Abstract
Romiplostim and eltrombopag, the first thrombopoietic receptor-agonists with demonstrated efficacy against immune thrombocytopenia in prospective controlled studies, were recently authorized in most countries for adults with chronic immune thrombocytopenia. So far, no data are available about the potential contribution of switching from romiplostim to eltrombopag or vice versa in terms of efficacy or tolerance. Efficacies and tolerance profiles were evaluated for 46 patients who sequentially received both drugs, switching from one to the other. The reasons for switching were: lack of efficacy for 23 patients, platelet-count fluctuations for 11, side effects for 4, and 8 patients’ preferences. For 50–80% of the patients, switching from romiplostim to eltrombopag or eltrombopag to romiplostim effectively impacted the platelet count, with fluctuations disappearing in 54% and side effects resolved in 100%. In 80% of the patients, the 2 thrombopoietic receptor-agonists achieved similar response patterns. Our results confirmed that switching from one thrombopoietic receptor-agonist to the other could be beneficial in clinical practice for patients with severe chronic immune thrombopenia who failed to respond or experienced adverse events to the first. (Clinical Trials.gov identifier: NCT01618734).
Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts responsible for various degrees of mucocutaneous bleeding.1–6 ITP pathophysiology has long been considered to be only a matter of accelerated platelet destruction by platelet-bound antibodies but there is strong evidence to show that it is also associated with impaired platelet production.7–11 Most therapies commonly used to treat ITP (e.g. corticosteroids, intravenous immunoglobulins (IVIg), immunosuppressants and splenectomy) are mainly active by reducing the destruction of antibody-coated platelets. In contrast, the novel thrombopoietic receptor-agonists (TPO-RAs) stimulate platelet production.
Two TPO-RAs are now available. Romiplostim is a peptide TPO-RA composed of an IgG Fc fragment to which four 14-amino–acid TPO peptides are attached; one of them activates the TPO-R by binding to the extracytoplasmic domain, just like endogenous TPO.12 Romiplostim is administered as a weekly subcutaneous injection.13 Eltrombopag is a non-peptide TPO-RA that is a 442-Da drug that binds to a transmembrane site on the TPO-R, thereby activating it. It is administered daily as an oral tablet.14 In randomized-controlled trials, the reported response rates to romiplostim and eltrombopag were 59–88% and this high efficacy was achieved in splenectomized and non-splenectomized ITP patients.15–19 In view of these robust data, both drugs have been approved for adult chronic ITP in more than 80 countries and some groups consider them second-line treatment for chronic ITP.5,20 However, in Europe, they are only authorized for use after splenectomy failure or when splenectomy is contraindicated.
In contrast to these very good results, in an observational study on romiplostim we showed that inefficacy or side effects led approximately one-third of the patients to discontinue treatment.21 Because romiplostim and eltrombopag bind to different sites on the TPO-R and the 2 molecules have not yet been directly compared, the relevance of switching from one TPO-RA to the other in clinical practice has not been established. To determine the pertinence of switching, a retrospective multicenter pilot trial was conducted throughout the network of French National Referral Centers for Adult ITP.
Design and Methods
Patients
All patients followed by specialists in the network of National ITP Referral Centers who have been sequentially treated with the TPO-RAs, romiplostim and eltrombopag, were enrolled. Inclusion criteria were: 1) age 18 years or over; 2) primary ITP diagnosed according to the current definitions;4,22 3) previous romiplostim and eltrombopag treatment, regardless of the switching sequence, with at least three months of follow up with each molecule. Patients treated for secondary ITP were excluded.
The trial protocol was approved by the Henri-Mondor University Hospital Institutional Review Board and Ethics Committee; all patients received a written information form before screening and data analysis.
Treatment design
According to the European Medicines Agency authorization of the 2 TPO-RAs, patients received a weekly subcutaneous injection of romiplostim, usually started at 1 μg/kg/week, or daily oral eltrombopag, at a starting dose of 50 mg/day; the dose was then adjusted, as needed (up to a maximum 10 μg/kg/week and 75 mg/day, respectively) based on the patient’s platelet count.
Patients could continue to receive concurrent ITP therapies, including rescue interventions, defined as any agent administered to transiently increase the platelet count, e.g. IVIg, corticosteroids, anti-IgD and/or platelet transfusions. The need to increase the dose of concurrent ITP medication to levels above those used at baseline was also considered a rescue intervention.
