Abstract
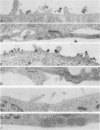
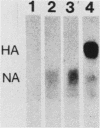
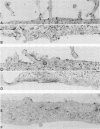
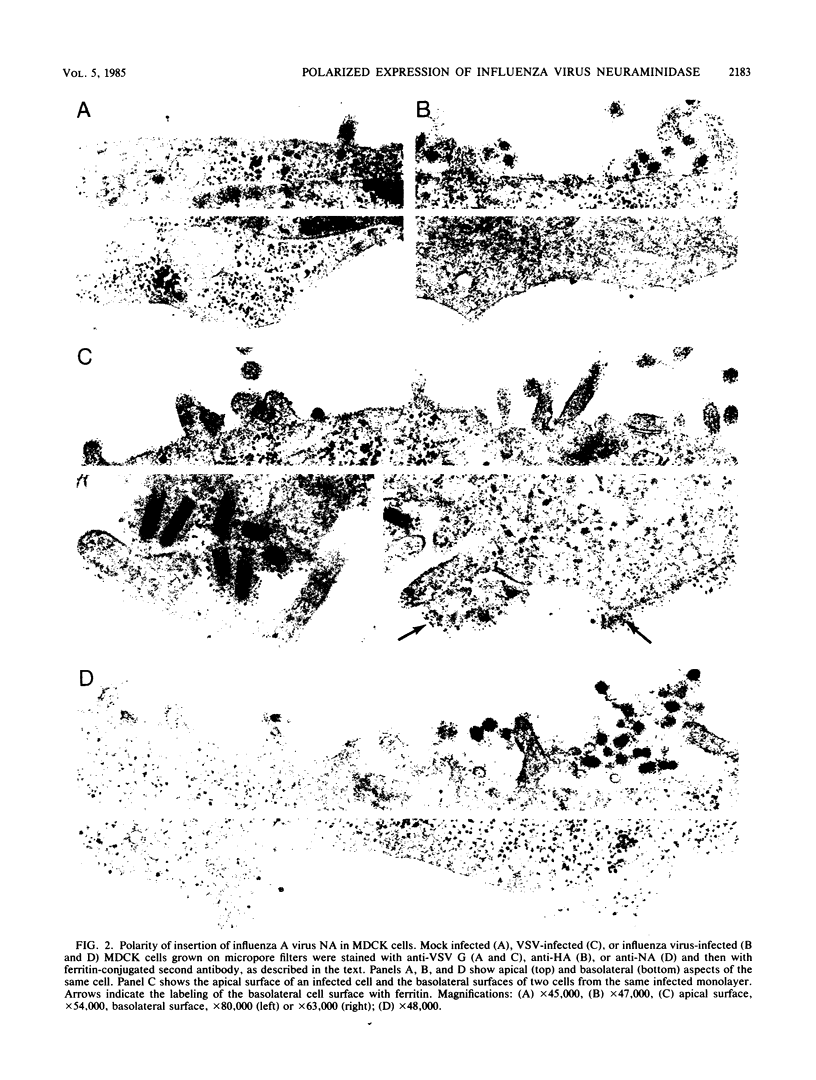
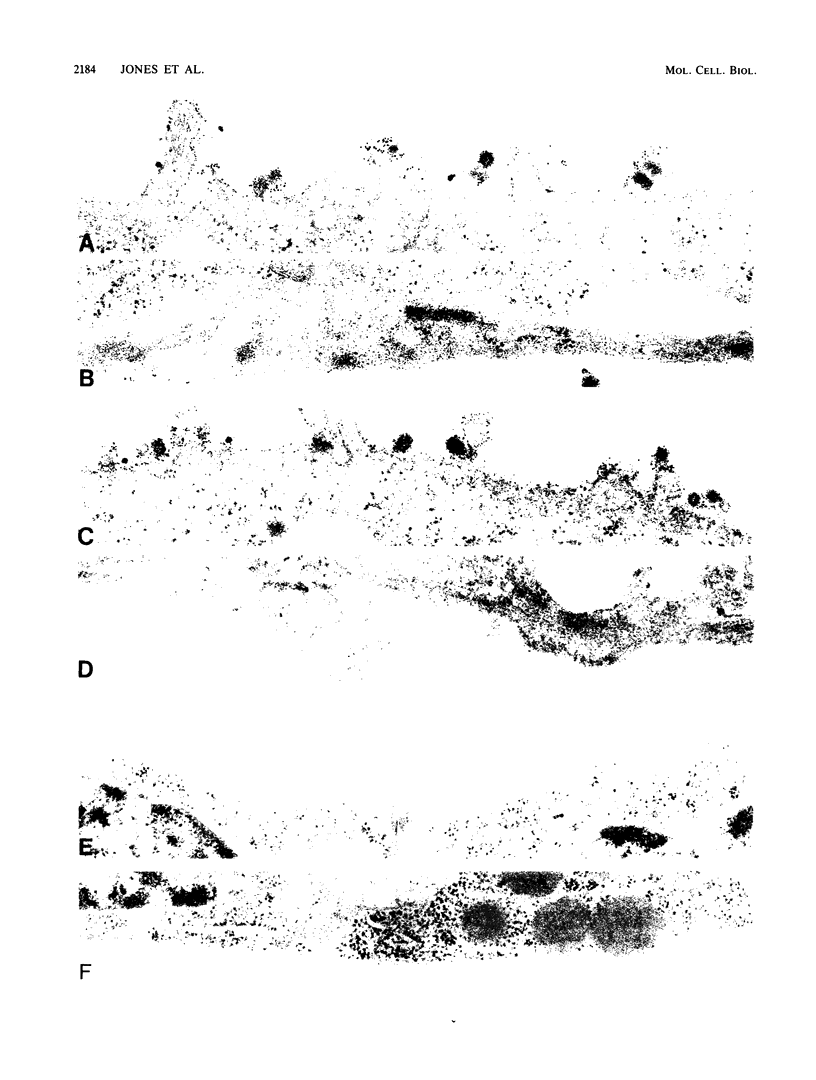
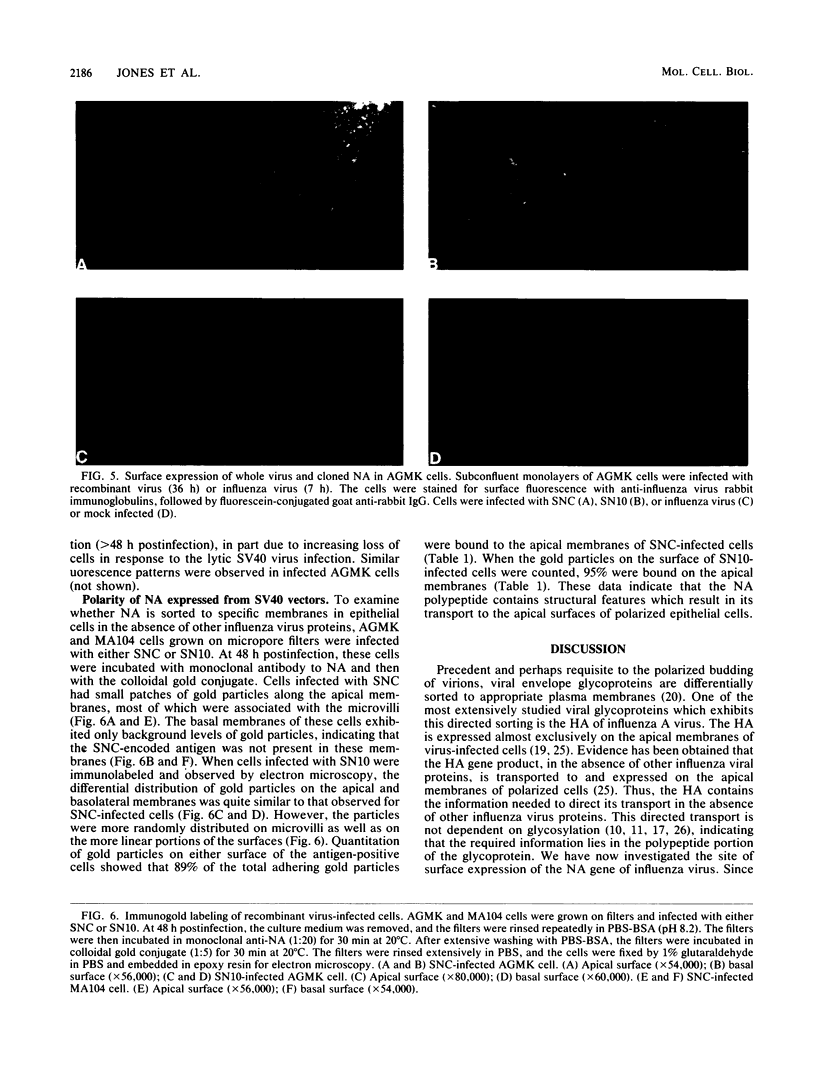
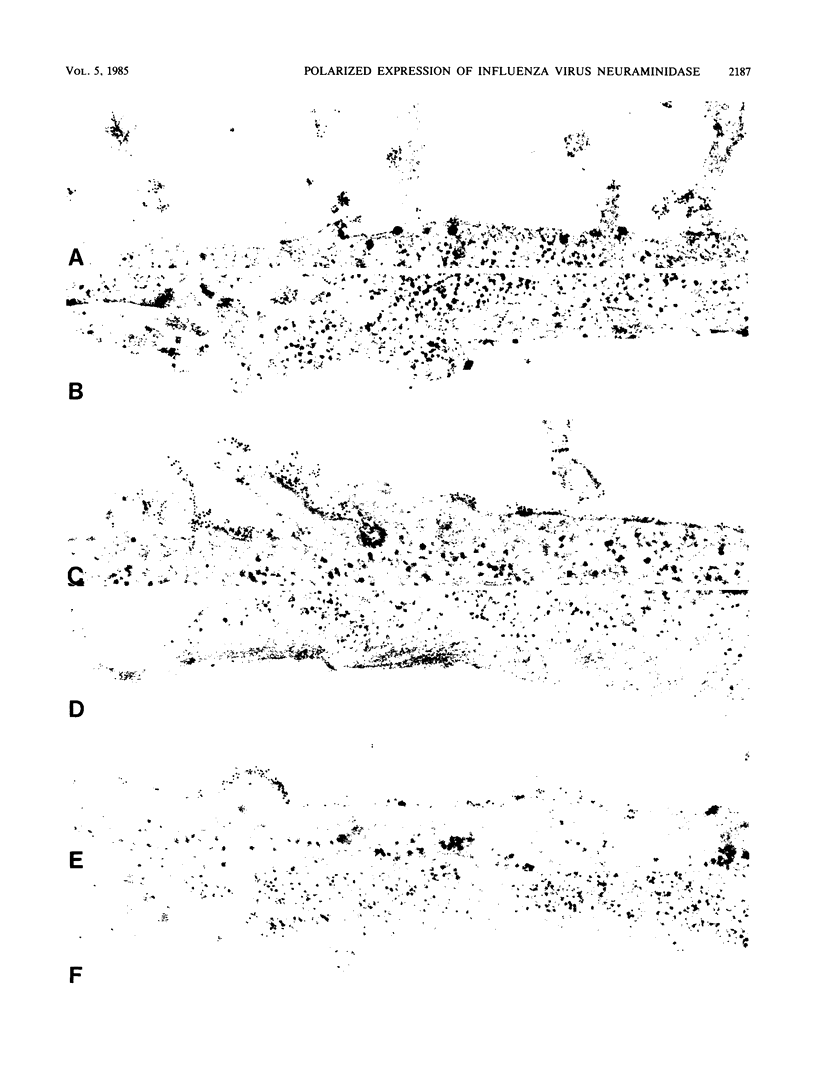
We have investigated the site of surface expression of the neuraminidase (NA) glycoprotein of influenza A virus, which, in contrast to the hemagglutinin, is bound to membranes by hydrophobic residues near the NH2-terminus. Madin-Darby canine kidney or primary African green monkey kidney cells infected with influenza A/WSN/33 virus and subsequently labeled with monoclonal antibody to the NA and then with a colloidal gold- or ferritin-conjugated second antibody exhibited specific labeling of apical surfaces. Using simian virus 40 late expression vectors, we also studied the surface expression of the complete NA gene (SNC) and a truncated NA gene (SN10) in either primary or a polarized continuous line (MA104) of African green monkey kidney cells. The polypeptides encoded by the cloned NA cDNAs were expressed on the surface of both cell types. Analysis of [3H]mannose-labeled polypeptides from recombinant virus-infected MA104 cells showed that the products of cloned NA cDNA comigrated with glycosylated NA from influenza virus-infected cells. Both the complete and the truncated glycoproteins were found to be preferentially expressed on apical plasma membranes, as detected by immunogold labeling. These results indicate that the NA polypeptide contains structural features capable of directing the transport of the protein to apical cell surfaces and the first 10 amino-terminal residues of the NA polypeptide are not involved in this process.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alonso-Caplen F. V., Compans R. W. Modulation of glycosylation and transport of viral membrane glycoproteins by a sodium ionophore. J Cell Biol. 1983 Sep;97(3):659–668. doi: 10.1083/jcb.97.