Abstract
Multipotent stromal cells have immunomodulatory capacities and have been used in transplantation and autoimmune diseases. One of the effects of multipotent stromal cells involves the inhibition of dendritic cell differentiation. Since interleukin-6 and interleukin-10 are known to play a role in inhibiting immature dendritic cell differentiation, we hypothesized that these cytokines may also mediate the inhibitory effect of human multipotent stromal cells in immature dendritic cell differentiation. In order to test this hypothesis monocytes were cultured with interleukin-4 and granulocyte-monocyte colony-stimulating factor in the presence or absence of culture-expanded bone marrow-derived multipotent stromal cells. Neutralization and cytokine-depletion strategies were applied to reveal the cellular source and effect of interleukin-6 and interleukin-10. Addition of multipotent stromal cells to monocyte cultures significantly reduced the generation of immature dendritic cells (CD14−CD1a+) and resulted in the generation of CD14+CD1a− cells that displayed a significantly reduced immunostimulatory effect. We found that culture supernatants of co-cultures of multipotent stromal cells and monocytes contained higher concentrations of interleukin-6 and interleukin-10. Multipotent stromal cells produced interleukin-6 and neutralizing this interleukin-6 reversed the inhibitory effect of the multipotent cells. Interleukin-10 was not produced by multipotent stromal cells, but exclusively by monocytes after exposure to multipotent stromal cell-produced interleukin-6. In conclusion, through constitutive production of interleukin-6, multipotent stromal cells prevent the differentiation of monocytes towards antigen-presenting immunogenic cells and skew differentiation towards an anti-inflammatory interleukin-10-producing cell type.
Introduction
Multipotent stromal cells (MSC) are non-hematopoietic progenitor cells that have been identified in different tissues and are capable of differentiation towards adipogenic, osteogenic and chondrogenic lineages. Importantly, MSC have immunoregulatory properties. These properties have been the subject of many studies over the years and make MSC interesting candidates for further clinical application.
In several experimental disease models, MSC have been shown to modulate immune responses in vivo. Administration of MSC has been shown to prevent graft-versus-host disease1 and the development of experimental autoimmune encephalomyelitis in mice2 and results in prolonged skin graft survival in baboons.3 The use of MSC as a cellular therapy is currently being explored in clinical trials, including treatment of graft-versus-host disease,4 promotion of engraftment following hematopoietic stem cell transplantation5 and treatment of Crohn’s disease.6
The mechanisms underlying the immune suppression by MSC are still unclear. It has been widely shown that MSC suppress the proliferation of T cells upon allogeneic or mitogenic stimulation.7,8 Soluble factors proposed to be involved in this effect include indoleamine 2,3-dioxygenase, prostaglandin E2, transforming growth factor-β1, interleukin (IL)-6 and heme oxygenase 1.9–13 MSC also inhibit the proliferation of B cells and IL-2-stimulated natural killer cells14,15 and have been described to promote the formation of regulatory T cells (Treg).16–18
MSC can also modulate immune responses through inhibition of monocyte-derived immature dendritic cell (iDC) differentiation.19,20 Monocyte differentiation towards iDC can be inhibited by IL-6 and IL-1021,22 and several studies have suggested that IL-6 may play a role in the inhibitory effect of MSC on the differentiation of monocytes to dendritic cells.19,20 These data are, however, derived from in vitro co-culture experiments of MSC and monocytes in which the cellular source of cytokine production was not identified. The specific role of these cytokines in inhibiting MSC functionality is, therefore, still unclear.
In this study, we investigated the mechanism of inhibition by MSC during the differentiation of monocytes towards iDC and focused on the exact source of the cytokines involved. To this end we performed co-culture, transwell and supernatant-supplemented experiments of monocytes and MSC and applied neutralization and depletion strategies to reveal the cellular interactions that are crucial for the immunomodulating effect of MSC on monocyte differentiation.
Design and Methods
Generation of human multipotent stromal cells
Adult bone marrow was harvested from healthy donors or from orthopedic patients after obtaining their written informed consent, according to procedures that were approved by the medical ethical committee. Mononuclear cells were isolated and plated at a density of 1.3×105/cm2 in DMEM-low glucose (Invitrogen Corp., Paisley, UK) supplemented with 10% fetal calf serum (Greiner Bio-one) and penicillin/streptomycin (Invitrogen Corp.). When MSC reached confluency the cells were detached, characterized by FACS analysis and used in the co-culture experiments at passages 2–5. Further details are given in the Online Design and Methods.
Isolation and differentiation of monocytes
Human peripheral blood mononuclear cells from healthy donors were isolated from buffy coats obtained from Sanquin Blood Supply. CD14+ monocytes were purified with a MACS LS column using CD14 microbeads according to the manufacturer’s recommendations (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany).