Assessments and outcome measures
The following characteristics were recorded on a standardized form by the same investigator (MK): age, gender; and ITP history: disease duration, previous and/or ongoing medications and bleeding assessment within one month prior to starting TPO-RA. Reasons for non-splenectomy were also noted for those patients not splenectomized. The need for concurrent and/or rescue interventions during follow up was also recorded. Platelet counts were obtained on average weekly until the TPO-RA dose was stabilized and then at least once monthly.
From a clinical point of view, only a sustained response was taken into account. It was defined as lasting for at least 2 consecutive platelet counts at the end of a minimum follow up of three months of treatment and never receiving rescue treatment. In accordance with the usual standards,22 complete response was defined as a platelet count of 100×109/L and a response as a platelet count of 30×109/L or overwith at least twice the initial (pre-treatment) value.
Adverse events were retrospectively entered on a standard case-report form and then analyzed by the same investigator (MK).
Statistical analysis
Quantitative data are presented as means±standard deviation (SD) or range, or medians (range or interquartile range, IQR), as appropriate, and qualitative data as numbers (%). Quantitative data were compared using the Mann-Whitney non-parametric test and qualitative data with Fisher’s exact test. Analyses were computed using SPSS version 18 statistical package. P≤0.05 was considered significant.
Results
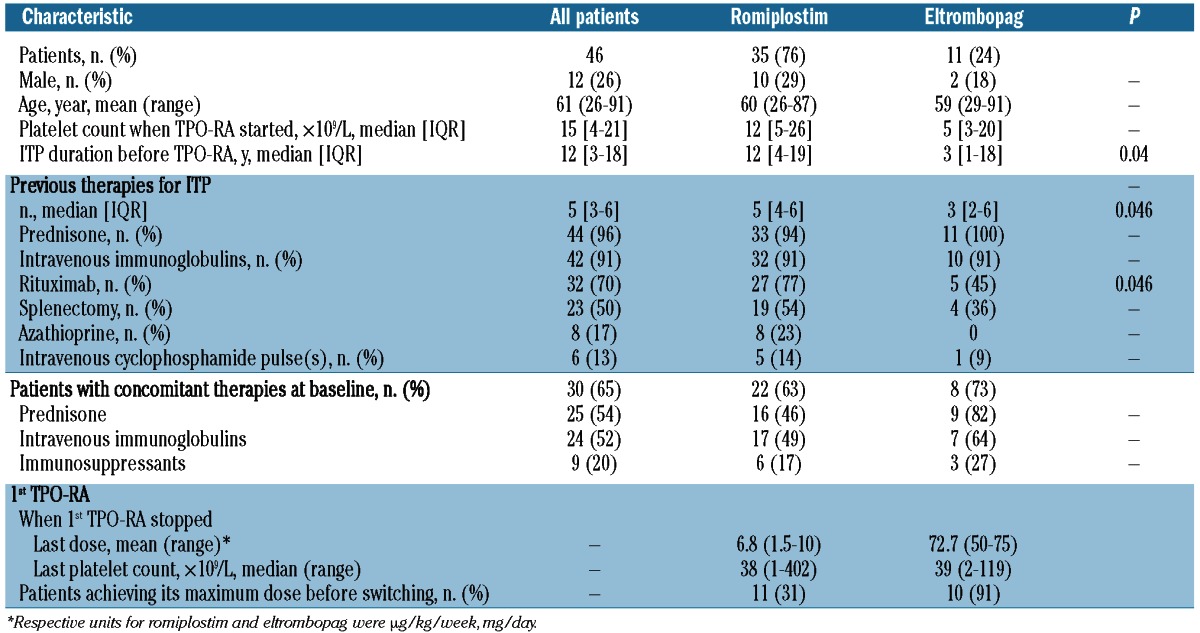
Forty-six patients fulfilling the inclusion criteria were enrolled in the study (Table 1). Thirty-five patients first received romiplostim (romiplostim group), whereas eltrombopag (eltrombopag group) was the first TPO-RA for 11 patients. All but 2 patients had previously received corticosteroids and IVIg, and 70% had received rituximab without responding. The splenectomy rate was 50% (23 of 46). Among the 23 non-splenectomized patients, 6 had refused to undergo surgery, whereas treating physicians considered splenectomy contraindicated for the remaining cases. Comparing romiplostim-group patients versus the eltrombopag group, respectively, the first had previously been given rituximab more frequently: 27 of 35 (77%) versus 5 of 11 (45%); (P=0.046). At the time of the first TPO-RA administration, 30 of 46 (65%) patients were receiving concomitant medication(s) for ITP, including mainly corticosteroids (n=25, 54%) with a mean dose of 50 (range 5–120) mg/day, repeated IVIg infusions (n=24, 52%) and/or immunosuppressant(s) (n=9, 20%). Thirty-one (67%) patients had bleeding symptoms during the month preceding the first TPO-RA administration. The median platelet count at the first administration of the first TPO-RA was 15×109/L (IQR 4–21×109/L). The base-line platelet count was 30×109/L or over for 6 patients, 4 of whom were receiving concomitant treatment(s) for ITP.
