Abstract
Earlier we identified PPP2CA, which encodes for the alpha-isoform of protein phosphatase 2A (PP2A) catalytic subunit, as one of the downregulated genes in androgen-independent prostate cancer. PP2A is a ser/thr phosphatase and a potent tumor suppressor involved in broad cellular functions; however, its role in prostate cancer has not yet been determined. Here, we have investigated the effect of PP2A activity modulation on the androgen-independent growth of prostate cancer cells. Our data show that the PPP2CA expression and PP2A activity is downregulated in androgen-independent (C4-2) prostate cancer cells as compared to androgen-dependent (LNCaP) cells. Downregulation of PP2A activity by pharmacological inhibition or siRNA-mediated PPP2CA silencing sustains the growth of LNCaP cells under androgen-deprived condition by relieving the androgen-deprivation-induced cell-cycle arrest and preventing apoptosis. Immunoblot analyses reveal enhanced phosphorylation of Akt, ERK, BAD, increased expression of cyclins (A1/D1) and decreased expression of cyclin inhibitor (p27) upon PP2A downregulation. Furthermore, our data demonstrate that androgen-receptor (AR) signaling is partially maintained in PP2A inhibited cells through increased AR expression and ligand-independent phosphorylation. Pharmacological inhibition of Akt, ERK and AR suggest a role of these signaling pathways in facilitating the androgen-independent growth of LNCaP cells. These observations are supported by the effect of ceramide, a PP2A activator, on androgen-independent C4-2 cells. Ceramide inhibited the growth of C4-2 cells upon androgen-deprivation, an effect that could be abrogated by PP2A downregulation. Altogether, our findings suggest that modulation of PP2A activity may represent an alternative therapeutic approach for the treatment of advanced androgen-independent prostate cancer.
Keywords: PP2A, prostate cancer, androgen-independent growth, androgen receptor, fostriecin, ceramide
INTRODUCTION
Prostate cancer (PCa) is the most common malignancy in men and the second leading cause of male cancer deaths in the United States (1). According to the estimate by American Cancer Society, nearly 192,280 patients were diagnosed with PCa and approximately 27,360 died due to this malignancy in the year 2009 (2). Considering the central role of androgen-receptor (AR) signaling in PCa, surgical or medical castration [referred as androgen-deprivation therapy (ADT)] is the first line of treatment for the advanced disease. Most patients treated with ADT initially exhibit a dramatic regression of the androgen-dependent (AD) cancer cells; however, the tumors eventually progress to an androgen-independent (AI) stage, resulting in a poor prognosis (1). The molecular mechanisms responsible for the failure of ADT are not yet clearly understood. It is believed that AR abnormalities, altered expression of AR co-regulators, and dysregulation of non-AR signaling cascades may be associated with the acquisition of hormone-refractory phenotype (3–5). A cross-talk of AR with other cell signaling pathways has also been demonstrated, which leads to its aberrant activation and thus compensate for androgen-ablation (6, 7). Once the prostate cancer has recurred, it progresses to a highly aggressive disease with frequent metastasis and poses an increased risk of morbidity and death (1). Importantly, this relapsed disease (AI PCa), unlike other cancers, also does not respond well to alternative approaches such as chemotherapy and radiotherapy (8–10). Therefore, high rate of mortality from prostate cancer is linked with its progression to hormone-refractory phenotype and a lack of effective alternative therapeutic approaches.
In an earlier study, we characterized the transcriptomic variation associated with androgen-sensitive and androgen-refractory phenotypes though a genome-wide expression profiling and identified many differentially-expressed genes (11). PPP2CA, which encodes the catalytic-subunit (alpha-isoform) of the protein phosphatase 2A (PP2ACα), was one of the genes of interest that exhibited a downregulated expression in AI PCa cells. The level of PP2ACα was decreased in majority of AI PCa cell lines and in cancer lesions as compared to the adjacent normal/benign tumor tissues. Interestingly, our study also demonstrated an inverse correlation of PP2ACα expression with stage (early vs. late) and Gleason grade (low vs. high) (11). In another study, the downregulated expression of β-isoform of PP2A catalytic subunit (PP2ACβ) in PCa has also been reported (12). PP2ACα and PP2ACβ share 97% identity and are ubiquitously expressed; however, PP2ACα is about 10 times more abundant than PP2ACβ (13). PP2ACα/β is a well conserved subunit of PP2A serine/threonine phosphatases, and the in vivo activity of PP2A is provided by related complexes that exist either as hetero-dimers or hetero-trimers with scaffold (A) and regulatory (B) subunits (14).
PP2A performs broad cellular functions and the functional diversity of PP2A is determined by different scaffold and regulatory subunits. In fact, PP2A has been shown to interact with a wide range of proteins via its three subunits (14). These interactions facilitate the cross-talk of PP2A with multiple cell signaling pathways including MAPKs, Akt/PKB, PKC, IkB kinases, etc. (15–17). Most common role of PP2A catalytic activity in different organisms is in cell survival (18–20). More recently, important roles of PP2A in stem-cell pluripotency, cell migration and invasion, DNA repair, translation, stress-response, etc. have been implicated (14, 21, 22). In the present study, we have investigated the functional significance of downregulated PPP2CA expression in androgen-independent growth of prostate cancer cells. Using lineage-associated androgen-dependent (LNCaP) and –independent (C4-2) prostate cancer cell lines, we demonstrate that decreased PP2A activity is associated with enhanced potential to sustain under androgen-deprived condition. Specifically, our data reveal that the androgen-independent growth of prostate cancer cells upon PP2A inhibition is sustained through a concerted action of Akt, ERK and AR signaling pathways.
MATERIALS AND METHODS
Reagents
RPMI media, penicillin, streptomycin and Vybrant MTT cell proliferation assay kit were from Invitrogen (Carlsbad, CA). Fetal-bovine serum (FBS) was from Atlanta Biologicals (Lawrenceville, GA). FuGENE transfection reagent and phosphatase/protease inhibitors cocktail were from Roche Diagnostics (Mannheim, Germany). PP2A immunoprecipitation phosphatase assay kit was from Upstate Biotechnology (Lake Placid, NY). Human PPP2CA-specific siRNAs (Cat. # L-003598-01), non-target siRNAs (Cat. # D-001810-10) and DharmaFECT transfection reagent were from Dharmacon (Lafayette, CO). Charcoal/Dextran-stripped serum (CSS) was from Gemini Bio-Products (West Sacramento, CA). Propidium iodide/RNAse staining buffer was from BD Bioscience (San Diego, CA). Fostriecin was from Enzo Life Science (Plymouth Meeting, PA). PI3/Akt inhibitor (LY294002) and ERK inhibitor (PD98059) and antibodies against ERK1/2 (rabbit monoclonal), pERK1/2 (mouse monoclonal), BAD (rabbit monoclonal), pBAD (rabbit polyclonal), Bcl-xl (rabbit monoclonal) and Bax (rabbit polyclonal) were from Cell Signaling Technology (Beverly, MA). Antibodies (rabbit monoclonal) against PP2Ac, Akt, p-Akt, androgen-receptor, and PSA were from Epitomics (Burlingame, CA). Anti-phospho-androgen receptor (Ser81, rabbit polyclonal) and (Ser213/210, mouse monoclonal) antibodies were from Millipore (Temecula, CA) and Imgenex (San Diego, CA), respectively. Antibodies against p21 (mouse monoclonal), p27, cyclin A1, cyclin D1 (rabbit polyclonal) and horseradish peroxidase-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Dihydrotestosterone (DHT), anti-androgen Bicalutamide (Casodex) and C2 dihydroceramide were from Sigma-Aldrich (St. Louis MO). CaspACE FITC-VAD-FMK and Dual Luciferase Assay System kit were from Promega (Madison, WI). VECTASHIELD® mounting medium with DAPI was from Vector Laboratories Inc. (Burlingame, CA). ECL plus Western Blotting detection kit was from Thermo Scientific (Logan, UT). Cignal™ AR Androgen Receptor Assay Kit was purchased from SA Biosciences (Frederick, MD).
