), and additionally adenine and histidine (SD-AHWL, Cyto-Y2H) or tryptophan (SD-UWL, Split-Trp-Y2H) for protein interaction analysis. Spotted plates were incubated 72 h at 30°C (Cyto-Y2H) or up to 9 days at 27°C (Split-Trp-Y2H).
Rat liver extracts, protein
Rat liver was obtained from animals anesthetized with sodium pentobarbital (40 mg/kg, i.p.) according to the protocol approved by the Grenoble Ethics Committee for Animal Experimentation (no. 36_LBFA-LK-01). Liver tissue was immediately extracted in 10 mM HEPES pH 7.4 (containing 220 mM mannitol, 70 mM sucrose, 0,1% bovine serum albumin (BSA), 0.2 mM EDTA) and centrifuged twice (1000 g and 12 000 g for 10 min each) to obtain soluble proteins in the supernatant for pull-down and immunoprecipitation. Protein concentrations were determined according to Bradford [50] with the Biorad microassay (Biorad, Reinach, Switzerland) and BSA as standard.
GST pull-down, immunoprecipitation and immunoblotting
For pull-down assays, either 30 µg of purified recombinant protein (GST-Sj, rat GSTM1 and -P1, or GST-tagged acetyl-CoA carboxylase, GST-ACC [51]) or 1 mg proteins from liver extract were incubated with 30 µg recombinant AMPK (α2β2γ1 wild-type, 221WT; or T172Dα2β2γ1 mutant, 221TD) for 1 h in PD-buffer (20 mM HEPES pH 7.4, 50 mM NaCl, 2,5 mM MgCl2, 10% glycerol, 6 g/L BSA, 0,5% Tween 20, 0,02% NaN3) before addition of Glutathione Sepharose beads and incubation for an additional hour at 4°C. Where indicated, 1 mM glutathione was included. Sepharose beads were washed eight times and resuspended in SDS sample buffer. For immuno precipitation, 1 mg protein from liver extracts were reacted with anti-GSTM (ab53942, Abcam) or GSTP1 (ab53943, Abcam) antibody (1∶240) in PD-buffer overnight at 4°C. Protein A Sepharose was added, incubated for another hour at 4°C, and washed 8 times before being resuspended in SDS-PAGE sample buffer. Solubilized, denaturated proteins were subjected to SDS-PAGE and immunoblotting using anti-AMPKα primary antibody (dilution 1: 1000, 2532, Cell Signaling Technology, Danvers, MA, USA) and anti-rabbit secondary antibody (1: 5000, NA934, GE Healthcare) for detection with a chemiluminescence kit (ECL plus, GE Healthcare) and a CCD camera (ImagerQuant LAS 4000, GE Healthcare). Bands were quantified densitometrically by Image J (imagej.nih.gov/ij) and normalized. Statistical analysis was done by students T-test. Where indicated, proteins stained in PAGE gels with colloidal Coomassie Blue were identified by MALDI-TOF/TOF mass spectrometry.
Surface Plasmon Resonance (SPR) and mass spectrometry (MS)
For SPR with BIAcore (GE Healthcare), GSTs were covalently immobilized by standard amine coupling (GE Healthcare) on the carboxylic functions of two different chips. Gold chips functionalized by mixed self-assembled monolayers as described [52] were kindly provided by Wilfrid Boireau (FEMTO-ST, CNRS Besançon, France). They used 97% 11-mercapto-1-undecanol to reduce non-specific adsorption of proteins to the surface, and 3% 16-mercaptohexadecanoic acid for protein immobilization to obtain well controlled, low ligand densities as convenient for initial experiments with GST-Sj. CM5 chips (GE Healthcare) allowing higher ligand surface densities were used for sequential analyte injection during detailed kinetic analysis. GST (30 µg/ml) in 10 mM acetate buffer pH 6 (GST-Sj), pH 5 (GSTM1) or pH 4 (GSTP1) were injected at 5 µl/min to immobilize ≈2.5 ng GST/mm2. Interaction measurements were carried out in running buffer (10 mM HEPES pH 7.4, 100 mM NaCl, 50 μM EDTA, 0.005% Surfactant P20) at a flow rate of 20 µl/min. AMPK diluted to different concentrations just prior to measurements was injected onto the GST surfaces for 180 to 300 s at 20 or 30 µl/min which excludes mass transfer limitations (not shown). Experimental curves were corrected for bulk refractive index changes. Fitting of association and dissociation curves for kinetic analysis was done with BIAevaluation software. GSTs were identified by MALDI-TOF/TOF and peptide mass fingerprinting, potential phosphosites by LC-MS/MS as described in Methods S1.
AMPK glutathionylation
AMPK 221WT (1 μM, stocks preserved at −80°C) in 0.1 M phosphate buffer pH 6.5 was incubated with or without 10 mM glutathione alone or together with GSTM1 or -P1 (0.5 μM) for 10 min at 30°C. Alternatively, AMPK (1 μM, reduced by overnight incubation with 1 mM ß-mercaptoethanol in phosphate buffer as above) was incubated with EDTA (1 mM) at 30°C alone or together with GSTM1 or -P1 (10 μM, added after 5 min). After 15 min, 0,1 mM glutathione was added for 2 or 4 min. The reaction was stopped by heating in SDS sample buffer and samples separated by non-reducing SDS-PAGE and immunoblotted using primary anti-glutathione antibody (1∶1000, MAB5310, Millipore Corporation, Billerica, USA) and anti-mouse secondary antibody (1: 4000, 31430, Pierce, Rockford, USA) for luminescent detection and quantification as described above. Blotted proteins were also visualized by Ponceau staining to reveal Mr shifts due to glutathionylation.
AMPK phosphorylation
AMPK 221WT (25 nM) was incubated for 10 min at 30°C with or without glutathione (10 mM) and in presence or absence of GSTM1 or -P1 (125 nM) in kinase buffer containing 200 μM [γ-32P]ATP (specific activity 400 mCi/mmol ATP), 50 μM AMP, 5 mM MgCl2, 1 mM DTT, and 10 mM HEPES (pH 7.4). Recombinant CamKKβ (1.25 nM) was added and samples were incubated for 3 min at 30°C. The reaction was stopped by heating in SDS sample buffer and AMPK phosphorylation at Thr-172 as an indicator of AMPK activity was probed by SDS-PAGE and immunoblotting with anti-phospho-T172 AMPKα primary antibody (1: 1000, 2531, Cell Signaling Technology, Danvers, MA, USA) and anti-rabbit secondary antibody for luminescent detection and quantification as described above.