Table 1.
Characteristics of the patients receiving romiplostim or eltrombopag as the first thrombopoietic receptor-agonist (1st TPO-RA).
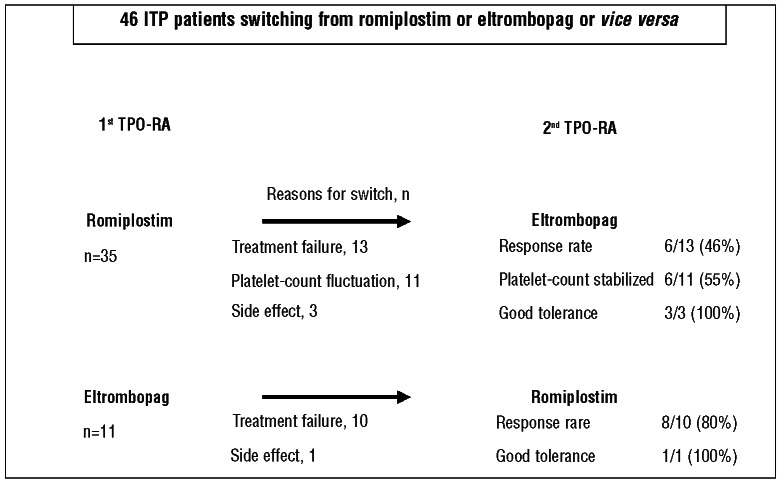
Reasons for switching from one TPO-RA to the other
The 4 reasons for switching from the first TPO-RA to the other were: lack of efficacy of the first agonist (n=23), platelet-count fluctuation (n=11), side effect (n=4) and patient’s preference (n=8).
Switched because of inefficacy
From romiplostim to eltrombopag
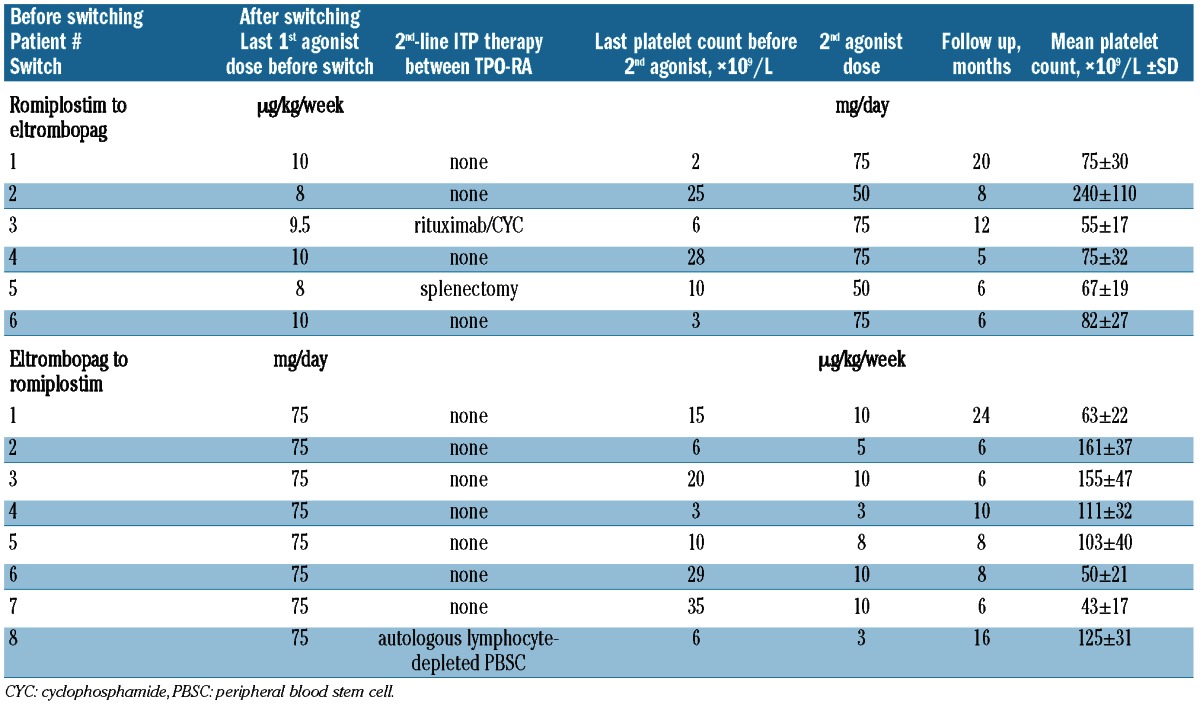
The characteristics of the 13 patients who failed to respond to romiplostim and switched to eltrombopag are detailed in Table 2. The median duration of romiplostim use before switching was 8 (IQR 4–16) months. The reasons for such a delay before switching to eltrombopag were: escalation dose period, patients without a definite platelet response