Cell culture
Adherent monolayer cultures of androgen-dependent (AD) LNCaP (ATCC, Manassas, VA) and androgen-independent (AI) C4-2 (UroCor Inc., Oklahoma City, OK) human prostate cancer cell lines were maintained in RPMI-1640 medium supplemented with 5.0% fetal bovine serum (FBS) and 100 μM each of penicillin and streptomycin. Cells were grown at 37°C with 5% CO2 in humidified atmosphere, and media was replaced every third day. Cells were split (1:3), when they reached near confluence. To authenticate the cell lines, we performed short tandem repeats (STR) genotyping. Furthermore, their response to androgens for growth and androgen-receptor activity was also monitored intermittently during the study.
Treatments and transfections
For various treatments, cells were cultured either in 10 cm Petri-dishes or 6/24/96-well plates to ~60–80% confluence as specified above. Thereafter, media was replaced with steroid-reduced charcoal:dextran-stripped serum (CSS)-containing media and cells were treated with i) DHT, ii) Fostriecin, iii) LY294002, iv) PD98059, v) Bicalutamide/Casodex, vi) Ceramide alone or in combination at doses and times specified in figure legends. For the knockdown of PPP2CA, cells were cultured in 6/96-well plates to ~50–70% confluence and transiently transfected with 0.05 mM of human PPP2CA-specific or non-target control-siRNAs using DharmaFECT (Dharmacon Lafayette, CO) as per the manufacturer’s protocol. Following 24 h after transfection, cells were treated as described earlier.
Western blot analysis
Cells were processed for protein extraction and western blotting as described earlier (23). Briefly, the cells were washed twice with PBS and cell lysates were prepared in NP-40 lysis buffer (150 mM NaCl, 1% NP-40, 50 mM Tris-Cl, pH 7.4, and 5 mM EDTA) containing protease and phosphotase inhibitors. Cell lysates were passed through a needle syringe to facilitate the disruption of the cell membranes and centrifuged at 14,000 rpm for 20 min at 4°C, and supernatants collected. Protein lysates (10–60 μg) were resolved by electrophoresis on 10% SDS-PAGE, transferred onto polyvinylidene difluoride (PVDF) membrane and subjected to standard immunodetection procedure using specific antibodies: PP2Ac, Akt, pAkt, ERK1/2, pERK1/2, BAD, pBAD, AR, pAR (S81), Bcl-xl, Bax (1:1000), pAR (S213/210), PSA (1:2500), p21, p27, cyclin A1, cyclin D1 (1: 200) and β-actin (1:20000). All secondary antibodies were used at 1:2500 dilutions. Blots were processed with ECL Plus Western Blotting detection kit and the signal detected using an LAS-3000 image analyzer (Fuji Photo Film Co., Tokyo, Japan).
PP2A activity assay in vitro
PP2A activity was determined using PP2A immunoprecipitation phosphatase assay kit according to the manufacturer’s instructions. Briefly, PP2ACα was immunoprecipitated with anti-PP2ACα monoclonal antibody and protein A-agarose beads. PP2ACα -bound beads were collected by the centrifugation and washed with ser/thr assay buffer. Thereafter, phosphopeptide (K-R-pT-I-R-R) was added to the washed beads (at final concentration 250 μM), followed by incubation at 30°C for 15 min. After centrifugation, 25 μl of supernatant was transferred to an assay plate; 100 μl of Malachite Green phosphate detection solution was added and incubated at 30°C for 15 min for the color development. The relative absorbance was measured at 630 nm in a microplate reader (BioTek, Winooski, VT).
Cell-growth assay
Cells were seeded at a density of 5×103 cells per well in 96-well plate. After various treatments, cell viability was determined by using Vybrant MTT cell proliferation assay kit. Growth was calculated as percent (%) = [{(A / B)−1} × 100], where A and B are the absorbance of treatment and control cells, respectively.
Cell-cycle analysis
Following various treatments, cells were trypsinized and washed twice in PBS. Subsequently, 70% ethanol was added and cells were fixed overnight at 4°C. Fixed cells were washed with PBS and stained with propidium iodide using PI/RNase staining buffer for 1 h at 37°C. Stained cells were analyzed by flow-cytometry on a BD-FACS Canto™ II (Becton-Dickinson, San Jose, CA) and percentage of cell population in various phases of cell cycle was calculated using Mod Fit LT software (Verity Software House, Topsham, ME).
Apoptosis assay
Cells cultured on glass bottom FluoroDish (World Precision Inst., FL) were subjected to various treatments as described in figure legend. Apoptosis was detected by staining the cells with CaspACE FITC-VAD-FMK solution in PBS for 2 h at 37°C. CaspACE™ FITC-VAD-FMK In Situ Marker is a fluorescent analog of the pan-caspase inhibitor Z-VAD-FMK (carbobenzoxy-valyl-alanyl-aspartyl-[O-methyl]-fluoromethyl-ketone), which irreversibly binds to activated caspases and is a surrogate for caspase activity in situ. Following staining, cells were fixed with 4% paraformaldehydede at room temperature, washed with PBS and mounted with VECTASHIELD®. The bound fluorescent marker was detected under a Nikon Eclipse TE2000-U fluorescent microscope (Nikon Instruments Inc, Melville, NY). The number of apoptotic cells per field (100×) was counted and results expressed as the mean ± SD of apoptotic cells in 10 random view-fields.
Androgen receptor transcriptional activity assay
Androgen receptor transcriptional activity was determined by Cignal™ AR androgen-receptor assay kit according to the manufacturer’s protocol. Briefly, cells were grown in 24-well plate to ~50–60% confluence and thereafter transiently transfected with AR reporter-, negative- and positive-control- plasmids using FuGENE transfection reagent as per manufacturer’s instructions. After 24 h of transfection, cells were treated as described in figure legend for next 24 h and total protein was isolated in passive lysis buffer. Firefly (for AR activity) and Renilla (for internal normalization) luciferase activities were measured using a Dual-Luciferase assay kit. All experiments were done in triplicate and relative luciferase units (RLUs) were reported as mean ± SD from triplicates.
Statistical analysis
Each experiment was performed at least three times and all the values were expressed as mean ± SD. The differences between the groups were compared using student’s t-tests. A p value of equal or less than 0.05 was considered statistically significant.