AMPK substrate phosphorylation
To analyze GST phosphorylation in vitro, AMPK 221WT (4 pmol) was activated by incubation with CamKKβ (1 pmol) for 20 min at 30°C in kinase buffer with cold ATP. Purified GSTs and ACC [51] (200 pmol each) were then incubated for 3–60 min at 37°C in the presence or absence of pre-activated AMPK 221WT (4 pmol) in kinase buffer. For negative controls, GSTs were incubated with 1 pmol CamKKβ alone without AMPK. To analyze effects of GST/AMPK complexes on in vitro phosphorylation of AMPK substrates, AMPK 221WT (4 pmol, reduced as above) was pre-activated with CamKKβ in kinase buffer with cold ATP and glutathionylated with 0,1 mM glutathione in presence or absence of GSTM1 or -P1, both as described above. Then, ACC (200 pmol) [51] and [γ-32P]ATP were added and the mixture incubated for 2 min at 37°C. Kinase reactions were stopped as above, separated on SDS-PAGE and analyzed by Typhoon phosphoimager (GE Healthcare).
Results
GST-Mu and -Pi isoforms interact with AMPK in vitro
In the course of our interactomic research on AMPK, we repeatedly pulled down recombinant heterotrimeric AMPK with recombinant proteins fused to a GST-tag that is derived from S. japonicum GST (GST-Sj). In fact, GST-Sj alone can pull down AMPK 221WT (Fig. 1). We first examined whether such heterologous interaction of GST-Sj with rat AMPK reflects an interaction that evolved with homologous rat GSTM1 (closest homologue of GST-Sj, 44% sequence identity) and GSTP1 (30% sequence identity). Both enzymes were cloned from a rat cDNA library, bacterially expressed and purified. In an assay with five fold molar excess of GST, both GSTM1 and –P1 were able to pull down AMPK 221WT even after extensive washing (Fig. 1). These results suggest that the GST/AMPK interaction evolved in at least two different eukaryotic GST classes: the Mu and Pi families.
Figure 1. GST isoforms and GST-tag interact with full-length AMPK in pull-down assays.

Pull-down of recombinant AMPK 221WT with GST-Sj (S. japonicum), GSTM1 or GSTP1 (R. norvegicus). In all assays, AMPK (0.075 mg/ml) was incubated with or without (negative control) GST proteins (0.075 mg/ml). Pull-down with Glutathione Sepharose 4B was subjected to immunoblot analysis using anti-AMPKα antibody. Left: representative data; right: quantification (mean ± SD, n = 3; * p<0,01 and # p<0,05 versus no GST).
GST-Mu and -Pi isoforms directly interact with AMPK β-subunits in Y2H assays
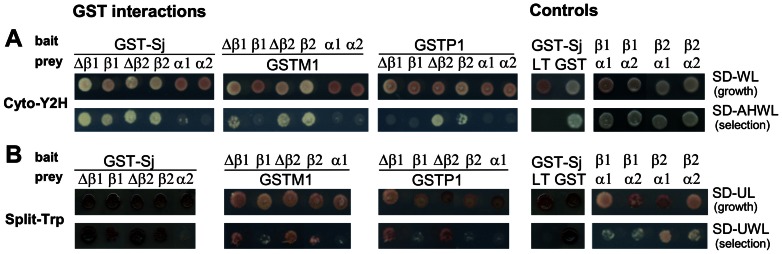
A potential direct interaction of GSTs with AMPK in vivo was verified by two different last-generation Y2H assays [53]. Here, bait/prey interaction leads to reconstitution of split proteins in the yeast cytosol, either of ubiquitin (Cyto-Y2H) or an enzyme in tryptophan biosynthesis (Split-Trp-Y2H). Readout is provided via transcription factor release by ubiquitin-specific proteases that triggers transcription of reporter genes (Cyto-Y2H), or more directly by allowing growth on Trp-deficient medium (Split-Trp-Y2H) [49]. While the transcriptional amplification of the Cyto-Y2H read-out makes it very sensitive to detect even weak or transient interactions, the direct readout of the Split-Trp-Y2H is more proportional to interaction strength.
The three examined GSTs (GST-Sj, GSTM1, GSTP1) did not interact with AMPK α -subunits in the sensitive Cyto-Y2H assay (Fig. 2A). However, all three showed interaction with AMPK β-subunits: β1 and β2 in case of GST-Sj, and preferentially β2 in case of GSTM1 and -P1. The N-terminal domain of the β-subunits (Δβ1 or Δβ2, amino acids 1–54) was sufficient for GST/AMPK binding, suggesting that it is part of the interaction domain. Control experiments confirmed expected GST homodimerization and AMPK α/β-subunit interaction, while no binding to unrelated protein Large T (LT) was detected. The Split-Trp-Y2H assay confirmed these data (Fig. 2B), although the readout was weaker in some cases, as e.g. in case of the AMPK α-/β-subunit interaction. In both assays, results were similar, irrespective whether GST-Sj was used as bait or prey (not shown).
Figure 2. Y2H analysis identifies GST isoforms as AMPK interaction partners and the AMPK interaction domain.
Two different cytosolic Y2H systems were applied to analyze interaction of AMPK with Schistosoma japonicum GST (GST-Sj) and mammalian (rat) GSTM1 and GSTP1. (A) Cyto-Y2H: interacting proteins lead to reconstitution of ubiquitin and a transcriptional readout allowing growth on medium lacking adenine and histidine (SD-AHWL). Spots represent yeast grown for 72 h at 30°C. (B) Split-Trp-Y2H: interacting proteins lead to reconstitution of Trp1p, an enzyme in tryptophan biosynthesis, and allow growth on medium lacking tryptophan (SD-UWL). Yeast was grown for 8–9 days at 27°C. Δβ, N-terminal domain of AMPK β-subunit. Controls: LT, Large T Antigen of Simian Virus (amino acids 84–704; negative control); GST, GST-Sj (positive control). A representative data set out of three independent experiments is shown. For more details see Materials and Methods and Supporting Information.