but with a clinical benefit21 on romiplostim and/or the need to wait for eltrombopag to be licensed and available. Most patients, 9 of 13 (69%), received the highest dose (9–10 μg/kg/week) before switching to eltrombopag. Four of those 13 patients received one or several new treatment lines before starting eltrombopag (rituximab for 2, cyclophosphamide for 2, splenectomy for one). Six (46%) of the 13 responders to eltrombopag were receiving a mean±SD dose of 67±6 mg with a mean follow up of 7±4 months. Two of those 6 patients had not received the maximum romiplostim dose of 9–10 μg/kg/week (Table 3). All non-responders to eltrombopag had taken the maximum dose of 75 mg/day.
Table 2.
Characteristics of the patients first given romiplostim or eltrombopag, then switched because of primary failure or transient response.
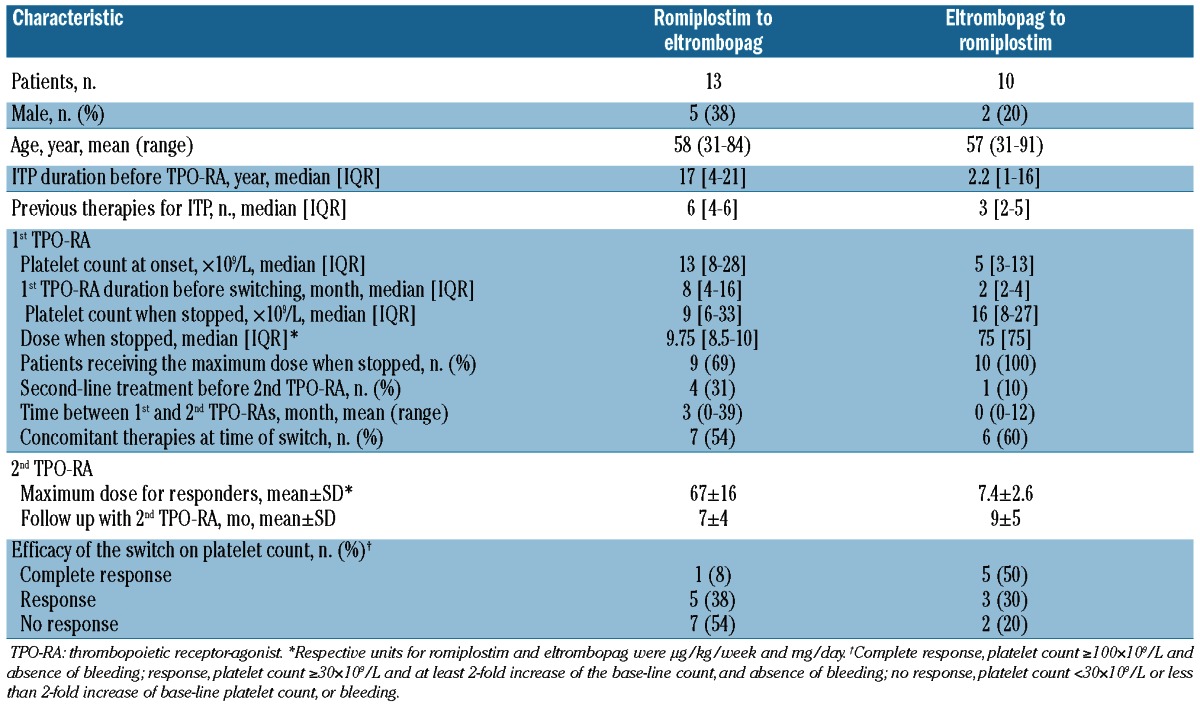
Table 3.