RESULTS
Inhibition of PP2A enables androgen-dependent prostate cancer cells to grow under steroid-depleted condition
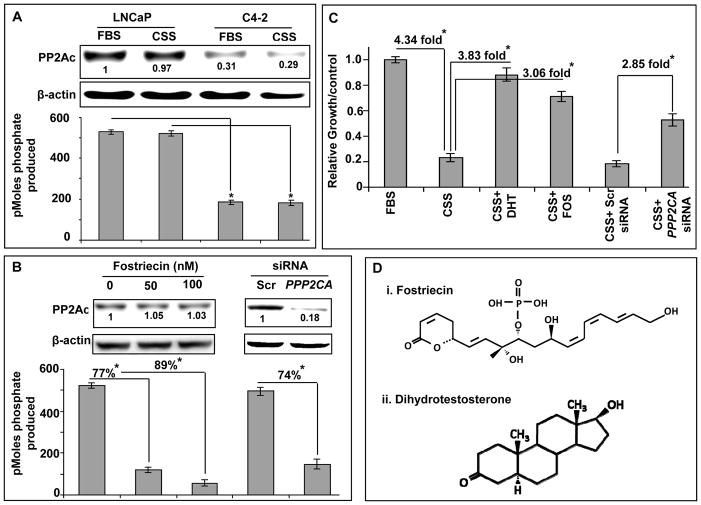
Previously, we have reported the downregulated expression of PP2ACα in AI PCa cells as compared to the AD PCa cells (11). Here, we examined the expression and activity of PP2ACα in two AR-expressing, lineage-associated human prostate cancer cell lines, LNCaP (AD) and C4-2 (AI) under regular or steroid-reduced conditions. Our Immunoblot and in vitro phosphatase activity data show that both the expression and activity of PP2ACα is significantly downregulated in C4-2 (AI) cells as compared to LNCaP (AD) cells and there is no significant change in the expression or activity of PP2ACα upon steroid depletion (Figure 1A). Next, we examined the effect of fostriecin (a potent inhibitor of PP2A) and siRNA-mediated silencing of PPP2CA on the activity of PP2A in LNCaP cells. Our data demonstrated that PP2A activity was decreased following treatment with fostriecin (~77.27% and 89.32% at 50nM and 100nM, respectively) or transfection with PPP2CA-specific siRNA (~74%) that resulted in over 80% reduction in gene expression (Figure 1B). In next set of experiments, we analyzed the effect of PP2A inhibition on the growth of LNCaP cells under steroid-depleted condition. LNCaP cells were treated with fostriecin (100 nM) or DHT (1 nM) under steroid-reduced condition. Alternatively, following transfection with scrambled- or PPP2CA-specific siRNAs for 24 h, LNCaP cells were placed in steroid-reduced growth media. Growth of the LNCaP cells was analyzed by MTT assay after 96 h of treatments (Figure 1C). We observed that LNCaP cells under steroid-depleted condition had ~4.3 fold decreased cell growth as compared to the cells grown in regular-media. The treatment with either DHT or fostreicin had a rescue effect exhibiting ~3.83 fold and ~3.06 fold growth induction, respectively. Similarly, siRNA-mediated silencing of PPP2CA also resulted in increased growth (~2.85 fold) as compared to the scrambled-siRNA transfected control cells under steroid-depleted condition (Figure 1C). These findings suggest that the down-modulation of PP2A enables androgen-dependent prostate cancer cells to grow under steroid-deprivation and thus may have an important role in androgen-independent growth of prostate cancer.
Figure 1. PP2A activity is downregulated in androgen-independent prostate cancer cells and its inhibition sustains the growth of androgen-dependent prostate cancer cells under steroid-depleted condition.
A, Total protein, from LNCaP (AD) and C4-2 (AI) prostate cancer cells, was resolved and immunoblotted for PP2Ac and β-actin (internal control). PP2A activity was determined by malachite green based phosphatase assay. PP2Ac was expressed at low level in AI PCa (C4-2) cells in comparison with AD PCa (LNCaP) cells and correlated with decreased activity (≥ 70%) under both steroid-supplemented and –reduced conditions. B, AD PCa (LNCaP) cells were treated with different doses (50 and 100nM) of fostriecin (Fos) in steroid-reduced (CSS) media for 72 h. In parallel, PPP2CA expression was silenced by transient transfection of LNCaP cells with PPP2CA-specific siRNA for 72 h. Cells were also transfected with non-targeted scrambled siRNAs to serve as control. Activity of PP2Ac was decreased in LNCaP cells after treatment with Fos (~77.27% and 89.32% at 50 nM and 100 nM, respectively) and knockdown of PP2Ac with specific siRNA (≥ 74%). C, To investigate the effect of PP2A inhibition on androgen-independent growth, LNCaP cells were incubated in steroid-reduced (CSS) media and treated with DHT (1.0 nM), Fos (100 nM) and PPP2CA-specific or scrambled siRNAs. Cell growth was assessed by MTT assay after 96 h of treatment. Numbers below the bands, represent the fold ratio of densitometric quantification relative to corresponding control. Bars represent the means ± S.D. (n=3); *, statistically significant (p<0.05). D, Chemical structures of fostriecin (i) and dihydrotestosterone (ii).
Downregulation of PP2A sustains growth of LNCaP cells by preventing steroid depletion-induced cell-cycle arrest and apoptosis
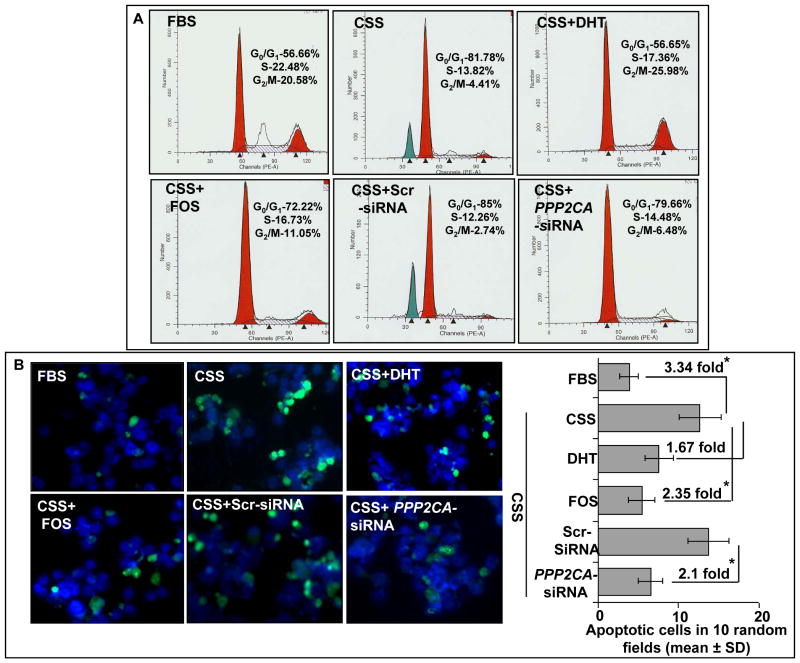
Earlier, it has been shown that steroid depletion induces arrest of cell cycle and apoptosis in androgen-dependent LNCaP cells, which leads to overall deceased growth (24–26). Therefore, we examined the effect of PP2A inhibition on cell cycle progression and apoptosis under steroid-depleted (CSS) condition. The proliferation index was determined by DHT or fostriecin treatments of synchronized LNCaP cells followed by propidium-iodide staining and flow cytometry (Figure 2A). In accordance with previously published reports (24, 25), our data showed arrest of LNCaP cells in G0/G1 phase of cell cycle under steroid-reduced condition, an effect that was abrogated upon treatment with DHT (1 nM) (Figure 2A). Furthermore, we observed that the inhibition of PP2A by either fostriecin or siRNA-mediated silencing of PPP2CA also led to the release of steroid depletion-induced cell cycle arrest of LNCaP cells. The total percentage of LNCaP cells that entered S-phase and then progressed to G2/M phase was 27.78% upon fostriecin treatment as compared to 18.22% in CSS-only treated LNCaP cells. Similarly, 20.96 % of PPP2CA-silenced LNCaP cells were in S and G2/M phases as compared to 15.0% in scrambled-siRNA transfected cells (Figure 2A). To analyze the apoptotic index, we stained the cells with CaspACE FITC-VAD-FMK, a fluorescent analog of a pan-caspase inhibitor that binds to the active caspases. As a measure of activity of caspases or apoptosis, we counted the fluorescently-stained LNCaP cells in 10 random fields of view under a fluorescence microscope (Figure 2B). Our data showed that steroid-depletion led to enhanced apoptosis of LNCaP cells (3.34 fold), which could be suppressed up to 1.67 and 2.35 folds by treatment with DHT and fostriecin, respectively. Similarly, PPP2CA-silencing also led to the reduction of apoptosis (2.1 fold) under steroid-deprived condition as compared to the scrambled siRNA-transfected cells. Altogether, our data demonstrate that PP2A inhibition supports the growth of LNCaP cells under androgen-depleted condition by preventing cell cycle arrest and apoptosis.
Figure 2. PP2A inhibition relieves hormone-deprivation-induced G0/G1 arrest and suppresses apoptosis.