GST/AMPK interaction occurs in rat liver
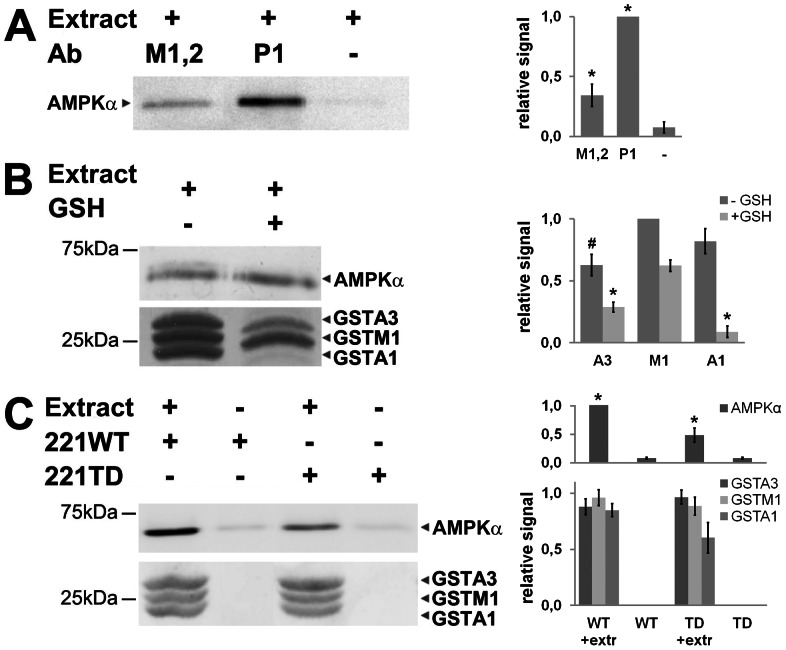
To test whether also endogenous rat GST isoforms bind to AMPK, we used crude rat liver extracts for co-immunoprecipitation and GST pull-down assays. Liver contains mainly GST-Alpha and -Mu isoforms and few GST-Pi. Endogenous rat AMPK indeed co-immunoprecipitated with antibodies specific for GSTM1/2 and GSTP1 (Fig. 3A) and pulled down together with three major endogenous GST isoforms, GSTA1, GSTA3 and GSTM1, as identified by MALDI mass spectrometry (Fig. 3B). If glutathione is then added to the extract, the pull-down assay becomes more stringent. Mainly GSTM1 is now pulled down, while GSTA1 and GSTA3 are strongly reduced, without affecting the quantity of associated AMPK. Finally, when liver extracts were spiked with additional recombinant AMPK 221WT or 221TD, even more AMPK was pulled down (Fig. 3C). More inactive AMPK 221WT was recovered as compared to AMPK 221TD, a mutant mimicking active AMPK [54].
Figure 3. AMPK interacts with endogenous GST isoforms in rat liver.
GST immunoprecipitation (A) or pull-downs (B, C) were performed with rat liver extract. AMPK in immunoprecipitates or pull-down fractions was detected by immunoblot analysis with anti-αAMPK antibody. The main liver GST isoforms in pull-down fractions were detected by Ponceau staining and mass spectroscopy. (A) Immunoprecipitation of endogenous AMPK by anti-GSTM1/2 or anti-GSTP1 antibodies. (B) GST pull-down of endogenous liver AMPK by liver GST isoforms in absence or presence of glutathione. Note: Addition of glutathione reduces pull-down of GSTA isoforms without affecting pull-down of AMPK. (C) GST pull-down of added AMPK 221WT or constitutively active 221TD. Left: representative data sets; right: quantification (mean ± SD, n = 3; * p<0,01 and # p<0,05 versus no GST (A), GSTM1 (B) or no extract (C)). Extr, liver extract.
GST/AMPK interaction is direct and rapid
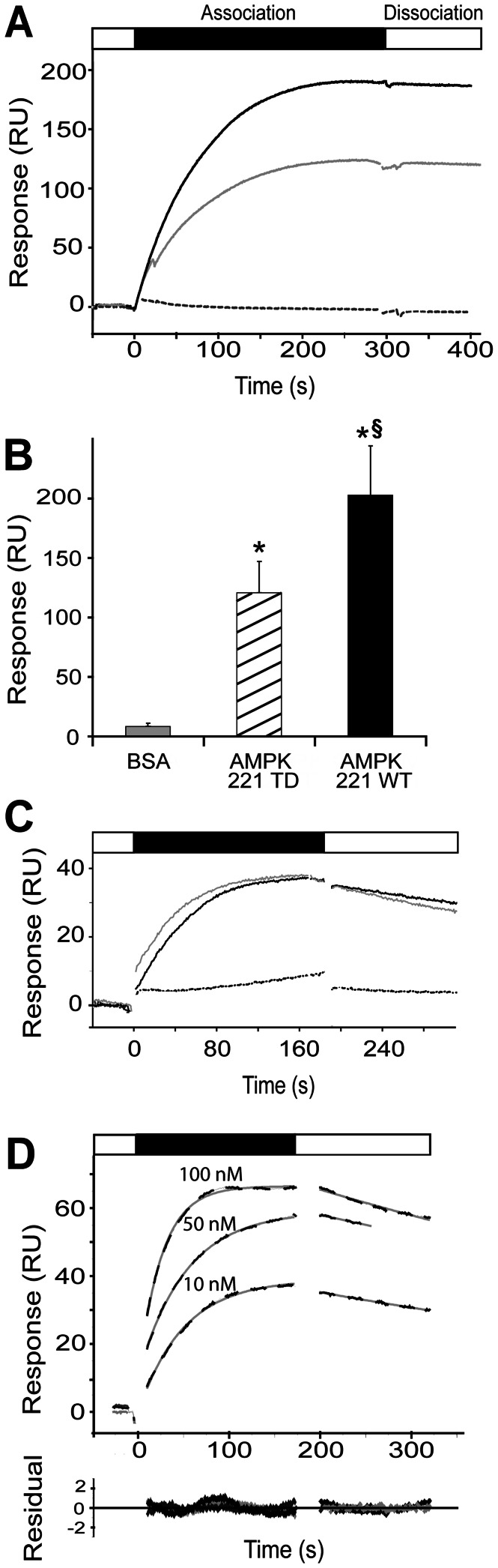
To obtain quantitative data on the GST/AMPK interaction in respect to kinetics and affinity, we performed a series of in vitro experiments with surface plasmon resonance spectroscopy (SPR). The GST interaction partner was chosen for covalent immobilization since it appeared more stable in this setup. We first used a sensor chip where the gold surface had been functionalized with a self assembled monolayer that reduces non-specific adsorption to the surface and allows immobilization of low ligand densities for analyzing GST-Sj. Sensorgrams with AMPK 221WT, 221TD or BSA (negative control) injected onto this surface confirmed a direct GST/AMPK interaction (Fig. 4A) that was not affected by glutathione (not shown). The equilibrium response was 203±41.5 RU for AMPK 221WT, which was reduced to 121±27 RU for AMPK 221TD, as compared to 8±3.7 RU for BSA (Fig. 4B). Conventional CM5 sensor chips were then used to confirm a specific interaction of rat GSTM1 or GSTP1 with rat AMPK (Fig. 4C) and to extract affinity data for GSTM1 by injecting an AMPK concentration series (Fig. 4D). The simple kinetics could be very well fitted to a Langmuir 1∶1 model (Fig. 4D) as seen by the very low residuals of the fit (<1 RU). The fast association (ka = 3,1·105 M−1 s−1) and the very slow dissociation (kd = 1,6·10−3 s−1) resulting in a KD of about 5 nM.
Figure 4. Surface plasmon resonance identifies high affinity interactions between GSTs and AMPK.