Characteristics of the responders first given romiplostim or eltrombopag, then switched because of primary failure or transient response.
From eltrombopag to romiplostim
Ten patients who failed to respond to the maximum eltrombopag dose were switched to romiplostim (Table 2). Only one of them received another treatment (autologous hematopoietic stem cell transplantation) before switching. Only 2 (20%) patients refractory to eltrombopag failed to respond to romiplostim given at its maximum dose (10 μg/kg/week). The mean±SD romiplostim dose for the 8 sustained responders was 7.4±2.6 μg/kg/week.
Switched because of platelet-count fluctuation
Eleven romiplostim-group patients were switched to eltrombopag because their treating physicians judged that their platelet counts were too unstable. They suddenly developed profound thrombocytopenia (<30×109/L), occasionally complicated by bleeding episodes requiring rescue therapy, e.g. corticosteroids and/or IVIg (Table 4). For 6 of 11 (55%) patients, this strategy was effective, achieving platelet-count stabilization within the narrow range of 50–200×109/L, thereby avoiding the use of emergency treatments with a mean±SD follow up of 9±3 months (Figure 1). For 5 (46%) of these patients, the switch to eltrombopag did not prevent platelet-count fluctuations, eventually leading TPO-RA discontinuation, except for 2 patients who were switched back to romiplostim as it was considered slightly more effective on the platelet count. In relation to this, no patient was switched from eltrombopag to romiplostim for this reason.
Table 4.
Characteristics of the patients switching from romiplostim to eltrombopag because of platelet-count fluctuation or personal preference.
Figure 1.
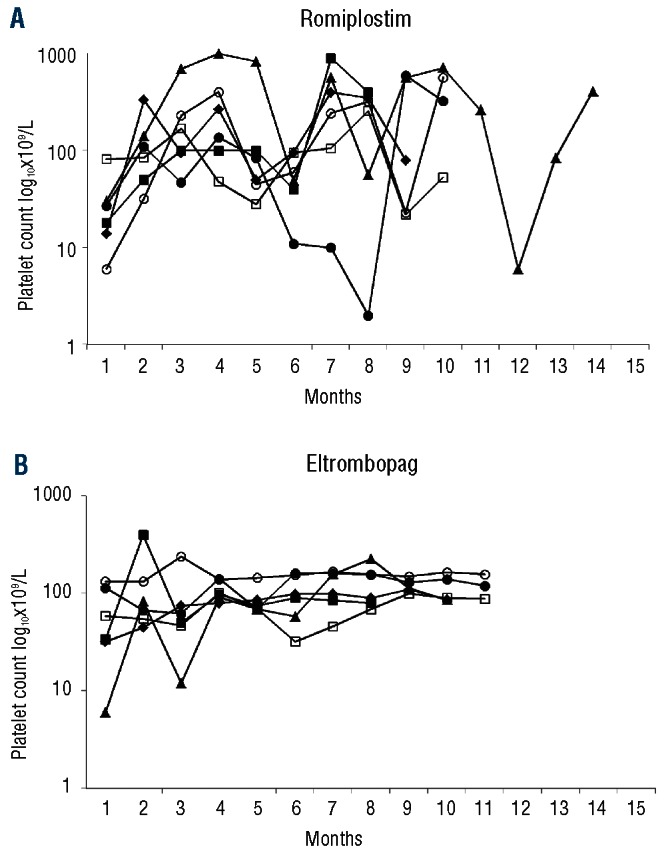
Platelet-count fluctuations of 6 patients under romiplostim (A) and their attenuation after switching to eltrombopag (B).
Switched because of side effects
Three romiplostim responders switched to eltrombopag because they experienced arthralgias, and one eltrombopag responder was switched to romiplostim because of persistent bone pain and moderately increased liver enzymes. The adverse events of all 4 patients’ resolved rapidly and all of them responded to the second TPO-RA. For the 46 patients, no blood-count abnormalities, i.e. pancytopenia or blood smear modifications that could suggest the onset of a bone-marrow reticulin fibrosis, were observed. This explains why no bone-marrow biopsy was taken.