Freshly isolated monocytes (CD14+) were cultured at a concentration of 1.0×106 cells/well in 6-well plates in RPMI containing penicillin/streptomycin, L-glutamine (Invitrogen Corp.) and 10% fetal calf serum supplemented with the growth factors IL-4 (10 ng/mL) and granulocyte-monocyte colony-stimulating factor (GM-CSF) (5 ng/mL) (both from Invitrogen Corp.) for 6 days, resulting in the generation of iDC (CD14−/CD1a+). To examine the effect of MSC on monocyte differentiation, irradiated MSC (60 Gy) were added enabling direct cell-cell contact or in a transwell system (pore size 0.4 μM; Corning Inc., Lowell, MA, USA) at a MSC:monocyte ratio of 1:10, as described previously.23,24 In some experiments IL-10 (20 ng/mL), IL-6 (100 ng/mL), anti-IL-10 (2–20 μg/mL) or anti-IL-6 (2.5 μg/mL) (all from R&D systems Europe Ltd., Abingdon, UK) was added. The involvement of secreted factors was further assessed by addition of conditioned medium from MSC alone (MSC-CM), MSC cultured with growth factors (MSC+GF CM) and from the monocyte-MSC co-cultures (MSC+mono CM) to the monocyte differentiation culture. In some experiments, IL-6 was depleted from the MSC-CM using the μMACS Streptavidin kit (Miltenyi Biotec) according to the manufacturer’s recommendations. MSC-CM was used in the monocyte differentiation in a 2:1 ratio with fresh medium. Further details are given in the Online Supplementary Design and Methods.
FACS analysis
The flow cytometry analysis is described in the Online Supplementary Design and Methods.
Analysis of cytokines
The concentrations of IL-6 and IL-10 were measured in cell-free supernatants collected on day 6 from cultures of monocytes with or without MSC. To distinguish between cytokines produced by MSC or monocytes, the cells from the transwell co-cultures were separated on day 6 and cultured further in fresh medium for an additional 2 days and IL-6 protein concentrations were determined. Further details are given in the Online Supplementary Design and Methods.
Gene expression
The mRNA expression of IL-6, IL-10 and β-actin was measured in MSC and monocyte populations on day 6 of the co-culture. All data were normalized using β-actin as a reference gene. Further details are given in the Online Supplementary Design and Methods.
Allogeneic mixed lymphocyte reaction assay
Monocytes cultured for 6 days in the presence or absence of MSC in a transwell co-culture were tested for their ability to stimulate the proliferation of allogeneic CD4+CD25− T cells that were isolated from peripheral blood mononuclear cells by MACS using the CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec) according to the manufacturer’s instructions. The CD4+CD25− T cells were stimulated with iDC or the monocyte-derived cells from the monocyte-MSC co-culture at various ratios. After 4 days of incubation, cells were pulsed overnight with 0.5 μCi of [3H]-thymidine to determine T-cell proliferation. Further details are given in the Online Supplementary Design and Methods.
Statistical analysis
All data represent the average and standard deviation from multiple MSC donors. Unless otherwise specified, statistical analyses were conducted using the Student’s t test for two groups and by a two-way ANOVA with a Bonferroni post-test for comparison of three groups. Prism5 software (GraphPad Software, Inc., CA, USA) was used.
Results
Multipotent stromal cells skew the differentiation of monocytes to the formation of CD14+ cells with low allostimulatory capacity
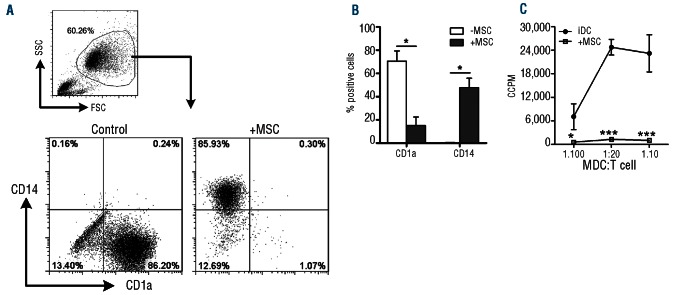
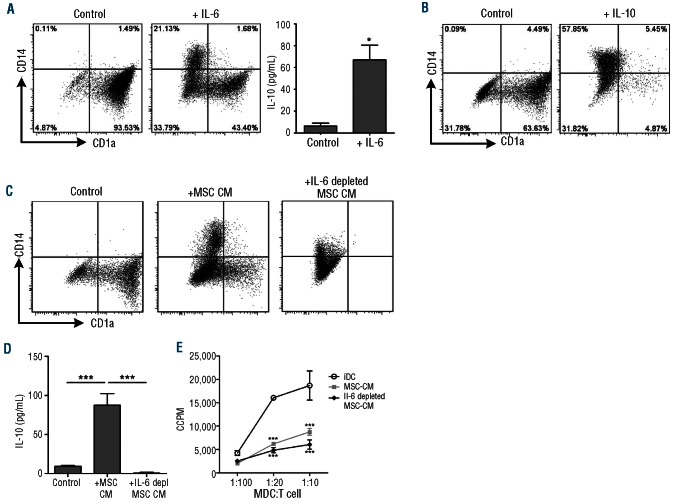
Monocytes (purity after MACS isolation was more than 90% CD14+ cells) cultured in the presence of IL-4 and GM-CSF differentiated into CD1a+CD14− iDC. Following addition of MSC to these cultures, monocyte differentiation towards iDC was inhibited (Figure 1A,B). In the presence of MSC, a significantly lower fraction of monocytes acquired CD1a (15.1±7.4% versus 70.6±8.8%, P=0.002, n=7) and a significantly lower fraction lost CD14 expression (52.2±8.1% versus 99.5±0.1%, P=0.001, n=7) (Figure 1B).