Freshly diluted, recombinant full-length AMPK was injected onto immobilized GST. (A) GST-Sj binding of 10 nM AMPK 221WT (black full line), constitutive active AMPK 221TD (grey full line) or BSA (grey dotted line) at a flow rate of 20 µl/min (surface: self-assembled monolayer). (B) Equilibrium response from (A), mean ± SD, 12 (221TD), 6 (221WT) or 3 (BSA) independent experiments (* p<0,01 versus control; § p<0,01 versus AMPK-TD). (C) Comparison of GSTM1 (black) or GSTP1 (grey) association and dissociation kinetics of 10 nM AMPK 221WT (full lines) or 100 nM of BSA (dotted lines) and a flow rate of 30 µl/min (surface: CM5). (D) GSTM1 association and dissociation kinetics of AMPK 221WT at different concentrations (dashed black lines) and a flow rate of 30 µl/min (surface: CM5), single exponential fit of experimental data (grey lines) and corresponding residuals (to assess the quality of the fit, lower panel). Representative sensorgrams of at least two repetitions are shown. Bars on the top of sensorgrams indicate protein injection (association, black) or injection of running buffer (white).
GST/AMPK complexes do not lead to relevant GST phosphorylation but increase GST activity
To gain insight into the putative role(s) of GST/AMPK complexes, we first examined whether they lead to GST phosphorylation and/or affect GST activity. In vitro phosphorylation assays with CamKKβ-activated AMPK and a 50-fold excess of GSTs revealed no phosphorylation of GST-Sj and slow, very low level phosphorylation of GSTM1 and GSTP1 as compared to ACC (Fig. 5), reaching less than 7% of the ACC phosphorylation level within 1 hour (Fig. S2). Presence of glutathione did not further increase this phosphorylation (not shown) as it was reported for PKA and PKC [43], and no specific phosphosites could be identified by mass spectrometry (not shown). However, in an activity assay using the model substrate 1-chloro-2,4-dinitrobenzene (CDNB), addition of AMPK 221WT to GSTM1 or GSTP1 led to a moderate increase of vmax by about 25% at almost unchanged apparent Km (Table 1, Fig. S3). This increase occurred only after mixing GST with AMPK, not with BSA, and did not require addition of active AMPK 221TD (Table 1). Thus, GST activation is not due to unspecific stabilization by protein addition and unrelated to the faint and slow GST phosphorylation. Rather, specific GST/AMPK complex formation itself altered the catalytic properties of GST, since GST activation also depended on the amount of AMPK 221WT added (Fig. S3).
Figure 5. GST is a poor AMPK substrate.
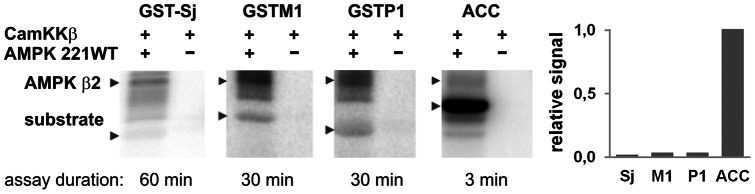
AMPK 221WT (4 pmol) pre-activated by CamKKβ (1 pmol) does not phosphorylate GST-Sj and phosphorylates GSTM1 and -P1 only at low levels as compared to ACC (all at 200 pmol). In vitro phosphorylation assays were run for 3 min (GST-ACC), 30 min (GSTP1, GSTM1) or 60 min (GST-Sj) and analyzed by SDS-PAGE and Typhoon phosphoimager. Note the autophosphorylation of the AMPK β-subunit. Representative data with quantification are shown. Detailed phosphorylation kinetics is shown in Fig. S2.
Table 1. Enzyme kinetic parameters of GSTP1 in presence or absence of AMPK or BSA.
| vmax | kcat | Km (CDNB) | |
| (U mg−1) | (s−1) | (mM) | |
| GSTM1 | 21,6±0.8 | 16,9±0,5 | 0,037±0.005 |
| GSTM1 + BSA | 20,9±0.8 | 16,4±0,5 | 0,033±0.006 |
| GSTM1 + AMPK 221 | 25,5±0.7 | 20,0±0,5 | 0,045±0,005 |
| GSTM1 + AMPK 221TD | 25,9±0,9 | 20,3±0,6 | 0,043±0,006 |
| GSTP1 | 20.4±2.9 | 16,0±2,3 | 1.9±0.4 |
| GSTP1 + AMPK 221 | 24.6±1.1 | 19,3±0,9 | 1.5±0.1 |
| GSTP1 + AMPK 221-P 1) | 26,8±0.5 | 21,0±0,8 | 1,7±0.1 |
GST enzyme activity was determined with variable concentrations of model substrate 1-chloro-2,4-dinitrobenzene at a fixed glutathione concentration (10 mM for GSTM1, 2 mM for GSTP1) at 25°C. Vmax and Km values were obtained by direct fitting of values to Michaelis-Menten kinetics. Enzyme activity given in U is equivalent to µmol/min. Values are means ± SD, n = 3. 1) AMPK221 pre-activated by phosphorylation with CamKKβ.
GST/AMPK complexes lead to AMPK glutathionylation and activation
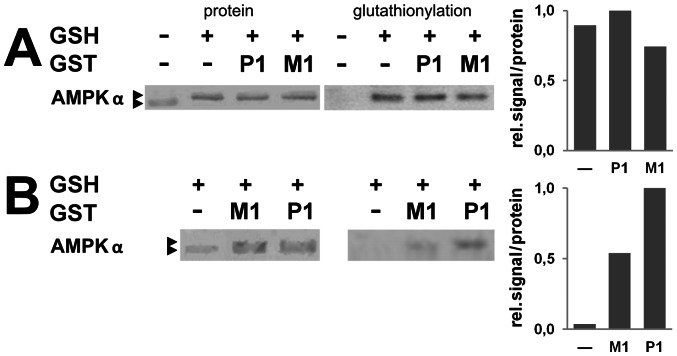
It has previously been shown that GSTM1 and -P1 were able to modulate signal transduction through an interaction with JNK and/or other stress activated kinases ([40], [44], reviewed in [39]). Hence, we hypothesized that GST could modulate AMPK activity by glutathionylation as it was shown recently [20]. At very high glutathione concentrations of 10 mM, spontaneous auto-glutathionylation occurred with AMPK 221WT already in absence of mammalian GSTs (Fig. 6A). However, at more limiting conditions using 0,1 mM glutathione and AMPK pre-reduced with β-mercaptoethanol, auto-glutathionylation was almost absent (Fig. 6B). In this case, presence of GSTM1 and -P1 increased glutathionylation of AMPK to immunodetectable levels, also visible as a partial shift of AMPK to a higher molecular mass (Fig. 6B).
Figure 6. Glutathionylation of AMPK is facilitated by GST.
Glutathionylation assays were performed (A) with AMPK 221WT (1 μM) in absence or presence of GSTM1 or -P1 (0,5 μM) and 10 mM glutathione or (B) with AMPK 221WT (1 μM, additionally pre-reduced with ß-mercaptoethanol) in absence or presence of GSTM1 or -P1 (10 μM) and 0,1 mM glutathione. AMPK modification was detected either as a molecular mass shift of GST protein in SDS-PAGE (see arrows in Ponceau protein stain, “protein”) or by direct detection of glutathione by immunoblotting (“glutathionylation”). Note: As soon as glutathione is present, AMPK is almost quantitatively glutathionylated in (A), while additional presence of GST is needed for glutathionylation in (B). Left: representative data; right: quantification (mean, n = 2).