A, LNCaP cells were synchronized by serum-starvation and treated with DHT (1 nM) or fostriecin (Fos) (100 nM) for 24 h in steroid-reduced (CSS) media. After treatments, distribution of cells in different phases of cell cycle was analyzed by propidium iodide (PI) staining followed by flow cytometry. Cell cycle analysis was also performed on control and PPP2CA-silenced LNCaP cells incubated in steroid-reduced media. Both the treatment with DHT or PP2A inhibition relieved the androgen-deprivation induced G0/G1 cell cycle arrest, although the effect was more prominent with DHT. B, To determine the effect of PP2A inhibition on apoptosis, subconfluent cultures of LNCaP cells were treated with DHT (1.0 nM), Fos (100 nM) and PPP2CA-specific or scrambled siRNAs under steroid-reduced condition for 96 h. Apoptosis was detected by staining the cells with CaspACE FITC-VAD-FMK solution in PBS for 2 h at 37°C. Following fixation, bound marker was visualized by fluorescent detection under a Nikon microscope. Inhibition of PP2A suppressed the apoptosis as evident by the decreased fluorescence intensity and number of positively (dark green florescent) -stained cells. Representative picture is from one of the random fields of view. Bars represent the means ± S.D of apoptotic cells in 10 random view fields; *, statistically significant (p<0.05).
Downregulation of PP2A leads to the activation of survival signaling and alters the expression of cell-cycle associated proteins
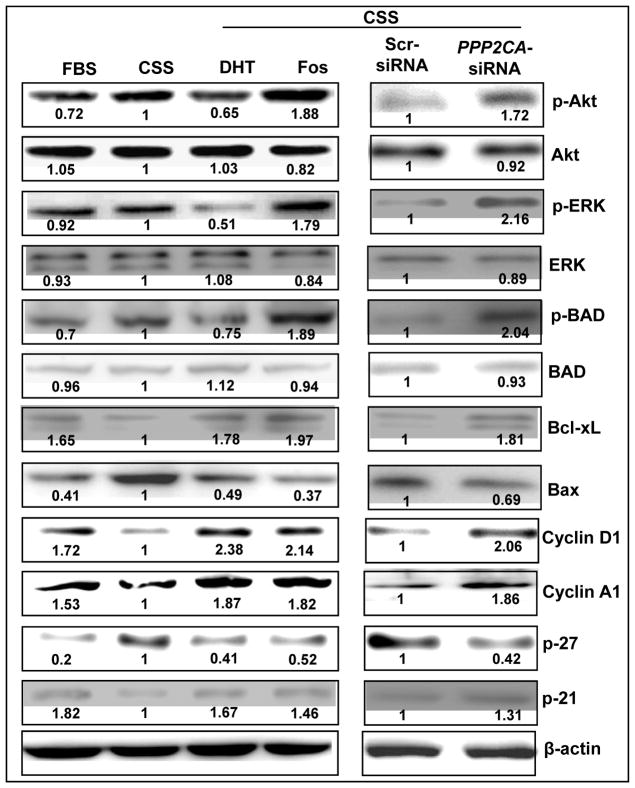
PP2A impacts multiple cell signaling pathways by causing dephosphorylation of the signaling proteins (14). Akt and ERK are among the most significant signaling proteins that are regulated by PP2A and have also been shown to be involved in androgen-independent growth of human prostate cancer cells (15, 27, 28). To determine if the sustained growth of LNCaP cells under steroid-depleted condition was due to the activation of Akt and ERK, we monitored the change in their phosphorylation upon PP2A inhibition. Our immunoblot data with total and phospho-form-specific antibodies (Figure 3) showed an increased phosphorylation of both Akt and ERK. Similarly, silencing of PPP2CA also resulted in an increased Akt and ERK phosphorylation. Furthermore, we observed that PP2A inhibition induced the phosphorylation of BAD protein, which causes the loss of its pro-apoptotic effect. Interestingly, treatment with DHT led to a decrease in Akt, ERK and BAD phosphorylation, while both DHT and fostriecin induced the expression of anti-apoptotic Bcl-xL and suppressed the expression of pro-apoptotic Bax. Effect of DHT on Akt is in corroboration with earlier studies (28, 29); however, DHT has also been shown to cause non-genomic activation of PI3K/Akt in AR (ectopic)-expressing PC3 prostate cancer cells (30). Therefore, it will be of interest to investigate these observations further to identify the underlying molecular mechanism(s). Nonetheless, our findings suggest that a balance of pro- and anti-apoptotic signaling during steroid-deprivation determines the overall effect of DHT or PP2A inhibition in potentiating the survival of prostate cancer cells. Cell cycle is controlled by actions of various cyclins and their inhibitors. As we observed the effect of steroid-deprivation and PP2A inhibition on cell cycle arrest in G0/G1 phase, we examined the expression of cyclins (D1 and A1) and their inhibitors (p27 and p21), which are involved during G1/S transition. Our data showed that androgen-deprivation led to the downregulation of both cyclin D1 and A1 expression in LNCaP cells, whereas the treatment with DHT or PP2A inhibition (by fostriecin or silencing of PPP2CA) caused their induction (Figure 3). Furthermore, the expression of p27, inhibitor of cyclin D1, was upregulated upon androgen-deprivation and downregulated upon treatment with DHT or PP2A inhibition. Interestingly, our data showed that the expression of p21 was changed in an opposite manner (Figure 3). The functional significance of such observation is not clear; however, this data is consistent with a previous finding (28). Altogether, our data suggest that PP2A inhibition potentiates proliferation and survival signaling and thus maintains androgen-independent growth of prostate cancer cells.
Figure 3. Inhibition of PP2A alters the expression and/or activation of survival and cell cycle-associated proteins.
LNCaP cells under steroid-reduced condition were treated with DHT (1.0 nM) or fostriecin (Fos) (100 nM) or silenced for PPP2CA expression. Following treatment, immunoblot analyses were performed for p-Akt/Akt, p-ERK/ERK, p-BAD/BAD, Bcl-xl, Bax, cyclin A1, cyclin D1, p27, p21 and β-actin (used as internal control). Phosphorylation of Akt, ERK and BAD was increased upon treatment with fostriecin or PPP2CA-specific siRNAs. Moreover, expression of anti-apoptotic Bcl-xL protein, cyclin A1 and D1 was increased, whereas expression of pro-apoptotic Bax protein and cyclin inhibitor p27 was decreased upon PP2A downregulation. Interestingly, treatment with DHT exhibited contrasting effects on Akt, ERK and BAD, while the expression of cyclin inhibitor, p21, was increased in both DHT-treated and PP2A-inhibited cells. Numbers below the bands, represent the fold ratio of densitometric quantification relative to corresponding control.