Figure 1.
MSC prevent the differentiation of iDC. (A) Representative FACS plots of the differentiation of CD14+ monocytes towards iDC with or without MSC in a direct co-culture. (B) Cumulative data of seven independent experiments with six different MSC donors and seven different monocyte donors (data are mean ± SEM): significance is relative to –MSC (*P<0.05). (C) Corrected counts per minute (CCPM) of iDC and day 6 MDC from transwell co-cultures that were incubated with CD4+CD25− T cells to assess their allostimulatory capacity. Data are from two different MSC donors and the experiment was performed in triplicate; significance is relative to iDC (*P<0.05, **P<0.01, ***P<0.001).
The MSC were analyzed for expression of surface markers before and after co-culture with monocytes. MSC expressed HLA-class I, CD73, CD105 and CD90 and did not show expression of HLA-class II, CD31, CD45, CD80 or CD34. No changes were observed after co-culture with monocytes.
The monocyte-derived populations, formed in the absence or presence of MSC in transwell co-cultures, were functionally tested for their ability to induce proliferation of allogeneic T cells (Figure 1C). iDC effectively induced proliferation of allogeneic CD4+CD25− T cells. The monocyte-derived population that was generated in the presence of MSC showed a significantly reduced allostimulatory capacity compared to the iDC.
The concentrations of both interleukin-6 and interleukin-10 are increased in co-cultures of monocytes and multipotent stromal cells
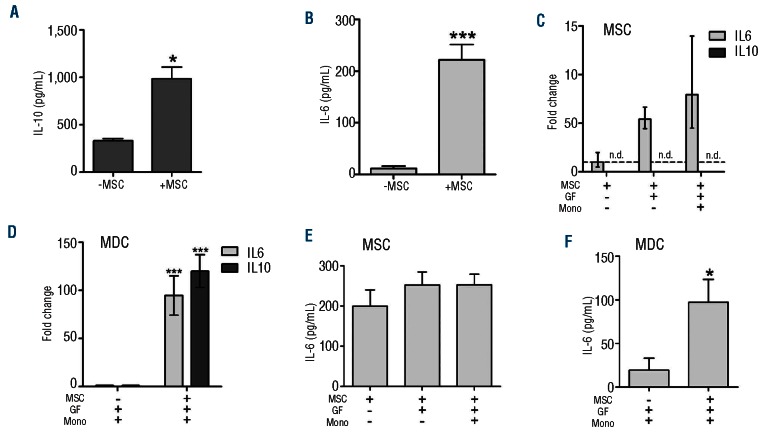
Monocytes were cultured in the presence of IL-4 and GM-CSF and in the presence or absence of MSC and cytokine concentrations were measured in cell-free culture supernatants of the direct co-cultures. Supernatants from monocytes that were cultured in the presence of MSC contained significantly higher concentrations of IL-6 and IL-10 than control supernatants derived from cultures without MSC (IL-6: 984.6±122.6 pg/mL versus 333.4±15.1 pg/mL, n=3, P<0.0001; IL-10: 221.6±30.0 pg/mL versus 11.9±4.4 pg/mL, n=3, P<0.05; Figure 2A,B).
Figure 2.
Cytokine expression in MSC and monocytes in the co-cultures. Cytokine protein levels in the supernatant of monocyte cultures at day 6 for IL-10 (A) and IL-6 (B) in the presence or absence of MSC (IL-10: mean±SEM of three independent experiments with three different monocyte donors and six different MSC donors; IL-6: mean of six independent experiments with six different monocyte donors and seven different MSC donors; *P<0.05, **P<0.01, ***P<0.001). (C) IL-6 and IL-10 mRNA expression in MSC after 6 days of culture with and without monocytes and growth factors in a transwell co-culture system. Fold change is relative to MSC alone. (D) IL-6 and IL-10 mRNA expression in the monocyte-derived cell population from the transwell co-culture at day 6 of differentiation in the absence or presence of MSC. Fold change is relative to MDC differentiated in the absence of MSC. (E) IL-6 secretion of MSC after 2 days of culture in fresh medium following 6 days of culture with or without growth factors and monocytes. (F) IL-6 secretion of monocyte-derived-cells (MDC) after 2 days of culture in fresh medium following differentiation in the absence or presence of MSC. (C-F: data are mean±SEM of three different MSC donors, *P<0.05, ** P<0.01, ***P<0.001).