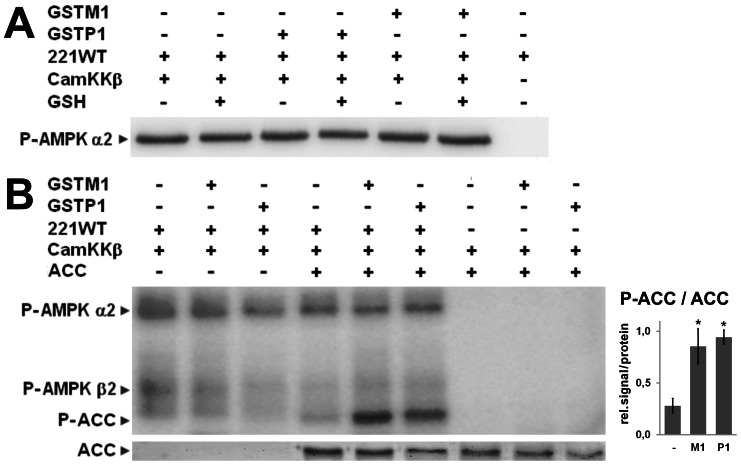
We then analyzed the effect of AMPK S-glutathionylation for AMPK signaling. In vitro phosphorylation of AMPK 221WT at αT172 by its upstream kinase CamKKβ was identical in presence or absence of glutathione, and also not further modified by GSTM1 or -P1 (Fig. 7A). However, phosphorylation of the AMPK downstream substrate ACC was clearly increased with AMPK 221WT preparations that had been glutathionylated before in presence of glutathione by GSTM1 and -P1 as above, compared to controls lacking mammalian GSTs (Fig. 7B). Activation of AMPK by CamKKβ was a prerequisite for this ACC phosphorylation. CamKKβ (Fig. 7B) and AMPK alone (Fig. S4) or combined with GSTs did not affect ACC phosphorylation. These results suggest that GST-dependent S-glutathionylation of AMPK in vitro indeed increases kinase activity in the same way as previously shown for H2O2-dependent AMPK glutathionylation [20].
Figure 7. AMPK glutathionylation does not affect its phosphorylation by CamKKβ, but increases phosphorylation of downstream substrate.
(A) GSTM1 or -P1 (62,5 pmol) were pre-incubated with AMPK 221WT (12.5 pmol) with or without 10 mM glutathione, in presence of ATP prior to addition of CamKKβ (0.63 pmol). Phosphorylation assays were subjected to immunoblot analysis using anti-P-T172-α AMPK antibody. (B) AMPK 221WT pre-activated with CamKKβ in kinase buffer with cold ATP and glutathionylated with 0,1 mM glutathione in presence or absence of GSTM1 or -P1, both as described above and in Fig. 6, were incubated with ACC (200 pmol) and [γ-32P]ATP. In vitro phosphorylation assays were analyzed by SDS-PAGE, Ponceau protein staining (lower panel) and Typhoon phosphoimager (upper panel) are shown. Left: representative data; right: quantification of lanes in presence of glutathione (mean ± SD, n = 4; * = p<0,01 versus no GST). A control experiment lacking CamKKβ is shown in Fig. S4. Note: AMPK autophosphorylation of α- and β-subunits.
Discussion
The energy stress sensor AMPK can be activated by ROS and RNS, possibly via different AMP-dependent and -independent mechanisms [12]–[14] and is involved in cellular redox regulation [16] and antioxidative defense via induced expression of various antioxidative pathways [17]–[19]. Exposure of AMPK to the strong oxidant hydrogen peroxide at high glutathione concentrations induces non-enzymatic S-glutathionylation of AMPK α- and β- subunits which in turn activates the kinase [20]. Our study adds another element to such redox regulation: activation of AMPK via GST-facilitated glutathionylation in the absence of exogenous oxidant that may be relevant to normal physiological conditions. We provide evidence that mammalian GSTM1 and -P1 can rapidly interact with AMPK, become enzymatically activated by this interaction, and assist in turn in glutathionylation and activation of AMPK as we show in vitro.
It has previously been demonstrated that GSTM1 and -P1 were able to modulate signal transduction through interactions with JNK and/or other stress activated kinases ([40], [44], reviewed in [39]), and that this can involve GST phosphorylation or modification of the interacting kinase [40]–[44]. However, interaction with AMPK led only to slow and low-level phosphorylation of GSTM1 and -P1; its importance (if any) remains to be elucidated. By contrast, complex formation alone was sufficient to increase activity of bound GST and, importantly, to glutathionylate and activate AMPK under in vitro conditions where auto-glutathionylation is low, i.e. in the absence of strong oxidants. It is worth noting that protein interaction partners of GST were mostly also identified as targets for S-glutathionylation (e.g. [29], [31], [55]). The residues glutathionylated within AMPK, α-Cys299 and α-Cys304 [20], activate AMPK rather due to direct conformational changes as those produced by allosteric AMP regulation. AMPK glutathionylation did not make AMPK a better substrate for the upstream kinase CamKKβ, at least with the recombinant enzymes in the reconstituted in vitro system applied here.
Reversible protein modification by cysteine glutathionylation is increasingly recognized as an important signaling mechanism by which cells can respond effectively and reversibly to redox inputs [21], [23]. S-glutathionylation inhibits or activates a number of protein kinases (PKA, PKC, MEKK1, ASK1) and phosphatases (reviewed in [23]). Although the understanding of the S-glutathionylation cycle is still limited, there is evidence for the participation glutaredoxins and GST isoforms, including GSTM1 and -P1 [28], [31], [35]. GSTP1P2 knockout mice and cells expressing dead mutants of GSTP have a diminished capacity to S-glutathionylate proteins [28]. GSTP plays an essential role in the S-glutathionylation of 1-cys peroxiredoxin [29], [30], [56]. Even more, a glutathionylation cycle was recently described to regulate aldose reductase [31]. Here, sequential glutathiolyation/deglutathioylation is catalyzed by GSTP and GRx in vitro and in vivo, correlated with physical association of the reductase with either GSTP or GRx [31]. Since the GST catalytic activity is necessary for lowering the pKa of the glutathione cysteine thiol [45], altered catalytic properties as we observed with GSTM1 and -P1 in complex with AMPK may be relevant for the GST glutathionylation function.
These findings fit very well into the emerging role of AMPK as a redox switch in oxidative stress and redox signaling. AMPK is activated by ROS/RNS via different mechanisms: impaired mitochondrial ATP generation will translate into increased cytosolic AMP/ATP and ADP/ATP ratios that activate AMPK [12], but ROS/RNS may also directly affect upstream mediators and kinases [13], [14] or AMPK itself by glutathionylation [20]. Activated AMPK in turn up-regulates the cellular antioxidative defense machinery, mainly via the FOXO3 transcription factor: manganese superoxide dismutase [17]–[19], catalase [17], [18], thioredoxin [17], [57], metallothioneins [58], or uncoupling protein 2 [15]. Among AMPK-FOXO3-induced genes are also γ-glutamylcysteine synthase, the first enzyme in glutathione biosynthesis [17], glutathione peroxidase [18] that uses glutathione to reduce lipid and hydrogen peroxides, as well as GSTM1 [58].