Switched to comply with patient’s preference
Eight (17%) romiplostim responders asked to switch to eltrombopag mainly because they considered oral intake easier for them. No difference in terms of efficacy was observed for any 8 patients after switching.
For patients who switched for a reason other than the lack of efficacy, the concordance of response rates after switching from one TPO-RA to the other was 19 of 23 (83%).
Discussion
The authors of pivotal studies17,23,24 reported romiplostim and eltrombopag to be highly effective against chronic ITP, with average immediate responses exceeding 70% and obtaining 50% sustained responses. However, a French study conducted on unselected patients showed that, after two years of follow up, 35% of the patients had to stop romiplostim, mainly because of inefficacy.21 In agreement with this, our present results showed that the lack of response was the main reason that led physicians to consider switching the TPO-RA.
Romiplostim and eltrombopag stimulate the TPO-R but some differences related to molecular structure and pharmacokinetic characteristics could explain their different response patterns: romiplostim is a peptibody that is injected subcutaneously,25 while eltrombopag is a small molecule taken orally.26 To date, no pharmacokinetic studies are available comparing these 2 molecules. But these differences suggest that the efficacies of these 2 compounds might not necessarily be the same. Our results support this hypothesis by clearly showing that, in the absence of response with one TPO-RA, switching to the other can be effective: 80% of the patients who failed to respond to eltrombopag eventually responded to romiplostim, and 46% of patients whose disease did not respond to romiplostim responded to eltrombopag.
Due to the small number of patients in the 2 groups, it is not yet possible to draw definitive conclusions concerning TPO-RA efficacy or to ascertain if one of the 2 available agonists is superior to the other. The tendency towards a better response to romiplostim after switching compared to eltrombopag as the second TPO-RA could have several explanations. It could simply be due to a dose phenomenon, which could be overcome by giving a higher eltrombopag dose. The median romiplostim dose for ITP patients was 4 μg/kg/week in the pivotal studies.17,19 Hence, the patients receiving the maximum dose of 10 μg/kg/week had a 2.5-fold higher TPO-RA level. The median eltrombopag dose used for ITP patients in the pivotal study18 was 51 mg/day. Therefore, with the maximum dose of 75 mg allowed in this pilot study, there was only a 1.5-fold increase in the TPO-RA, suggesting that a higher eltrombopag dose might be useful for non-responders. According to this insufficient dose hypothesis, it was recently shown that some patients with refractory aplastic anemia responded to 150 mg of eltrombopag after failing to respond to 75 mg of that agonist.27 Another explanation could be that patients whose first TPO-RA was romiplostim had more frequently been previously treated with rituximab and tended to have received immunosuppressants more frequently, suggesting that their ITP could be more severe and refractory, leading logically to a poorer response to eltrombopag as the second TPO-RA. Indeed, we previously demonstrated the lower probability of patients with severe ITP responding to TPO-RA.21
Lack of TPO-RA efficacy can be associated with the development of bone marrow fibrosis that can be reversed by stopping TPO-RA.28 In this situation, discontinuation of one TPO-RA could allow a response to another. However, because none of the patients included in this study developed blood-count abnormalities, like pancytopenia or blood smear modifications suggesting reticulin bone-marrow deposition, no bone marrow biopsies were taken that could support this hypothesis.
A limitation of our study is that some patients considered resistant to romiplostim by their treating physicians had not received the maximum authorized dose (i.e. 10 μg/kg/week) before being switched to eltrombopag. Therefore, one cannot be certain that these patients were fully resistant to romiplostim.17,19,21 On the other hand, 4 patients who switched to eltrombopag received another treatment line after stopping romiplostim and a synergistic effect with subsequent eltrombopag use cannot be excluded.
A matter of concern about use of TPO-RA for both physicians and patients is the occurrence of significant and sometimes unexpected platelet-count fluctuations necessitating recourse to rescue therapy and/or to the reduction, transient withdrawal or increase of the TPO-RA dose, thereby running the risk of rebound thrombocytopenia and bleeding or thrombocytosis. In our study, only romiplostim-treated patients were switched for this reason, suggesting that this problem is mainly observed with this agent. Another explanation could be that experience with eltrombopag is limited, as it has only recently become available in France. Such fluctuations, observed with both TPO-RAs in pivotal and extension studies,17,19,24 remain poorly understood. These fluctuations could suggest several possibilities: subcutaneous romiplostim administration might be responsible for variable pharmacokinetics, depending on the body mass index or other factors; drug preparation and dilution errors made by the patient (in France 50% of patients self-inject romiplostim) or the nurse; and platelet-count fluctuations might reflect the heterogeneity of platelet turnover among ITP patients. However, in the study reported here, the platelet-count fluctuation was better controlled for “only” half of the patients switched to eltrombopag for this reason, suggesting the involvement of mechanisms other than the route of administration.