Cytokine expression by multipotent stromal cells and monocytes
The MSC-induced changes in surface marker phenotype and cytokine concentrations in the monocyte cultures were also observed when the experiments were performed in a transwell system (data not shown). To assess which population was responsible for the observed increase in cytokine concentrations, the MSC and the monocyte-derived cell populations from such transwell cultures were assessed at day 6 for expression of IL-6 and IL-10 mRNA. Neither unstimulated MSC nor MSC cultured in the presence of IL-4 and GM-CSF and in the presence or absence of monocytes, were found to express IL-10 mRNA (Figure 2C). In agreement with this observation, IL-10 protein was not detected in culture supernatants from unstimulated MSC or from MSC cultured in the presence of IL-4 and GM-CSF (data not shown). Monocyte-derived cells, however, expressed detectable levels of IL-10 mRNA (fold change of 3.47×10−4±8.5×10−5 relative to β-actin) and these expression levels were considerably increased in the presence of MSC (P<0.05; Figure 2D). These data indicate that the IL-10 protein detected in the supernatants of monocyte-MSC co-cultures was produced by the monocyte-derived cell population.
Next, we analyzed expression levels of IL-6. Figure 2C shows that unstimulated MSC express IL-6 mRNA. A 5-fold increase in the average level of IL-6 mRNA expression was observed in MSC from cultures containing IL-4 and GM-CSF, but this increase did not reach statistical significance (P=0.06), which was probably due to the high variation that was observed between the different MSC samples. No further increase in IL-6 mRNA expression was observed in MSC that were co-cultured with monocytes. After the 6 days of co-culture, MSC and monocytes were separated and cultured for another 2 days to measure the cytokine concentrations secreted by both populations after co-culture. As for the IL-6 mRNA expression in MSC, the concentrations of IL-6 protein in culture supernatants of MSC cultured in the presence of IL-4 and GM-CSF and in the presence or absence of monocytes were not significantly increased compared to those in the culture supernatants of unstimulated MSC (Figure 2E).
IL-6 mRNA was also expressed in the monocyte-derived iDC. Significantly increased expression levels (P<0.001) were found in monocyte-derived populations that were generated in the presence of MSC (Figure 2D). Concordantly, the concentrations of IL-6 in the supernatant of monocytes cultured for an additional 2 days after the 6 days of MSC-monocyte co-culture were significantly increased compared to those of the control culture of monocytes with IL-4 and GM-CSF (P<0.05; Figure 2F).
Secreted factors from unstimulated multipotent stromal cells are responsible for the inhibition of monocyte differentiation
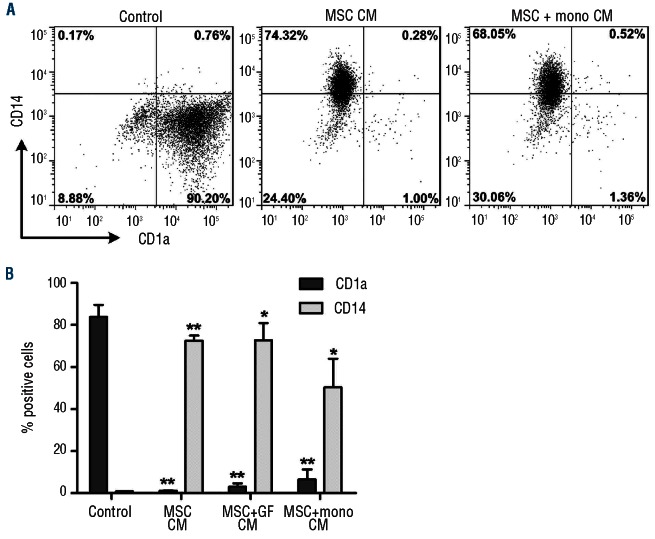
The observation that MSC also displayed their inhibitory effect on the differentiation of monocytes when the co-cultures were performed in a transwell system showed that cell-cell contact between monocytes and MSC was not required and that soluble factors were responsible for this effect. We investigated whether these soluble factors were secreted by culture-expanded MSC or only by MSC stimulated in the co-cultures. To do this, three types of conditioned medium were collected and tested for their ability to inhibit monocyte differentiation: (i) conditioned medium from unstimulated MSC (MSC-CM), (ii) conditioned medium from MSC cultured with the growth factors IL-4 and GM-CSF (MSC+GF CM) and (iii) conditioned medium from the monocyte-MSC direct co-cultures (MSC+mono CM). Figure 3 shows that the differentiation of CD1a−CD14+ monocytes into CD1a+CD14− iDC was completely inhibited in the presence of CM derived from stimulated, but also from unstimulated MSC. This shows that MSC constitutively produce and secrete the essential factor(s) that cause their inhibitory effect on monocyte differentiation to iDC.
Figure 3.
Secreted factors from unstimulated MSC are responsible for the inhibition of monocyte differentiation. Monocytes were differentiated in the presence of different conditioned media. (A) Representative dotplots of monocytes differentiated for 6 days in the presence of various conditioned media. (B) Cumulative data of CD1a and CD14 expression on day 6 monocytes differentiated in the presence of various conditioned media (data were collected from two different MSC donors and one monocyte donor). MSC-CM, MSC conditioned medium; MSC+GF CM, conditioned medium from MSC cultures containing the growth factors IL-4 and GM-CSF; MSC+mono CM, conditioned medium from the monocytes differentiated with growth factors in the presence of MSC in a direct co-culture. Statistical significance is compared to control (*P<0.05,**P<0.01).