PP2A inhibition upregulates the expression of androgen receptor and partially sustains its transcriptional activity
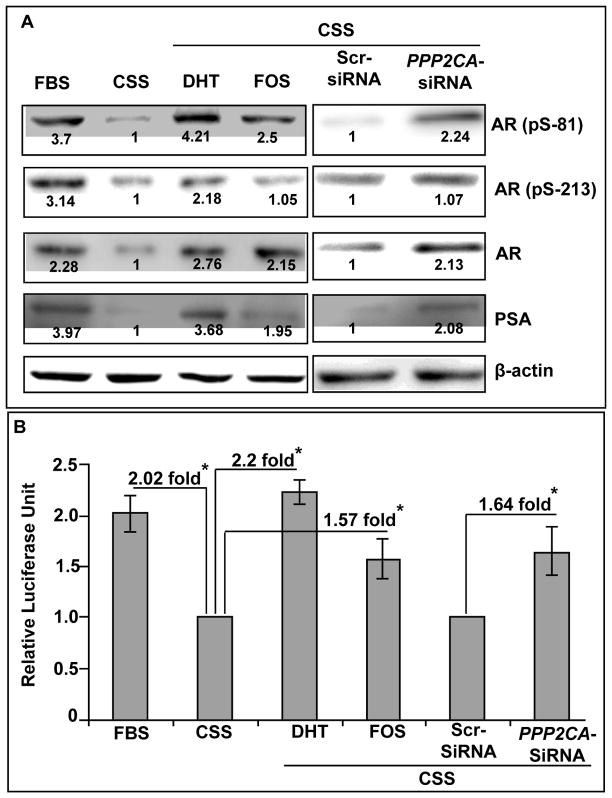
Androgen receptor (AR) plays important roles in both androgen-dependent and –independent growth of prostate cancer cells (1). It has been established that AR can maintain its transcriptional activity even under androgen-deprived condition through ligand-independent activation (28). Notably, it has been shown earlier that both Akt and ERK can induce phosphorylation of AR at serine residues leading to its activation (28, 31). Therefore, we examined the effect of PP2A inhibition on the phosphorylation of AR in LNCaP cells under steroid-depleted condition (Figure 4A). We observed that the inhibition of PP2A either by fostriecin or siRNA led to an increased phosphorylation of AR at serine-81 residue, while no change was detected at the serine-213. In contrast, stimulation with DHT induced phosphorylation at both the serines (81 and 213). Our immunoblotting data also demonstrated an induced expression of AR and its target gene, PSA/KLK3 upon treatment with DHT or PP2A inhibition (Figure 4A). To substantiate the activation of AR pathway, we conducted promoter-reporter assay to measure the transcription activity of an AR-responsive promoter. LNCaP cells were transfected with promoter-reporter and control plasmids (negative and positive), and 24 h post-transfection, treated with either DHT or fostriecin under steroid-depleted condition for next 24 h. In parallel, cells also co-transfected with scrambled or PPP2CA-specific siRNAs for 48 h. Transcriptional activity of AR is presented as the relative luciferase units (RLUs), which is the ratio between firefly (for AR activity) and renilla (transfection efficiency control) luciferase activity (Figure 4B). Our data show a limited induction of AR activity in LNCaP cells treated with fostriecin (1.57 fold) or silenced for PPP2CA expression (1.64 fold) under steroid-depleted condition as compared to the cells grown in normal FBS (2.02 fold) or cells treated with DHT (2.2 fold). Altogether, our findings suggest that the inhibition of PP2A partially sustains AR activity by inducing AR expression and ligand-independent phosphorylation.
Figure 4. Inhibition of PP2A leads to induction of androgen receptor expression and its ligand-independent activation.
A, LNCaP cells under steroid-reduced condition were treated with DHT (1.0 nM) or fostriecin (100 nM) or silenced for PPP2CA expression. Following treatment, immunoblot analyses were performed for AR, phospho-AR (Ser81 and Ser213/210) and PSA. β-actin was used as an internal control. Treatment with DHT or PP2A inhibition led to upregulation of AR and PSA and enhanced pSer(81)-AR phosphorylation. Phosphorylation at the Ser213/210 was only observed in DHT-treated cells. Numbers below the bands, represent the fold ratio of densitometric quantification relative to corresponding control. B, LNCaP cells were transfected with a mixture of control Renilla reporter and ARE-luciferase reporter plasmids. After 24 h of the transfection, cells were treated with either DHT (1 nM) or fostriecin (fos) (100 nM) in steroid-reduced medium for next 24 h. In parallel experiments, cells were co-transfected with scrambled or PPP2CA-specific siRNAs along with Renilla- or ARE-reporter plasmids for 48 h. Luciferase activities were estimated using a dual-luciferase kit. Relative luciferase units (RLUs), the ratio of firefly/Renilla luciferase) were calculated as a measure of AR transcriptional activity. Bars represent the means ± S.D. (n=3); *, statistically significant (p<0.05). A partial activation of AR is reported in PP2A inhibited LNCaP cells as compared to DHT-treated cells.
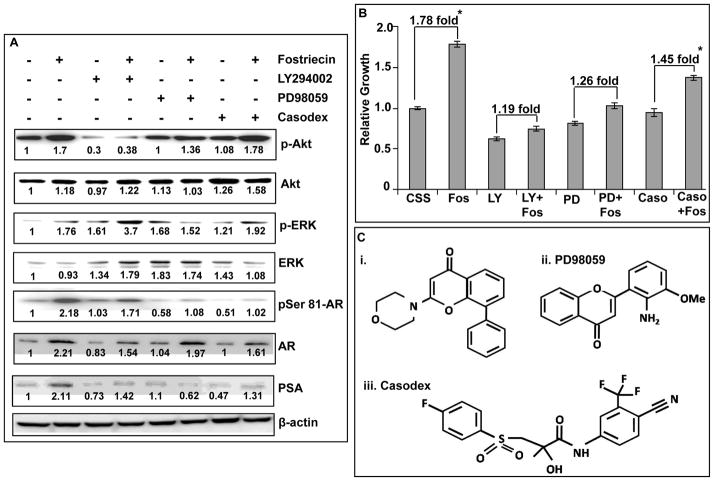
Androgen receptor activity is regulated by both Akt and ERK and their concerted action supports the androgen-independent growth of prostate cancer cells
Having evaluated the impact of PP2A inhibition on Akt, ERK and AR signaling pathways, we next evaluated the cross-talk of these signaling nodes and their involvement in androgen-independent growth of LNCaP cells. To examine this, we used pharmacological inhibitors of Akt (LY294002) and ERK (PD98059) and anti-androgen (Casodex) to obstruct their activation prior to PP2A inhibition under steroid-deprived condition. The blockade of Akt, ERK and AR activation was confirmed by monitoring their phosphorylation and PSA expression by immunoblotting (Figure 5A). Our data indicated that the induced expression of AR upon PP2A inhibition involves activation of Akt, while its phosphorylation at serine-81 is associated with ERK activation. Furthermore, inhibition of both Akt and ERK led to the reduced expression of PSA, thus indicating a role of these signaling pathways in ligand-independent activation of AR. Evaluation of LNCaP cell growth upon repression of Akt, ERK and AR prior to PP2A inhibition suggested a major role of Akt and ERK signaling pathways in supporting the androgen-independent growth of LNCaP cells (Figure 5B). Nonetheless, downregulation of AR also had a significant negative impact on the fostriecin-induced growth of LNCaP cells under androgen-deprived condition. These findings suggest that the inhibition of PP2A leads to the activation of Akt and ERK, which supports androgen-independent growth of LNCaP cells in AR-dependent (through partial activation) and –independent manners.
Figure 5. Pharmacological repression of Akt, ERK and AR signaling pathways suppresses androgen-independent growth of PP2A inhibited cells.
A, LNCaP cells were pre-treated for an hour with LY294002 (20 μM), PD98059 (25 μM) and Casodex (5 μM) followed by treatment with fostriecin (100 nM) for 24 h. Total protein was isolated and effect on the activation of Akt, ERK and AR was examined by immunoblotting with their total and phospho-form-specific antibodies. Data indicate that the induction of AR expression upon PP2A inhibition occurs through Akt pathway, whereas its ligand-independent phosphorylation involves ERK activation. Numbers below the bands, represent the fold ratio of densitometric quantification relative to corresponding control. B, In parallel experiments, effect of Akt, ERK and AR inhibition was observed on the growth of LNCaP cells under steroid-reduced condition following PP2A downregulation. Cell growth was assessed after 48 h of treatment by MTT assay. Bars represent the means ± S.D. (n=3). Our data indicates that androgen-independent growth of LNCaP cells upon PP2A inhibition is facilitated through a concerted action of Akt, ERK and AR signaling pathways. C. Chemical structures of LY294002, PI3K inhibitor (i), PD98059, ERK inhibitor (ii) and Casodex, anti-androgens (iii).