Figure 2.
Reasons for the thrombopoietic receptor-agonist (TPO-RA) switch and its outcomes for the 46 ITP patients.
Switches were rarely required for adverse events, confirming that the short-term tolerance of TPO-RAs is good, as found in pivotal studies17–19 and a study conducted on unselected patients.21 As expected, eltrombopag and romiplostim have different tolerance profiles that could be explained by their different molecular structures, administration routes and binding sites, and, thus, signaling pathways. In the case of intolerance of one of the TPO-RAs, the efficacy and tolerance of the other seemed to be excellent, strongly supporting switching in this setting.
Lastly, 8 romiplostim-treated patients requested the switch to eltrombopag because they preferred taking a pill once a day, rather than receiving a weekly subcutaneous injection. The possibility for patients to self-inject romiplostim and the recent modification of the injection kit should lower the percentage of romiplostim responders wanting to switch to eltrombopag.
In conclusion, in the absence of efficacy of a first TPO-RA, switching to the other agonist seemed highly relevant, as the likelihood of responding to the second agent was 50–80%. When the switch of a TPO-RA responder was motivated by another reason, the probability of response was very high. The fluctuation in platelet count observed in some TPO-RA treated patients remains a challenge for physicians. Switching from romiplostim to eltrombopag sometimes stabilized platelet count but the advantage of switching from eltrombopag to romiplostim for this specific reason requires further assessment.
Acknowledgments
The authors thank Janet Jacobson for editorial assistance. They would also like to thank all the other physicians who had included a patient in this study: Thierry Lamy de La Chapelle, Marc Bernard and Martine Escoffe-Barbe, Department of Hematology, CHU Rennes, Rennes; Eric Oksenhendler and Lionel Galicier, Department of Hematology, CHU Saint-Louis, APHP, Université de Paris-Diderot, Paris; Xavier Delbrel, Department of Rheumatology, CH de Pau, Pau; Marie-Françoise Le Coz, Department of Hematology, CH Bretagne-Sud, Lorient; Brigitte Pan-Petesch, Department of Hematology, Université de Brest, Brest; Anne Vekhoff and Elise Corre, Department of Hematology, CHU Saint-Antoine, APHP, Université Pierre-et-Marie-Curie, Paris; Antoinette Perlat, Department of Internal Medicine, CHU Rennes Sud, Rennes; Louis Terriou, Department of Hematology, CHU Claude-Huriez, Lille; Fabrice Jardin, Department of Hematology, CHU de Rouen, Rouen, France.
Footnotes
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Cines DB, Blanchette VS. Immune thrombocytopenic purpura. N Engl J Med. 2002;346 (13):995–1008 [DOI] [PubMed] [Google Scholar]
- 2.Cooper N, Bussel J. The pathogenesis of immune thrombocytopaenic purpura. Br J Haematol. 2006;133(4):364–74 [DOI] [PubMed] [Google Scholar]
- 3.George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, et al. Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood. 1996;88(1):3–40 [PubMed] [Google Scholar]
- 4.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207 [DOI] [PubMed] [Google Scholar]
- 5.Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood. 2010; 115(2):168–86 [DOI] [PubMed] [Google Scholar]
- 6.Stasi R, Provan D. Management of immune thrombocytopenic purpura in adults. Mayo Clin Proc. 2004;79(4):504–22 [DOI] [PubMed] [Google Scholar]
- 7.Ballem PJ, Segal GM, Stratton JR, Gernsheimer T, Adamson JW, Slichter SJ. Mechanisms of thrombocytopenia in chronic autoimmune thrombocytopenic purpura. Evidence of both impaired platelet production and increased platelet clearance. J Clin Invest. 1987;80(1):33–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isaka Y, Kambayashi J, Kimura K, Matsumoto M, Uehara A, Hashikawa K, et al. Platelet production, clearance and distribution in patients with idiopathic thrombocytopenic purpura. Thromb Res. 1990;60 (2):121–31 [DOI] [PubMed] [Google Scholar]