Role of interleukin-6 and interleukin-10 in the multipotent stromal cell-induced inhibition of monocyte differentiation
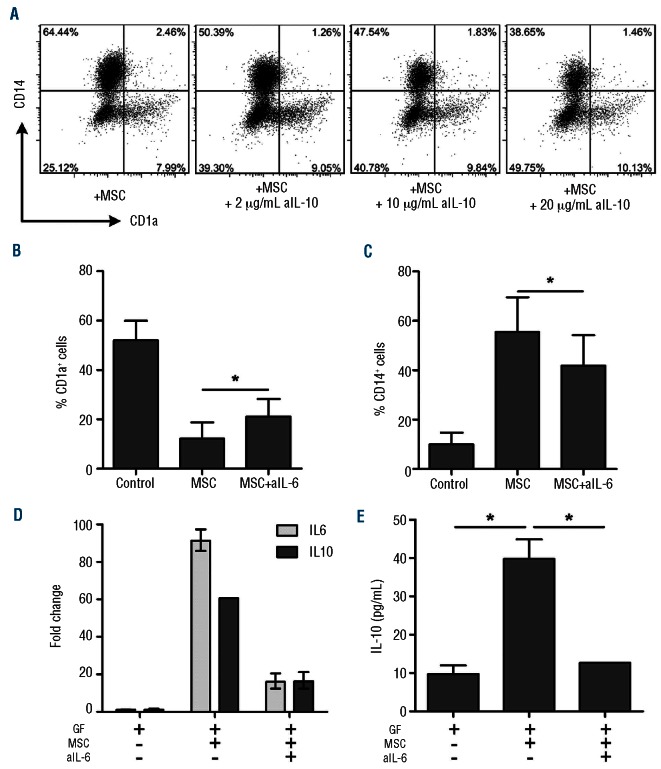
To study the exact role of IL-6 and IL-10 in the inhibition of monocyte differentiation by MSC, neutralizing antibodies against IL-6 and IL-10 were added to the transwell co-cultures. Addition of neutralizing antibodies against IL-10 did not result in restored iDC formation (Figure 4A). Addition of neutralizing antibodies against IL-6, however, caused a partial, but significant reduction of the inhibitory effect of MSC and resulted in increased CD1a expression and a decrease in the expression of CD14 on monocytes (Figure 4B,C). This neutralization of IL-6 substantially reduced the upregulation of IL-6 mRNA and IL-10 mRNA expression in the monocyte-derived population (Figure 4D) and reduced IL-10 protein concentrations (Figure 4E) in the monocyte cultures with MSC or MSC-CM.
Figure 4.
IL-6, but not IL-10, is directly involved in the inhibitory effect of MSC on monocyte differentiation. The role of IL-10 and IL-6 in the MSC-mediated inhibition of monocyte differentiation was investigated. (A) Adding a neutralizing antibody against IL-10 (aIL-10) did not restore iDC formation (data are representative dotplots from two independent experiments with two different MSC donors and two different monocyte donors). (B, C) The addition of a neutralizing antibody against IL-6 (aIL-6) (2.5 μg/mL) to the transwell co-culture significantly reduced the inhibitory effect of MSC (data are from three independent experiments with four different MSC donors and four different monocyte donors. Statistical analysis was performed using the Wilcoxon signed rank test *P<0.05). (D, E) The increase of IL-6 and IL-10 mRNA expression on day 6 monocytes (fold change is relative to iDC) (D) and protein secretion in the co-culture on day 6 (E) was reversed when an IL-6 neutralizing antibody was added to the culture (data are means±SD of two different MSC donors and one monocyte donor; * P<0.05).
We confirmed the observation that addition of either IL-6 or IL-10 resulted in inhibition of monocyte differentiation (Figure 5A-B). However, since MSC do not produce IL-10 (Figure 2), only IL-6 can be an MSC-derived factor that is relevant for the inhibitory effect of MSC. Indeed, we showed that addition of only IL-6 to the monocyte cultures induced IL-10 expression in monocytes, similar to the effect of MSC or MSC-CM (Figure 5A). To confirm that IL-6 is indeed the key factor produced by MSC, we depleted IL-6 from MSC-CM and studied the effect of this IL-6 depletion on monocyte differentiation. After depletion of IL-6, 84–96% of the IL-6 was depleted from the MSC-CM. Figure 5C shows that the inhibition of monocyte differentiation by MSC-CM was reversed by depletion of IL-6 from the CM. IL-6 depletion completely reversed the formation of a CD14+ monocyte-derived population, but did not reverse the MSC-CM-mediated inhibition of CD1a upregulation. Functional testing revealed that the MSC-CM-induced IL-10 secretion in the monocyte cultures was completely abolished following IL-6 depletion (Figure 5D). The allostimulatory capacity, however, was not restored by depleting IL-6 from the MSC-CM (Figure 5E). Overall, our results show that MSC-derived IL-6 is an essential factor for the inhibition of monocyte differentiation by MSC and for inducing an IL-10-producing, monocyte-derived CD14+CD1a− cell population. The neutralizing and depletion experiments show that IL-6 is an important factor, but not the only one, which is produced by MSC to exert an inhibitory effect on monocyte differentiation.