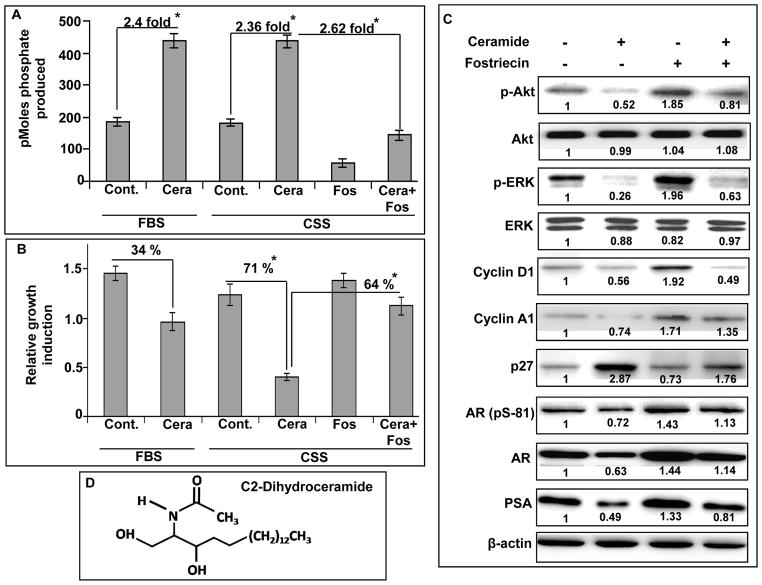
Activation of PP2A suppresses the androgen-independent growth of C4-2 prostate cancer cells
As C4-2 cells are androgen-independent and possess low PP2A activity, we examined if the activation of PP2A would diminish their growth under steroid-deprived condition. For this, we treated the C4-2 cells with ceramide, which is known to activate PP2A (32, 33) and observed its effect on their growth. Our data showed that ceramide treatment led to an increase (≥ 2.0 fold) in the activity of PP2A in C4-2 cells under both FBS and CSS conditions. Furthermore, we observed that the pretreatment of cells with fostriecin could arrest the ceramide-induced PP2A activity (Figure 6A). Treatment of C4-2 cells with ceramide decreased their growth (~34%) in regular media, whereas in steroid-deprived media, ceramide treatment showed even more potent effect (~ 71% decrease in growth) (Figure 6B). To confirm that the effect of ceramide on cellular growth was mediated through PP2A, we inhibited PP2A activity by pre-treating the C4-2 cells with fostriecin. Our data demonstrated that the inhibition of PP2A significantly attenuated ceramide-induced growth inhibition of C4-2 cells under steroid-depleted condition (Fig 6B). Our signaling data demonstrated that ceramide treatment decreased the phosphorylation of Akt and ERK, which could be reversed by pre-treatment with fostriecin (Figure 6C). It was also observed that the expression of cyclins (D1 and A1), AR, pS81-AR and PSA was downregulated, whereas, the expression of p27 was upregulated upon treatment of C4-2 cells with ceramide. Downregulation of PP2A with fostriecin abrogated ceramide-induced effect on cycin A1, D1, p27, AR and PSA (Figure 6C). Altogether, these findings provide additional support for a role of PP2A in modulating androgen-independent growth of prostate cancer cells.
Figure 6. Ceramide activates PP2A and suppresses the growth of androgen-independent prostate cancer C4-2 cells.
A, C4-2 prostate cancer cells under steroid-supplemented (FBS) or –reduced (CSS) conditions were treated with ceramide (20 μM) (with or without pre-treatment with fostriecin). PP2A activity was assessed after 24 h as previously described. Treatment with ceramide led to the activation of PP2A, which could be inhibited by pre-treatment with fostriecin. B, In parallel experiments, the effect of ceramide treatment was monitored on the growth of C4-2 cells under steroid-supplemented (FBS) or –reduced (CSS) conditions after 96 h using MTT assay. Ceramide led to the suppression of growth of C4-2 cells under both steroid-supplemented and –reduced conditions; however, the effect was more prominent under steroid-reduced condition. Pre-treatment with fostriecin attenuated ceramide-induced growth suppression. C, To examine the signaling changes, immunoblot analyses were performed for p-Akt/Akt, p-ERK/ERK, cyclin A1, cyclin D1, p27, phospho-AR (Ser81), AR, PSA and β-actin (used as internal control). Ceramide treatment led to the dephosphorylation of endogenously activated Akt and ERK, decreased the expression of cyclins, and induced the expression of p27. Moreover, reduced expression of AR and PSA, and decreased AR phosphorylation (Ser81) was also observed in ceramide treated cells. Pre-treatment of C4-2 cells with fostriecin abrogated the ceramide-induced changes in signaling/effector proteins. Numbers below the bands, represent the fold ratio of densitometric quantification relative to corresponding control. D. Chemical structure of C2-Dihydroceramide, a potent activator of PP2A.
DISCUSSION
Protein phosphorylation plays an important role in various biological processes and is regulated by a dynamic equilibrium between the protein kinases and phosphatases. Disruption of this balance often leads to various pathological conditions, including malignant transformation. Our earlier studies indicated that the downregulation of PP2A, a serine/threonine phosphatase, might be of clinical relevance in prostate cancer (11). Moreover, a recent phase I dose-escalation study of sodium selenate (an activator of PP2A) in patients with castration-resistant prostate cancer suggested that targeting of PP2A in combination with cytotoxic drug could be an effective therapeutic approach (34). In this study, our data demonstrate the functional role of PP2A in facilitating the androgen-independent growth of prostate tumor cells. Our data show that PP2A inhibition causes the release of steroid-depletion-induced cell-cycle arrest and prevents apoptosis. It has been reported earlier that androgen-withdrawal leads to cell-cycle arrest, and prostate cancer cells are able to bypass this checkpoint during the androgen-independent progression (26, 35). Furthermore, it has been shown that prostate cancer cells overexpress survival proteins, such as Bcl-2 or have deletion of tumor suppressor genes, such as PTEN, which enable them to resist apoptosis, and thus have a growth advantage under adverse conditions (36, 37). Therefore, our data is significant in explaining another possible mechanism by which prostate cancer cells gain apoptotic-resistance and escape cell-cycle arrest under androgen-deprivation.
Substantial body of evidence suggests that PP2A can impact cellular homeostasis by interacting with multiple signaling cascades (14). Many of these signaling pathways (Akt, MAPK, etc.) have functionally been implicated in the pathogenesis and androgen-independent nature of prostate cancer cells (15, 17, 28). We have observed that down-modulation of PP2A results in the activation of Akt and ERK, inactivation of BAD and induction of cell cycle-associated proteins in LNCaP cells. Akt is a downstream effector of phosphatidylinositol 3-kinase (PI3K) and has often been implicated in androgen-independent progression of prostate cancer (28, 38, 39). PI3K is upregulated in LNCaP cells due to the deletion of PTEN resulting in the hyperactivation of Akt (37). As the activity of Akt can also be controlled through PP2A-mediated dephosphorylation (40), our data indicates that the loss of this regulatory checkpoint further promotes Akt activation. PP2A has also been shown to suppress MEK-ERK pathway (15, 17), and both Akt and ERK have been shown to potentiate the proliferation and survival of cancer cells (38, 41). In fact, it has been reported that forced activation of either Akt or ERK signaling in an androgen-responsive prostate cancer cell line could induce hormone-independent growth in culture (42). Furthermore, it was observed that these pathways act synergistically in vivo to promote tumorigenicity and androgen-independence.