The interaction leading to GST/AMPK complexes seems to be rather specific for the GST-Mu and -Pi families, since it was not observed with GST-Alpha and -Omega isoforms (not shown). It does not involve the AMPK α-subunit, as we have recently reported for fumarate hydratase [59], but the β-subunit, as also seen with several other putative mammalian AMPK interactors (IntAct database, [60]) and the yeast and plants orthologs [61], [62]. Our data suggest interaction with the very N-terminal part of the β-subunit, a domain that is fairly well conserved across the AMPK protein family but lacks in the solved core structures of mammalian AMPK and its yeast ortholog ([10]; reviewed in [63]), possibly due to its high flexibility. The physical interaction of AMPK with GST-Sj calls for a note of caution for using GST fusion proteins in pull-down assays to identify AMPK interaction partners.
GST-mediated glutathionylation and activation of AMPK may be considered a possible additional layer of AMPK regulation linking the energy-stress sensor to redox regulation and anti-oxidative defense. Our present data are novel in that they provide a mechanism for glutathionylation-dependent AMPK activation at low oxidative capacity, as compared to the highly oxidative conditions used in an earlier study [20] which may not mimic peroxide concentrations generated intracellularly [22], [26]. Further studies have to show the specific importance of this mechanism for in vivo regulation of AMPK activity.
Supporting Information
The Strep-tag in Strep-GST constructs is phosphorylated by AMPK. Phosphorylation of GSTP1(200 pmol) and GSTM1 (40 pmol) in Strep-tagged (P1, M1) and Strep-tag-free forms (P1c, M1c) by AMPK221 (4 pmol) activated by CamKKβ (1 pmol). In vitro phosphorylation for 10 min at 37°C was analyzed by SDS-PAGE and Typhoon phosphoimager (top panel) and control Coomassie stain for protein loading (bottom panel). Control lanes lack AMPK221 but contain CamKKβ.
(PDF)
Substoichiometric phosphorylation of GSTP1 by AMPK in vitro . (A) Phosphorylation time course of GSTP1 or ACC (200 pmol each) by AMPK221 (4 pmol) activated by CamKKβ (1 pmol). In vitro phosphorylation for 5 to 60 min at 37°C was analyzed by SDS-PAGE and Typhoon phosphoimager. Control lanes lack AMPK221 but contain CamKKβ. (B) Quantification of (A) using Image Quant TL, using normalization to maximal ACC phosphorylation and fitting to phosphorylation enzyme kinetics.
(PDF)
GSTM1 and -P1 are activated in complexes with AMPK in vitro . Enzyme activity of or 20 μg GSTM1 (A) or 30 μg GSTP1 (B) in absence or presence of 5 or 15 µg AMPK221WT at different concentrations of the model substrate CDNB and saturating glutathione concentrations.
(PDF)
Increased phosphorylation of AMPK downstream substrate depends on the presence of AMPK-activating upstream kinase CamKKβ. AMPK 221WT preactivated with CamKKβ in kinase buffer with cold ATP and glutathionylated with 0,1 mM glutathione in presence or absence of GSTM1 or -P1, both as described in Figs. 6 and 7, were incubated with ACC (200 pmol) and [γ-32P]ATP. In vitro phosphorylation assays were analyzed by SDS-PAGE, Ponceau protein staining (lower panel) and Typhoon phosphoimager (upper panel) are shown. Note: AMPK autophosphorylation in particular of the α-subunit.
(PDF)
Acknowledgments
We thank Steve Calberson (Université Catholique de Louvain, Brussels, Belgium) for in vitro phosphorylation experiments, Laurence Kay (UJF, LBFA, Grenoble, France) for animal experimentation, Wilfrid Boireau (FEMTO-ST Institute, Besancon, France) for providing the functionalized gold chip, and Grahame Hardie (University of Dundee, UK) for providing the GST-ACC vector.
Funding Statement
This work was supported by EU FP6 contract LSHM-CT-2004-005272 (EXGENESIS), the Fondation pour la Recherche Médicale (given to A.K.) and the French Agence Nationale de Recherche (“chaire d'excellence” given to U.S.). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Hardie DG (2011) AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 25: 1895–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hardie DG, Ross FA, Hawley SA (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol 13: 251–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carling D, Thornton C, Woods A, Sanders MJ (2012) AMP-activated protein kinase: new regulation, new roles? Biochem J 445: 11–27. [DOI] [PubMed] [Google Scholar]
- 4. Viollet B, Lantier L, Devin-Leclerc J, Hebrard S, Amouyal C, et al. (2009) Targeting the AMPK pathway for the treatment of Type 2 diabetes. Front Biosci 14: 3380–3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steinberg GR, Kemp BE (2009) AMPK in Health and Disease. Physiol Rev 89: 1025–1078. [DOI] [PubMed] [Google Scholar]
- 6. Zhang BB, Zhou G, Li C (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab 9: 407–416. [DOI] [PubMed] [Google Scholar]
- 7. Neumann D, Woods A, Carling D, Wallimann T, Schlattner U (2003) Mammalian AMP-activated protein kinase: functional, heterotrimeric complexes by co-expression of subunits in Escherichia coli. Protein Expr Purif 30: 230–237. [DOI] [PubMed] [Google Scholar]
- 8. Fogarty S, Hardie DG (2010) Development of protein kinase activators: AMPK as a target in metabolic disorders and cancer. Biochim Biophys Acta 1804: 581–591. [DOI] [PubMed] [Google Scholar]
- 9. Oakhill JS, Steel R, Chen ZP, Scott JW, Ling N, et al. (2011) AMPK is a direct adenylate charge-regulated protein kinase. Science 332: 1433–1435. [DOI] [PubMed] [Google Scholar]
- 10. Xiao B, Sanders MJ, Underwood E, Heath R, Mayer FV, et al. (2011) Structure of mammalian AMPK and its regulation by ADP. Nature 472: 230–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Oakhill JS, Chen ZP, Scott JW, Steel R, Castelli LA, et al. (2010) beta-Subunit myristoylation is the gatekeeper for initiating metabolic stress sensing by AMP-activated protein kinase (AMPK). Proc Natl Acad Sci U S A 107: 19237–19241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hawley SA, Ross FA, Chevtzoff C, Green KA, Evans A, et al. (2010) Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab 11: 554–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zou MH, Kirkpatrick SS, Davis BJ, Nelson JS, Wiles WGt, et al. (2004) Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J Biol Chem 279: 43940–43951. [DOI] [PubMed] [Google Scholar]