Figure 5.
IL-6 is the key factor in the MSC-induced inhibition of monocyte differentiation. IL-6 (A) and IL-10 (B) were added to the monocyte differentiation cultures. Both IL-6 and IL-10 inhibited the differentiation to iDC in a manner similar to the effect of co-culture with MSC/MSC-CM and addition of IL-6 directly increased the IL-10 secretion by monocyte-derived cells (A) (data are means ± SD from two different MSC; *P<0.05). (C) Depletion of IL-6 from the MSC conditioned medium partly reversed the inhibiting effect of MSC on the monocyte-derived iDC differentiation (representative dotplot of three different MSC donors and two different monocyte donors. (D) MSC-derived IL-6 drives the IL-10 upregulation in the monocyte-derived cell population (C, D: data are means±SD from two different MSC; ***P<0.001). (E) Corrected counts per minute (CCPM) of iDC and day 6 MDC from cultures in MSC-CM and IL-6-depleted MSC-CM that were incubated with CD4+CD25− T cells to assess their allostimulatory capacity. Data are from two different MSC donors and the experiment was performed in triplicate; significance is relative to iDC (*P<0.05, **P<0.01, ***P<0.001).
Discussion
It has been acknowledged that MSC inhibit the differentiation of monocytes towards iDC. The cytokines IL-6 and IL-10 have been described to play a role in the differentiation of monocytes towards iDC.21,22 However, the role of these cytokines in the MSC-induced inhibition of this process is still unclear. In this study, we used an MSC-monocyte co-culture system to identify the cytokines that are crucial in this process and reveal their exact cellular source of production. The addition of MSC to monocyte cultures containing IL-4 and GM-CSF inhibited the monocyte-derived iDC differentiation and significantly increased the concentrations of the cytokines IL-6 and IL-10. Transwell experiments showed that cell-cell contact was not required for the inhibitory effect of MSC on monocyte differentiation, indicating that soluble factors were responsible for this effect. Subsequent experiments with MSC-CM showed that unstimulated MSC produced the soluble factors responsible for inhibition of monocyte differentiation. Previous studies suggested that IL-6 plays a role in the inhibitory effect of MSC on the differentiation of monocytes to dendritic cells.19,20 Whether this is a direct or indirect effect remained unclear since both monocytes and MSC are capable of IL-6 production and secretion. We show that IL-6 production by MSC is constitutive and not significantly enhanced upon contact with monocytes. Since the effect of MSC could be replaced by MSC-CM, we considered MSC-derived IL-6 to be responsible for the MSC-induced inhibition of monocyte differentiation. Indeed, addition of neutralizing antibodies to IL-6 significantly reversed the inhibitory effect of MSC and depletion of IL-6 reversed the inhibitory effect of MSC-CM. These data clearly indicate that MSC-derived IL-6 regulates in vitro monocyte differentiation, thereby skewing monocytes towards an IL-10-producing cell type with reduced allostimulatory capacity. Additional factors might however play a role in the complete inhibition of monocyte differentiation towards iDC by MSC, since the allostimulatory capacity of the monocyte-derived cells was not restored by depleting IL-6 from the MSC-CM.
We found that, in spite of a number of reports making this claim,10,25–32 MSC do not produce IL-10, but that IL-10 is exclusively produced by monocytes in this system. Several reports claiming that the immunomodulatory properties of MSC are mediated by IL-10 have used antibodies to neutralize IL-10 in co-cultures of MSC and other cell types, including monocytes. These studies were not, therefore, designed to identify the cells that produce IL-10. Many reports indicate that MSC need to be activated in order to exert their immunomodulatory activities or to produce IL-10.26,33,34 Our data clearly show that activation of MSC is not required for the IL-10 induction in monocytes.
Activated monocytes have been shown to increase their IL-10 expression upon IL-10 exposure.35 Such increased IL-10 production could further enhance the immunomodulatory effect of MSC. Increased IL-10 secretion is a characteristic of type II activated macrophages, which have an immunomodulatory, anti-inflammatory function.36,37 Type-II-activated macrophages have also been shown to be able to induce regulatory T cells,38 another potential additional indirect immunomodulatory effect of MSC.
Taken together, we propose the hypothesis that MSC, by inducing IL-10 production in monocyte-derived cells, play a powerful regulatory role in multiple anti-inflammatory mechanisms (Figure 6), which could explain their clinical benefits in immunotherapy such as the treatment of graft-versus-host disease. Thereby MSC may act as potent modulators of the (innate) immune response inducing an anti-inflammatory environment.
Figure 6.