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S., Compans R. W. Studies on the role of glycosylation in the functions and antigenic properties of influenza virus glycoproteins. Virology. 1983 Jul 15;128(1):77–91. doi: 10.1016/0042-6822(83)90320-3. [DOI] [PubMed] [Google Scholar]
- Blok J., Air G. M. Comparative nucleotide sequences at the 3' end of the neuraminidase gene from eleven influenza type A viruses. Virology. 1980 Nov;107(1):50–60. doi: 10.1016/0042-6822(80)90271-8. [DOI] [PubMed] [Google Scholar]
- Blok J., Air G. M., Laver W. G., Ward C. W., Lilley G. G., Woods E. F., Roxburgh C. M., Inglis A. S. Studies on the size, chemical composition, and partial sequence of the neuraminidase (NA) from type A influenza viruses show that the N-terminal region of the NA is not processed and serves to anchor the NA in the viral membrane. Virology. 1982 May;119(1):109–121. doi: 10.1016/0042-6822(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Bos T. J., Davis A. R., Nayak D. P. NH2-terminal hydrophobic region of influenza virus neuraminidase provides the signal function in translocation. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2327–2331. doi: 10.1073/pnas.81.8.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W. Replication of influenza virus in a continuous cell line: high yield of infective virus from cells inoculated at high multiplicity. Virology. 1969 Sep;39(1):130–134. doi: 10.1016/0042-6822(69)90354-7. [DOI] [PubMed] [Google Scholar]
- Davis A. R., Bos T. J., Nayak D. P. Active influenza virus neuraminidase is expressed in monkey cells from cDNA cloned in simian virus 40 vectors. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3976–3980. doi: 10.1073/pnas.80.13.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields S., Winter G., Brownlee G. G. Structure of the neuraminidase gene in human influenza virus A/PR/8/34. Nature. 1981 Mar 19;290(5803):213–217. doi: 10.1038/290213a0. [DOI] [PubMed] [Google Scholar]