As majority of androgen-independent prostate tumors retain androgen receptor expression and overexpress androgen-regulated genes (PSA, etc.), a pathogenic role of aberrant AR signaling is also considered central to AI progression of PCa (1, 6, 7). One of the important mechanisms proposed to explain the AI growth of PCa implicates an important role of ligand-independent activation of AR signaling. It has been shown that certain growth factors (IGF1, KGF and EGF) can activate the AR in the absence of androgen in PCa cells (43). In other studies, overexpression of ErbB2/HER2 has been shown to activate the expression of AR-dependent genes (6, 44). It is shown that such ligand-independent activation of AR signaling may involve MAP kinase pathway (44). However, the role of PI3K/Akt pathway in AR-mediated PC cell growth has been controversial and largely unclear. In some cases, Akt has been shown to suppress AR activity (45), while in other reports, it is also shown to potentiate AR action (46, 47). In this study, we report that PP2A downregulation leads to partially-sustained AR signaling. Our data indicate that AR signaling is maintained through induced expression of AR and its ligand-independent activation. These observations are in corroboration with recently published report, where PP2A inhibition was shown to cooperate with DHT to induce AR expression and phosphorylation (48). In addition, our studies utilizing pharmacological inhibitors against Akt and MEK/ERK indicate that induction of AR expression upon PP2A inhibition is mediated through the activation of Akt, whereas its ligand-independent phosphorylation (on serine-81) is caused by ERK activation. An earlier study also reported that AR phosphorylation at Ser-81 is mediated through ERK pathway (31). In other studies, AR phosphorylation on serine-213 by Akt has also been reported; however, we did not observe such phosphorylation despite activation of Akt in response to PP2A inhibition. Nonetheless, our data on AR transcriptional activity and PSA expression confirmed the partial activation of AR upon downregulation of PP2A under steroid-depleted condition, and thus holds mechanistic significance. Our data also highlighted the importance of these signaling pathways in sustaining androgen-independent growth of LNCaP cells upon PP2A inhibition. While we noted almost complete abrogation of AI growth in Akt- and ERK-inhibited cells, a minimal, but significant effect of AR inhibition was also observed. These findings are in accordance with an earlier report, where activation of AR signaling was found to be important in Akt or ERK-induced androgen-independent growth of prostate cancer cells (42).
In summary, our data provide first experimental evidence to support the functional significance of PP2A downregulation in androgen-independent progression of prostate cancer. Our findings demonstrate that PP2A is upregulated in LNCaP (AD) cells as compared to C4-2 (AI) prostate cancer cells, and the blockade of its activity sustains the growth of LNCaP cells under steroid-depleted condition. Our data clearly indicate that PP2A inhibition rescues LNCaP cells from steroid-deprivation-induced cell-cycle arrest and apoptosis. Mechanistic studies demonstrate that both Akt and ERK get activated upon PP2A inhibition and support the androgen-independent growth of LNCaP cells in AR-dependent and –independent manners. Our data reveal that the AR signaling is partially-sustained upon PP2A downregulation in LNCaP cells, in part, through induced expression of AR and its ligand-independent activation. These findings are further supported by our observations in androgen-independent C4-2 cells where activation of PP2A is shown to cause the suppression of their growth under steroid-reduced condition. Altogether, these findings may aid in the development of novel therapeutic strategies targeting the PP2A signaling network and/or better treatment planning against androgen-independent prostate cancer.
Acknowledgments
Grant support: Department of Defense/US Army (W81XWH-09-1-0137), NIH/NCI (CA137513) and USAMCI.
We thank Steve McClellan (USAMCI Flow Cytometry Core Facility) for technical support and Dr. Joel Andrews (USAMCI) for help with fluorescence microscopy.
Abbreviations
- ADT
androgen-deprivation therapy
- AD
androgen-dependent
- AI
androgen-independent
- AR
androgen-receptor
- CSS
charcoal-stripped serum
- DHT
dihydro-testosterone
- FBS
fetal-bovine serum
- PCa
prostate cancer
- PSA
prostate-specific antigen
References
- 1.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Culig Z, Hobisch A, Cronauer MV, Cato AC, Hittmair A, Radmayr C, et al. Mutant androgen receptor detected in an advanced-stage prostatic carcinoma is activated by adrenal androgens and progesterone. Mol Endocrinol. 1993;7:1541–50. doi: 10.1210/mend.7.12.8145761. [DOI] [PubMed] [Google Scholar]
- 4.Gregory CW, He B, Johnson RT, Ford OH, Mohler JL, French FS, et al. A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy. Cancer Res. 2001;61:4315–9. [PubMed] [Google Scholar]
- 5.Koivisto P, Kononen J, Palmberg C, Tammela T, Hyytinen E, Isola J, et al. Androgen receptor gene amplification: a possible molecular mechanism for androgen deprivation therapy failure in prostate cancer. Cancer Res. 1997;57:314–9. [PubMed] [Google Scholar]
- 6.Craft N, Shostak Y, Carey M, Sawyers CL. A mechanism for hormone-independent prostate cancer through modulation of androgen receptor signaling by the HER-2/neu tyrosine kinase. Nat Med. 1999;5:280–5. doi: 10.1038/6495. [DOI] [PubMed] [Google Scholar]
- 7.Kim O, Jiang T, Xie Y, Guo Z, Chen H, Qiu Y. Synergism of cytoplasmic kinases in IL6-induced ligand-independent activation of androgen receptor in prostate cancer cells. Oncogene. 2004;23:1838–44. doi: 10.1038/sj.onc.1207304. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Park S, Carroll PR. Prostate cancer 2004: insights from national disease registries. Oncology (Williston Park) 2004;18:1239–47. [PubMed] [Google Scholar]
- 9.Joly F, Tannock IF. Chemotherapy for patients with hormone-refractory prostate cancer. Ann Oncol. 2004;15:1582–4. doi: 10.1093/annonc/mdh445. [DOI] [PubMed] [Google Scholar]
- 10.Tannock IF, de WR, Berry WR, Horti J, Pluzanska A, Chi KN, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502–12. doi: 10.1056/NEJMoa040720. [DOI] [PubMed] [Google Scholar]
- 11.Singh AP, Bafna S, Chaudhary K, Venkatraman G, Smith L, Eudy JD, et al. Genome-wide expression profiling reveals transcriptomic variation and perturbed gene networks in androgen-dependent and androgen-independent prostate cancer cells. Cancer Lett. 2008;259:28–38. doi: 10.1016/j.canlet.2007.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Prowatke I, Devens F, Benner A, Grone EF, Mertens D, Grone HJ, et al. Expression analysis of imbalanced genes in prostate carcinoma using tissue microarrays. Br J Cancer. 2007;96:82–8. doi: 10.1038/sj.bjc.6603490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khew-Goodall Y, Hemmings BA. Tissue-specific expression of mRNAs encoding alpha-and beta-catalytic subunits of protein phosphatase 2A. FEBS Lett. 1988;238:265–8. doi: 10.1016/0014-5793(88)80493-9. [DOI] [PubMed] [Google Scholar]
- 14.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–39. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grethe S, Porn-Ares MI. p38 MAPK regulates phosphorylation of Bad via PP2A-dependent suppression of the MEK1/2-ERK1/2 survival pathway in TNF-alpha induced endothelial apoptosis. Cell Signal. 2006;18:531–40. doi: 10.1016/j.cellsig.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 16.Sontag E, Sontag JM, Garcia A. Protein phosphatase 2A is a critical regulator of protein kinase C zeta signaling targeted by SV40 small t to promote cell growth and NF-kappaB activation. EMBO J. 1997;16:5662–71. doi: 10.1093/emboj/16.18.5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Kanegan MJ, Adams DG, Wadzinski BE, Strack S. Distinct protein phosphatase 2A heterotrimers modulate growth factor signaling to extracellular signal-regulated kinases and Akt. J Biol Chem. 2005;280:36029–36. doi: 10.1074/jbc.M506986200. [DOI] [PubMed] [Google Scholar]