- 9.Louwes H, Zeinali Lathori OA, Vellenga E, de Wolf JT. Platelet kinetic studies in patients with idiopathic thrombocytopenic purpura. Am J Med. 1999;106(4):430–4 [DOI] [PubMed] [Google Scholar]
- 10.McMillan R, Wang L, Tomer A, Nichol J, Pistillo J. Suppression of in vitro megakaryocyte production by antiplatelet autoanti-bodies from adult patients with chronic ITP. Blood. 2004;103(4):1364–9 [DOI] [PubMed] [Google Scholar]
- 11.Nugent D, McMillan R, Nichol JL, Slichter SJ. Pathogenesis of chronic immune thrombocytopenia: increased platelet destruction and/or decreased platelet production. Br J Haematol. 2009;146(6):585–96 [DOI] [PubMed] [Google Scholar]
- 12.Cwirla SE, Balasubramanian P, Duffin DJ, Wagstrom CR, Gates CM, Singer SC, et al. Peptide agonist of the thrombopoietin receptor as potent as the natural cytokine. Science. 1997;276(5319):1696–9 [DOI] [PubMed] [Google Scholar]
- 13.Kuter DJ. New thrombopoietic growth factors. Blood. 2007;109(11):4607–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Erickson-Miller CL, Delorme E, Tian SS, Hopson CB, Landis AJ, Valoret EI, et al. Preclinical activity of eltrombopag (SB-497115), an oral, nonpeptide thrombopoietin receptor agonist. Stem Cells. 2009;27 (2):424–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, Kloczko J, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. N Engl J Med. 2007;357(22):2237–47 [DOI] [PubMed] [Google Scholar]
- 16.Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. N Engl J Med. 2006;355 (16): 1672–81 [DOI] [PubMed] [Google Scholar]
- 17.Bussel JB, Kuter DJ, Pullarkat V, Lyons RM, Guo M, Nichol JL. Safety and efficacy of long-term treatment with romiplostim in thrombocytopenic patients with chronic ITP. Blood. 2009;113(10):2161–71 [DOI] [PubMed] [Google Scholar]
- 18.Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. Lancet. 2011;377(9763):393–402 [DOI] [PubMed] [Google Scholar]
- 19.Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. Lancet. 2008;371(9610):395–403 [DOI] [PubMed] [Google Scholar]
- 20.Ghanima W, Godeau B, Cines DB, Bussel JB. How I treat immune thrombocytopenia (ITP): the choice between splenectomy or a medical therapy as a second-line treatment. Blood. 2012;120(5):960–9 [DOI] [PubMed] [Google Scholar]
- 21.Khellaf M, Michel M, Quittet P, Viallard JF, Alexis M, Roudot-Thoraval F, et al. Romiplostim safety and efficacy for immune thrombocytopenia in clinical practice: 2-year results of 72 adults in a romiplostim compassionate-use program. Blood. 2011;118(16): 4338–45 [DOI] [PubMed] [Google Scholar]
- 22.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: report from an international working group. Blood. 2009;113(11):2386–93 [DOI] [PubMed] [Google Scholar]
- 23.Cheng G. Eltrombopag for the treatment of immune thrombocytopenia. Expert Rev Hematol. 2011;4(3):261–9 [DOI] [PubMed] [Google Scholar]
- 24.Kuter DJ, Rummel M, Boccia R, Macik BG, Pabinger I, Selleslag D, et al. Romiplostim or standard of care in patients with immune thrombocytopenia. N Engl J Med 2011;363 (20):1889–99 [DOI] [PubMed] [Google Scholar]
- 25.Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. Clin Pharmacol Ther. 2004;76 (6):628–38 [DOI] [PubMed] [Google Scholar]
- 26.Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. Blood. 2007;109(11):4739–41 [DOI] [PubMed] [Google Scholar]
- 27.Olnes MJ, Scheinberg P, Calvo KR, Desmond R, Tang Y, Dumitriu B, et al. Eltrombopag and improved hematopoiesis in refractory aplastic anemia. N Engl J Med 2012;367(1):11–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuter DJ, Mufti GJ, Bain BJ, Hasserjian RP, Davis W, Rutstein M. Evaluation of bone marrow reticulin formation in chronic immune thrombocytopenia patients treated with romiplostim. Blood. 2009;114(18): 3748–56 [DOI] [PubMed] [Google Scholar]