- Gibson R., Leavitt R., Kornfeld S., Schlesinger S. Synthesis and infectivity of vesicular stomatitis virus containing nonglycosylated G protein. Cell. 1978 Apr;13(4):671–679. doi: 10.1016/0092-8674(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Green R. F., Meiss H. K., Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981 May;89(2):230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrler G., Nagele A., Meier-Ewert H., Bhown A. S., Compans R. W. Isolation and structural analysis of influenza C virion glycoproteins. Virology. 1981 Sep;113(2):439–451. doi: 10.1016/0042-6822(81)90173-2. [DOI] [PubMed] [Google Scholar]
- Hiti A. L., Nayak D. P. Complete nucleotide sequence of the neuraminidase gene of human influenza virus A/WSN/33. J Virol. 1982 Feb;41(2):730–734. doi: 10.1128/jvi.41.2.730-734.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Markoff L., Lin B. C., Sveda M. M., Lai C. J. Glycosylation and surface expression of the influenza virus neuraminidase requires the N-terminal hydrophobic region. Mol Cell Biol. 1984 Jan;4(1):8–16. doi: 10.1128/mcb.4.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen N. L., Nayak D. P., Jones L. V., Compans R. W. Chimeric influenza virus hemagglutinin containing either the NH2 terminus or the COOH terminus of G protein of vesicular stomatitis virus is defective in transport to the cell surface. Proc Natl Acad Sci U S A. 1984 Jan;81(2):395–399. doi: 10.1073/pnas.81.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Porter A. G., Barber C., Carey N. H., Hallewell R. A., Threlfall G., Emtage J. S. Complete nucleotide sequence of an influenza virus haemagglutinin gene from cloned DNA. Nature. 1979 Nov 29;282(5738):471–477. doi: 10.1038/282471a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980 May;20(1):45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez Boulan E., Sabatini D. D. Asymmetric budding of viruses in epithelial monlayers: a model system for study of epithelial polarity. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5071–5075. doi: 10.1073/pnas.75.10.5071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Paskiet K. T., Salas P. J., Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984 Jan;98(1):308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W. Antibody-resistant spread of vesicular stomatitis virus infection in cell lines of epithelial origin. J Virol. 1980 Aug;35(2):547–550. doi: 10.1128/jvi.35.2.547-550.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W. Delayed appearance of pseudotypes between vesicular stomatitis virus influenza virus during mixed infection of MDCK cells. J Virol. 1981 Dec;40(3):848–860. doi: 10.1128/jvi.40.3.848-860.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Compans R. W., Giusti L., Davis A. R., Nayak D. P., Gething M. J., Sambrook J. Influenza virus hemagglutinin expression is polarized in cells infected with recombinant SV40 viruses carrying cloned hemagglutinin DNA. Cell. 1983 Jun;33(2):435–443. doi: 10.1016/0092-8674(83)90425-7. [DOI] [PubMed] [Google Scholar]
- Roth M. G., Fitzpatrick J. P., Compans R. W. Polarity of influenza and vesicular stomatitis virus maturation in MDCK cells: lack of a requirement for glycosylation of viral glycoproteins. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6430–6434. doi: 10.1073/pnas.76.12.6430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth M. G., Srinivas R. V., Compans R. W. Basolateral maturation of retroviruses in polarized epithelial cells. J Virol. 1983 Mar;45(3):1065–1073. doi: 10.1128/jvi.45.3.1065-1073.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga M. C., Malissen B., McMillan M., Brayton P. R., Clark S. S., Forman J., Hood L. Expression and function of transplantation antigens with altered or deleted cytoplasmic domains. Cell. 1983 Sep;34(2):535–544. doi: 10.1016/0092-8674(83)90386-0. [DOI] [PubMed] [Google Scholar]