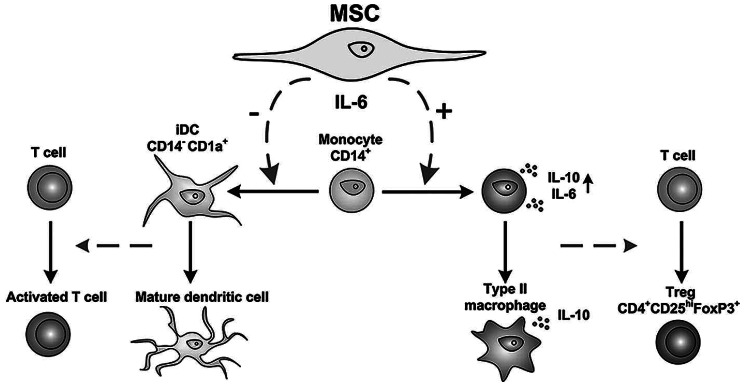
Hypothetical model of immunomodulating mechanisms exerted by MSC through production of IL-6. By secreting IL-6, MSC skew monocytes from differentiation towards an antigen-presenting dendritic cell towards an anti-inflammatory cell that produces IL-10.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This work received financial support from the Netherlands Organization for Scientific Research (NWO) ZonMW Translational Adult Stem Cell (TAS) program nr 11.600.1016.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Yanez R, Lamana ML, Garcia-Castro J, Colmenero I, Ramirez M, Bueren JA. Adipose tissue-derived mesenchymal stem cells have in vivo immunosuppressive properties applicable for the control of the graft-versus-host disease. Stem Cells. 2006;24(11): 2582–91 [DOI] [PubMed] [Google Scholar]
- 2.Zappia E, Casazza S, Pedemonte E, Benvenuto F, Bonanni I, Gerdoni E, et al. Mesenchymal stem cells ameliorate experimental autoimmune encephalomyelitis inducing T-cell anergy. Blood. 2005;106(5): 1755–61 [DOI] [PubMed] [Google Scholar]
- 3.Bartholomew A, Sturgeon C, Siatskas M, Ferrer K, McIntosh K, Patil S, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30(1):42–8 [DOI] [PubMed] [Google Scholar]
- 4.Le Blanc K, Frassoni F, Ball L, Locatelli F, Roelofs H, Lewis I, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: a phase II study. Lancet. 2008;371(9624):1579–86 [DOI] [PubMed] [Google Scholar]
- 5.Ball LM, Bernardo ME, Roelofs H, Lankester A, Cometa A, Egeler RM, et al. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood. 2007;110(7):2764–7 [DOI] [PubMed] [Google Scholar]
- 6.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut. 2011;60(6): 788–98 [DOI] [PubMed] [Google Scholar]
- 7.Marigo I, Dazzi F. The immunomodulatory properties of mesenchymal stem cells. SeminImmunopathol. 2011;33(6):593–602 [DOI] [PubMed] [Google Scholar]
- 8.Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, Matteucci P, et al. Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood. 2002;99(10):3838–43 [DOI] [PubMed] [Google Scholar]
- 9.Chabannes D, Hill M, Merieau E, Rossignol J, Brion R, Soulillou JP, et al. A role for heme oxygenase-1 in the immunosuppressive effect of adult rat and human mesenchymal stem cells. Blood. 2007;110(10):3691–4 [DOI] [PubMed] [Google Scholar]
- 10.Singer NG, Caplan AI. Mesenchymal stem cells: mechanisms of inflammation. Annu Rev Pathol. 2011;6(1):457–78 [DOI] [PubMed] [Google Scholar]
- 11.Meisel R, Zibert A, Laryea M, Gobel U, Daubener W, Dilloo D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood. 2004;103(12):4619–21 [DOI] [PubMed] [Google Scholar]
- 12.Najar M, Raicevic G, Boufker HI, Fayyad KH, De BC, Meuleman N, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: combined comparison of adipose tissue, Wharton’s jelly and bone marrow sources. Cell Immunol. 2010;264 (2):171–9 [DOI] [PubMed] [Google Scholar]
- 13.Yanez R, Oviedo A, Aldea M, Bueren JA, Lamana ML. Prostaglandin E2 plays a key role in the immunosuppressive properties of adipose and bone marrow tissue-derived mesenchymal stromal cells. Exp Cell Res. 2010;316(19):3109–23 [DOI] [PubMed] [Google Scholar]
- 14.Corcione A, Benvenuto F, Ferretti E, Giunti D, Cappiello V, Cazzanti F, et al. Human mesenchymal stem cells modulate B-cell functions. Blood. 2006;107(1):367–72 [DOI] [PubMed] [Google Scholar]
- 15.Sotiropoulou PA, Perez SA, Gritzapis AD, Baxevanis CN, Papamichail M. Interactions between human mesenchymal stem cells and natural killer cells. stem cells. 2006;24 (1):74–85 [DOI] [PubMed] [Google Scholar]
- 16.Maccario R, Podesta M, Moretta A, Cometa A, Comoli P, Montagna D, et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica. 2005;90(4):516–25 [PubMed] [Google Scholar]
- 17.Aggarwal S, Pittenger MF. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood. 2005;105(4): 1815–22 [DOI] [PubMed] [Google Scholar]
- 18.Crop M, Baan CC, Korevaar SS, Ijzermans JN, Weimar W, Hoogduijn MJ. Human adipose tissue-derived mesenchymal stem cells induce explosive T-cell proliferation. Stem Cells Dev. 2010;19(12):1843–53 [DOI] [PubMed] [Google Scholar]