- 14. Xie Z, Dong Y, Zhang M, Cui MZ, Cohen RA, et al. (2006) Activation of protein kinase C zeta by peroxynitrite regulates LKB1-dependent AMP-activated protein kinase in cultured endothelial cells. J Biol Chem 281: 6366–6375. [DOI] [PubMed] [Google Scholar]
- 15. Xie Z, Zhang J, Wu J, Viollet B, Zou MH (2008) Upregulation of mitochondrial uncoupling protein-2 by the AMP-activated protein kinase in endothelial cells attenuates oxidative stress in diabetes. Diabetes 57: 3222–3230. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16. Jeon SM, Chandel NS, Hay N (2012) AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485: 661–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Colombo SL, Moncada S (2009) AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J 421: 163–169. [DOI] [PubMed] [Google Scholar]
- 18. Wang S, Dale GL, Song P, Viollet B, Zou MH (2010) AMPKalpha1 deletion shortens erythrocyte life span in mice: role of oxidative stress. J Biol Chem 285: 19976–19985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kukidome D, Nishikawa T, Sonoda K, Imoto K, Fujisawa K, et al. (2006) Activation of AMP-activated protein kinase reduces hyperglycemia-induced mitochondrial reactive oxygen species production and promotes mitochondrial biogenesis in human umbilical vein endothelial cells. Diabetes 55: 120–127. [PubMed] [Google Scholar]
- 20. Zmijewski JW, Banerjee S, Bae H, Friggeri A, Lazarowski ER, et al. (2010) Exposure to hydrogen peroxide induces oxidation and activation of AMP-activated protein kinase. J Biol Chem 285: 33154–33164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Xiong Y, Uys JD, Tew KD, Townsend DM (2011) S-glutathionylation: from molecular mechanisms to health outcomes. Antioxid Redox Signal 15: 233–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pimentel D, Haeussler DJ, Matsui R, Burgoyne JR, Cohen RA, et al. (2012) Regulation of cell physiology and pathology by protein S-glutathionylation: lessons learned from the cardiovascular system. Antioxid Redox Signal 16: 524–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Pastore A, Piemonte F (2012) S-Glutathionylation signaling in cell biology: progress and prospects. Eur J Pharm Sci 46: 279–292. [DOI] [PubMed] [Google Scholar]
- 24. Riek U, Scholz R, Konarev P, Rufer A, Suter M, et al. (2008) Structural properties of AMP-activated protein kinase: dimerization, molecular shape, and changes upon ligand binding. J Biol Chem 283: 18331–18343. [DOI] [PubMed] [Google Scholar]
- 25. Chen L, Wang J, Zhang YY, Yan SF, Neumann D, et al. (2012) AMP-activated protein kinase undergoes nucleotide-dependent conformational changes. Nat Struct Mol Biol 19: 716–718. [DOI] [PubMed] [Google Scholar]
- 26. Arbault S, Pantano P, Sojic N, Amatore C, Best-Belpomme M, et al. (1997) Activation of the NADPH oxidase in human fibroblasts by mechanical intrusion of a single cell with an ultramicroelectrode. Carcinogenesis 18: 569–574. [DOI] [PubMed] [Google Scholar]
- 27. Anathy V, Roberson EC, Guala AS, Godburn KE, Budd RC, et al. (2012) Redox-based regulation of apoptosis: S-glutathionylation as a regulatory mechanism to control cell death. Antioxid Redox Signal 16: 496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Townsend DM, Manevich Y, He L, Hutchens S, Pazoles CJ, et al. (2009) Novel role for glutathione S-transferase pi. Regulator of protein S-Glutathionylation following oxidative and nitrosative stress. J Biol Chem 284: 436–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Manevich Y, Feinstein SI, Fisher AB (2004) Activation of the antioxidant enzyme 1-CYS peroxiredoxin requires glutathionylation mediated by heterodimerization with pi GST. Proc Natl Acad Sci U S A 101: 3780–3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ralat LA, Manevich Y, Fisher AB, Colman RF (2006) Direct evidence for the formation of a complex between 1-cysteine peroxiredoxin and glutathione S-transferase pi with activity changes in both enzymes. Biochemistry 45: 360–372. [DOI] [PubMed] [Google Scholar]
- 31. Wetzelberger K, Baba SP, Thirunavukkarasu M, Ho YS, Maulik N, et al. (2010) Postischemic deactivation of cardiac aldose reductase: role of glutathione S-transferase P and glutaredoxin in regeneration of reduced thiols from sulfenic acids. J Biol Chem 285: 26135–26148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. de Luca A, Moroni N, Serafino A, Primavera A, Pastore A, et al. (2011) Treatment of doxorubicin-resistant MCF7/Dx cells with nitric oxide causes histone glutathionylation and reversal of drug resistance. Biochem J 440: 175–183. [DOI] [PubMed] [Google Scholar]
- 33. Tew KD, Townsend DM (2011) Regulatory functions of glutathione S-transferase P1-1 unrelated to detoxification. Drug Metab Rev 43: 179–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Tew KD, Manevich Y, Grek C, Xiong Y, Uys J, et al. (2011) The role of glutathione S-transferase P in signaling pathways and S-glutathionylation in cancer. Free Radic Biol Med 51: 299–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tew KD, Townsend DM (2012) Glutathione-S-Transferases As Determinants of Cell Survival and Death. Antioxid Redox Signal. [DOI] [PMC free article] [PubMed]
- 36. Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45: 51–88. [DOI] [PubMed] [Google Scholar]
- 37. Smith DB (2000) Generating fusions to glutathione S-transferase for protein studies. Methods Enzymol 326: 254–270. [DOI] [PubMed] [Google Scholar]
- 38. Frova C (2006) Glutathione transferases in the genomics era: new insights and perspectives. Biomol Eng 23: 149–169. [DOI] [PubMed] [Google Scholar]
- 39. Lo HW, Ali-Osman F (2007) Genetic polymorphism and function of glutathione S-transferases in tumor drug resistance. Curr Opin Pharmacol 7: 367–374. [DOI] [PubMed] [Google Scholar]
- 40. Adler V, Yin Z, Fuchs SY, Benezra M, Rosario L, et al. (1999) Regulation of JNK signaling by GSTp. EMBO J 18: 1321–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cho SG, Lee YH, Park HS, Ryoo K, Kang KW, et al. (2001) Glutathione S-transferase mu modulates the stress-activated signals by suppressing apoptosis signal-regulating kinase 1. J Biol Chem 276: 12749–12755. [DOI] [PubMed] [Google Scholar]
- 42. Gilot D, Loyer P, Corlu A, Glaise D, Lagadic-Gossmann D, et al. (2002) Liver protection from apoptosis requires both blockage of initiator caspase activities and inhibition of ASK1/JNK pathway via glutathione S-transferase regulation. J Biol Chem 277: 49220–49229. [DOI] [PubMed] [Google Scholar]