- 18.Gotz J, Probst A, Ehler E, Hemmings B, Kues W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Calpha. Proc Natl Acad Sci U S A. 1998;95:12370–5. doi: 10.1073/pnas.95.21.12370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverstein AM, Barrow CA, Davis AJ, Mumby MC. Actions of PP2A on the MAP kinase pathway and apoptosis are mediated by distinct regulatory subunits. Proc Natl Acad Sci U S A. 2002;99:4221–6. doi: 10.1073/pnas.072071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Strack S, Cribbs JT, Gomez L. Critical role for protein phosphatase 2A heterotrimers in mammalian cell survival. J Biol Chem. 2004;279:47732–9. doi: 10.1074/jbc.M408015200. [DOI] [PubMed] [Google Scholar]
- 21.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–9. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 22.Miyabayashi T, Teo JL, Yamamoto M, McMillan M, Nguyen C, Kahn M. Wnt/beta-catenin/CBP signaling maintains long-term murine embryonic stem cell pluripotency. Proc Natl Acad Sci U S A. 2007;104:5668–73. doi: 10.1073/pnas.0701331104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Srivastava SK, Bhardwaj A, Owen LB, Singh AP. CXCL12-CXCR4 signalling axis confers gemcitabine resistance to pancreatic cancer cells: a novel target for therapy. Br J Cancer. 2010;103:1671–9. doi: 10.1038/sj.bjc.6605968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eto M, Bennouna J, Hunter OC, Hershberger PA, Kanto T, Johnson CS, et al. C16 ceramide accumulates following androgen ablation in LNCaP prostate cancer cells. Prostate. 2003;57:66–79. doi: 10.1002/pros.10275. [DOI] [PubMed] [Google Scholar]
- 25.Kazi A, Smith DM, Zhong Q, Dou QP. Inhibition of bcl-x(l) phosphorylation by tea polyphenols or epigallocatechin-3-gallate is associated with prostate cancer cell apoptosis. Mol Pharmacol. 2002;62:765–71. doi: 10.1124/mol.62.4.765. [DOI] [PubMed] [Google Scholar]
- 26.Knudsen KE, Arden KC, Cavenee WK. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J Biol Chem. 1998;273:20213–22. doi: 10.1074/jbc.273.32.20213. [DOI] [PubMed] [Google Scholar]
- 27.Carson JP, Kulik G, Weber MJ. Antiapoptotic signaling in LNCaP prostate cancer cells: a survival signaling pathway independent of phosphatidylinositol 3′-kinase and Akt/protein kinase B. Cancer Res. 1999;59:1449–53. [PubMed] [Google Scholar]
- 28.Murillo H, Huang H, Schmidt LJ, Smith DI, Tindall DJ. Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology. 2001;142:4795–805. doi: 10.1210/endo.142.11.8467. [DOI] [PubMed] [Google Scholar]
- 29.Rokhlin OW, Taghiyev AF, Guseva NV, Glover RA, Syrbu SI, Cohen MB. TRAIL-DISC formation is androgen-dependent in the human prostatic carcinoma cell line LNCaP. Cancer Biol Ther. 2002;1:631–7. doi: 10.4161/cbt.311. [DOI] [PubMed] [Google Scholar]
- 30.Baron S, Manin M, Beaudoin C, Leotoing L, Communal Y, Veyssiere G, et al. Androgen receptor mediates non-genomic activation of phosphatidylinositol 3-OH kinase in androgen-sensitive epithelial cells. J Biol Chem. 2004;279:14579–86. doi: 10.1074/jbc.M306143200. [DOI] [PubMed] [Google Scholar]
- 31.Shigemura K, Isotani S, Wang R, Fujisawa M, Gotoh A, Marshall FF, et al. Soluble factors derived from stroma activated androgen receptor phosphorylation in human prostate LNCaP cells: roles of ERK/MAP kinase. Prostate. 2009;69:949–55. doi: 10.1002/pros.20944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law B, Rossie S. The dimeric and catalytic subunit forms of protein phosphatase 2A from rat brain are stimulated by C2-ceramide. J Biol Chem. 1995;270:12808–13. doi: 10.1074/jbc.270.21.12808. [DOI] [PubMed] [Google Scholar]
- 33.Ruvolo PP, Deng X, Ito T, Carr BK, May WS. Ceramide induces Bcl2 dephosphorylation via a mechanism involving mitochondrial PP2A. J Biol Chem. 1999;274:20296–300. doi: 10.1074/jbc.274.29.20296. [DOI] [PubMed] [Google Scholar]
- 34.Corcoran NM, Hovens CM, Michael M, Rosenthal MA, Costello AJ. Open-label, phase I dose-escalation study of sodium selenate, a novel activator of PP2A, in patients with castration-resistant prostate cancer. Br J Cancer. 2010;103:462–8. doi: 10.1038/sj.bjc.6605798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agus DB, Cordon-Cardo C, Fox W, Drobnjak M, Koff A, Golde DW, et al. Prostate cancer cell cycle regulators: response to androgen withdrawal and development of androgen independence. J Natl Cancer Inst. 1999;91:1869–76. doi: 10.1093/jnci/91.21.1869. [DOI] [PubMed] [Google Scholar]
- 36.McDonnell TJ, Troncoso P, Brisbay SM, Logothetis C, Chung LW, Hsieh JT, et al. Expression of the protooncogene bcl-2 in the prostate and its association with emergence of androgen-independent prostate cancer. Cancer Res. 1992;52:6940–4. [PubMed] [Google Scholar]
- 37.Schmitz M, Grignard G, Margue C, Dippel W, Capesius C, Mossong J, et al. Complete loss of PTEN expression as a possible early prognostic marker for prostate cancer metastasis. Int J Cancer. 2007;120:1284–92. doi: 10.1002/ijc.22359. [DOI] [PubMed] [Google Scholar]
- 38.Graff JR, Konicek BW, McNulty AM, Wang Z, Houck K, Allen S, et al. Increased AKT activity contributes to prostate cancer progression by dramatically accelerating prostate tumor growth and diminishing p27Kip1 expression. J Biol Chem. 2000;275:24500–5. doi: 10.1074/jbc.M003145200. [DOI] [PubMed] [Google Scholar]
- 39.Malik SN, Brattain M, Ghosh PM, Troyer DA, Prihoda T, Bedolla R, et al. Immunohistochemical demonstration of phospho-Akt in high Gleason grade prostate cancer. Clin Cancer Res. 2002;8:1168–71. [PubMed] [Google Scholar]
- 40.Kim SW, Kim HJ, Chun YJ, Kim MY. Ceramide produces apoptosis through induction of p27(kip1) by protein phosphatase 2A-dependent Akt dephosphorylation in PC-3 prostate cancer cells. J Toxicol Environ Health A. 2010;73:1465–76. doi: 10.1080/15287394.2010.511553. [DOI] [PubMed] [Google Scholar]
- 41.Li B, Sun A, Youn H, Hong Y, Terranova PF, Thrasher JB, et al. Conditional Akt activation promotes androgen-independent progression of prostate cancer. Carcinogenesis. 2007;28:572–83. doi: 10.1093/carcin/bgl193. [DOI] [PubMed] [Google Scholar]
- 42.Gao H, Ouyang X, Banach-Petrosky WA, Gerald WL, Shen MM, Abate-Shen C. Combinatorial activities of Akt and B-Raf/Erk signaling in a mouse model of androgen-independent prostate cancer. Proc Natl Acad Sci U S A. 2006;103:14477–82. doi: 10.1073/pnas.0606836103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, et al. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–8. [PubMed] [Google Scholar]
- 44.Yeh S, Lin HK, Kang HY, Thin TH, Lin MF, Chang C. From HER2/Neu signal cascade to androgen receptor and its coactivators: a novel pathway by induction of androgen target genes through MAP kinase in prostate cancer cells. Proc Natl Acad Sci U S A. 1999;96:5458–63. doi: 10.1073/pnas.96.10.5458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin HK, Yeh S, Kang HY, Chang C. Akt suppresses androgen-induced apoptosis by phosphorylating and inhibiting androgen receptor. Proc Natl Acad Sci U S A. 2001;98:7200–5. doi: 10.1073/pnas.121173298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wen Y, Hu MC, Makino K, Spohn B, Bartholomeusz G, Yan DH, et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000;60:6841–5. [PubMed] [Google Scholar]
- 47.Xin L, Teitell MA, Lawson DA, Kwon A, Mellinghoff IK, Witte ON. Progression of prostate cancer by synergy of AKT with genotropic and nongenotropic actions of the androgen receptor. Proc Natl Acad Sci U S A. 2006;103:7789–94. doi: 10.1073/pnas.0602567103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen S, Kesler CT, Paschal BM, Balk SP. Androgen receptor phosphorylation and activity are regulated by an association with protein phosphatase 1. J Biol Chem. 2009;284:25576–84. doi: 10.1074/jbc.M109.043133. [DOI] [PMC free article] [PubMed] [Google Scholar]