- 19.Nauta AJ, Kruisselbrink AB, Lurvink E, Willemze R, Fibbe WE. Mesenchymal stem cells inhibit generation and function of both CD34+-derived and monocyte-derived dendritic cells. J Immunol. 2006; 177(4):2080–7 [DOI] [PubMed] [Google Scholar]
- 20.Djouad F, Charbonnier LM, Bouffi C, Louis-Plence P, Bony C, Apparailly F, et al. Mesenchymal stem cells inhibit the differentiation of dendritic cells through an inter-leukin-6-dependent mechanism. Stem Cells. 2007;25(8):2025–32 [DOI] [PubMed] [Google Scholar]
- 21.Allavena P, Piemonti L, Longoni D, Bernasconi S, Stoppacciaro A, Ruco L, et al. IL-10 prevents the differentiation of monocytes to dendritic cells but promotes their maturation to macrophages. Eur J Immunol. 1998;28(1):359–69 [DOI] [PubMed] [Google Scholar]
- 22.Chomarat P, Banchereau J, Davoust J, Palucka AK. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–4 [DOI] [PubMed] [Google Scholar]
- 23.Jiang XX, Zhang Y, Liu B, Zhang SX, Wu Y, Yu XD, et al. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood. 2005;105(10):4120–6 [DOI] [PubMed] [Google Scholar]
- 24.Spaggiari GM, Abdelrazik H, Becchetti F, Moretta L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: central role of MSC-derived prostaglandin E2. Blood. 2009;113(26): 6576–83 [DOI] [PubMed] [Google Scholar]
- 25.Haniffa MA, Wang XN, Holtick U, Rae M, Isaacs JD, Dickinson AM, et al. Adult human fibroblasts are potent immunoregulatory cells and functionally equivalent to mesenchymal stem cells. J Immunol. 2007;179(3):1595–604 [DOI] [PubMed] [Google Scholar]
- 26.Selmani Z, Naji A, Zidi I, Favier B, Gaiffe E, Obert L, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26(1):212–22 [DOI] [PubMed] [Google Scholar]
- 27.Zhukareva V, Obrocka M, Houle JD, Fischer I, Neuhuber B. Secretion profile of human bone marrow stromal cells: donor variability and response to inflammatory stimuli. Cytokine. 2010;50(3):317–21 [DOI] [PubMed] [Google Scholar]
- 28.Xu G, Zhang L, Ren G, Yuan Z, Zhang Y, Zhao RC, et al. Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 2007;17(3):240–8 [DOI] [PubMed] [Google Scholar]
- 29.Beyth S, Borovsky Z, Mevorach D, Liebergall M, Gazit Z, Aslan H, et al. Human mesenchymal stem cells alter antigen-presenting cell maturation and induce T-cell unresponsiveness. Blood. 2005;105(5):2214–9 [DOI] [PubMed] [Google Scholar]
- 30.Tolar J, Le BK, Keating A, Blazar BR. Hitting the right spot with mesenchymal stromal cells (MSCs). Stem Cells. 2010;28(8):1446–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bassi EJ, de Almeida DC, Moraes-Vieira PM, Camara NO. Exploring the role of soluble factors associated with immune regulatory properties of mesenchymal stem cells. Stem Cell Rev. 2012;8(2):329–42 [DOI] [PubMed] [Google Scholar]
- 32.Abumaree M, Al JM, Pace RA, Kalionis B. Immunosuppressive properties of mesenchymal stem cells. Stem Cell Rev. 2012;8 (2):375–92 [DOI] [PubMed] [Google Scholar]
- 33.Waterman RS, Tomchuck SL, Henkle SL, Betancourt AM. A New Mesenchymal stem cell (MSC) paradigm: polarization into a pro-Inflammatory MSC1 or an immunosuppressive MSC2 phenotype. PLoS ONE. 2010;5(4):e10088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groh ME, Maitra B, Szekely E, Koc ON. Human mesenchymal stem cells require monocyte-mediated activation to suppress alloreactive T cells. Exp Hematol. 2005;33 (8):928–34 [DOI] [PubMed] [Google Scholar]
- 35.de Waal Malefyt R, Abrams J, Bennett B, Figdor CG, de Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. J Exp Med. 1991;174(5):1209–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8(12):958–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosser DM. The many faces of macrophage activation. J Leukoc Biol. 2003; 73(2):209–12 [DOI] [PubMed] [Google Scholar]
- 38.Savage ND, TdB, Walburg KV, Joosten SA, van MK, Geluk A, et al. Human anti-inflammatory macrophages induce Foxp3+ GITR+ CD25+ regulatory T cells, which suppress via membrane-bound TGFbeta-1. J Immunol. 2008;181(3):2220–6 [DOI] [PubMed] [Google Scholar]