- 43. Lo HW, Antoun GR, Ali-Osman F (2004) The human glutathione S-transferase P1 protein is phosphorylated and its metabolic function enhanced by the Ser/Thr protein kinases, cAMP-dependent protein kinase and protein kinase C, in glioblastoma cells. Cancer Res 64: 9131–9138. [DOI] [PubMed] [Google Scholar]
- 44. Yin Z, Ivanov VN, Habelhah H, Tew K, Ronai Z (2000) Glutathione S-transferase p elicits protection against H2O2-induced cell death via coordinated regulation of stress kinases. Cancer Res 60: 4053–4057. [PubMed] [Google Scholar]
- 45. Graminski GF, Kubo Y, Armstrong RN (1989) Spectroscopic and kinetic evidence for the thiolate anion of glutathione at the active site of glutathione S-transferase. Biochemistry 28: 3562–3568. [DOI] [PubMed] [Google Scholar]
- 46. Nieslanik BS, Atkins WM (2000) The catalytic Tyr-9 of glutathione S-transferase A1-1 controls the dynamics of the C terminus. J Biol Chem 275: 17447–17451. [DOI] [PubMed] [Google Scholar]
- 47.Riek U, Ramirez S, Wallimann T, Schlattner U (2009) A versatile multidimensional protein purification system with full internet remote control based on a standard HPLC system. Biotechniques 46: ix–xii. [DOI] [PubMed]
- 48. Mockli N, Deplazes A, Hassa PO, Zhang Z, Peter M, et al. (2007) Yeast split-ubiquitin-based cytosolic screening system to detect interactions between transcriptionally active proteins. Biotechniques 42: 725–730. [DOI] [PubMed] [Google Scholar]
- 49. Tafelmeyer P, Johnsson N, Johnsson K (2004) Transforming a (beta/alpha)8 – barrel enzyme into a split-protein sensor through directed evolution. Chem Biol 11: 681–689. [DOI] [PubMed] [Google Scholar]
- 50. Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72: 248–254. [DOI] [PubMed] [Google Scholar]
- 51. Scott JW, Norman DG, Hawley SA, Kontogiannis L, Hardie DG (2002) Protein kinase substrate recognition studied using the recombinant catalytic domain of AMP-activated protein kinase and a model substrate. J Mol Biol 317: 309–323. [DOI] [PubMed] [Google Scholar]
- 52. Boireau W, Rouleau A, Lucchi G, Ducoroy P (2009) Revisited BIA-MS combination: entire “on-a-chip” processing leading to the proteins identification at low femtomole to sub-femtomole levels. Biosens Bioelectron 24: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 53. Bruckner A, Polge C, Lentze N, Auerbach D, Schlattner U (2009) Yeast two-hybrid, a powerful tool for systems biology. Int J Mol Sci 10: 2763–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Stein SC, Woods A, Jones NA, Davison MD, Carling D (2000) The regulation of AMP-activated protein kinase by phosphorylation. Biochem J 345 Pt 3: 437–443. [PMC free article] [PubMed] [Google Scholar]
- 55. Cross JV, Templeton DJ (2004) Oxidative stress inhibits MEKK1 by site-specific glutathionylation in the ATP-binding domain. Biochem J 381: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Noguera-Mazon V, Lemoine J, Walker O, Rouhier N, Salvador A, et al. (2006) Glutathionylation induces the dissociation of 1-Cys D-peroxiredoxin non-covalent homodimer. J Biol Chem 281: 31736–31742. [DOI] [PubMed] [Google Scholar]
- 57. Hou X, Song J, Li XN, Zhang L, Wang X, et al. (2010) Metformin reduces intracellular reactive oxygen species levels by upregulating expression of the antioxidant thioredoxin via the AMPK-FOXO3 pathway. Biochem Biophys Res Commun 396: 199–205. [DOI] [PubMed] [Google Scholar]
- 58. Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, et al. (2007) The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282: 30107–30119. [DOI] [PubMed] [Google Scholar]
- 59. Klaus A, Polge C, Zorman S, Auchlic Y, Brunisholzc R, et al. (2012) A two-dimensional screen for AMPK substrates identifies tumor suppressor fumarate hydratase as a preferential AMPKα2 substrate. J Proteomics 75: 3304–13. [DOI] [PubMed] [Google Scholar]
- 60. Aranda B, Achuthan P, Alam-Faruque Y, Armean I, Bridge A, et al. (2010) The IntAct molecular interaction database in 2010. Nucleic Acids Res 38: D525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vincent O, Carlson M (1999) Gal83 mediates the interaction of the Snf1 kinase complex with the transcription activator Sip4. EMBO J 18: 6672–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Polge C, Jossier M, Crozet P, Thomas M (2008) β subunits of the SnRK1 complexes share a common ancestral function together with expression and function specificities; physical interaction with nitrate reductase specifically occurs via AKINβ1 subunit. Plant Physiol 148: 1570–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Sanz P (2008) AMP-activated protein kinase: structure and regulation. Curr Protein Pept Sci 9: 478–492. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Strep-tag in Strep-GST constructs is phosphorylated by AMPK. Phosphorylation of GSTP1(200 pmol) and GSTM1 (40 pmol) in Strep-tagged (P1, M1) and Strep-tag-free forms (P1c, M1c) by AMPK221 (4 pmol) activated by CamKKβ (1 pmol). In vitro phosphorylation for 10 min at 37°C was analyzed by SDS-PAGE and Typhoon phosphoimager (top panel) and control Coomassie stain for protein loading (bottom panel). Control lanes lack AMPK221 but contain CamKKβ.
(PDF)
Substoichiometric phosphorylation of GSTP1 by AMPK in vitro . (A) Phosphorylation time course of GSTP1 or ACC (200 pmol each) by AMPK221 (4 pmol) activated by CamKKβ (1 pmol). In vitro phosphorylation for 5 to 60 min at 37°C was analyzed by SDS-PAGE and Typhoon phosphoimager. Control lanes lack AMPK221 but contain CamKKβ. (B) Quantification of (A) using Image Quant TL, using normalization to maximal ACC phosphorylation and fitting to phosphorylation enzyme kinetics.
(PDF)
GSTM1 and -P1 are activated in complexes with AMPK in vitro . Enzyme activity of or 20 μg GSTM1 (A) or 30 μg GSTP1 (B) in absence or presence of 5 or 15 µg AMPK221WT at different concentrations of the model substrate CDNB and saturating glutathione concentrations.
(PDF)
Increased phosphorylation of AMPK downstream substrate depends on the presence of AMPK-activating upstream kinase CamKKβ. AMPK 221WT preactivated with CamKKβ in kinase buffer with cold ATP and glutathionylated with 0,1 mM glutathione in presence or absence of GSTM1 or -P1, both as described in Figs. 6 and 7, were incubated with ACC (200 pmol) and [γ-32P]ATP. In vitro phosphorylation assays were analyzed by SDS-PAGE, Ponceau protein staining (lower panel) and Typhoon phosphoimager (upper panel) are shown. Note: AMPK autophosphorylation in particular of the α-subunit.
(PDF)