Abstract
Thalidomide and bortezomib are extensively used to treat elderly myeloma patients. In these patients, treatment-related side effects are frequent and full drug doses difficult to tolerate. We retrospectively analyzed data from 1435 elderly patients enrolled in 4 European phase III trials including thalidomide and/or bortezomib. After a median follow up of 33 months (95%CI: 10–56 months), 513 of 1435 patients (36%) died; median overall survival was 50 months (95%CI: 46–60 months). The risk of death was increased in patients aged 75 years or over (HR 1.44, 95%CI: 1.20–1.72; P<0.001), in patients with renal failure (HR 2.02, 95%CI: 1.51–2.70; P<0.001), in those who experienced grade 3–4 infections, cardiac or gastrointestinal adverse events during treatment (HR 2.53, 95%CI: 1.75–3.64; P<0.001) and in those who required drug discontinuation due to adverse events (HR 1.67, 95%CI; 1.12–2.51; P=0.01). This increased risk was restricted to the first six months after occurrence of adverse events or drug discontinuation and declined over time. More intensive approaches, such as the combination of bortezomib-thalidomide, negatively affected outcome. Bortezomib-based combinations may overcome the negative impact of renal failure. Age 75 years or over or renal failure at presentation, occurrence of infections, cardiac or gastrointestinal adverse events negatively affected survival. A detailed geriatric assessment, organ evaluation and less intense individualized approaches are suggested in elderly unfit subjects.
Introduction
Multiple myeloma (MM) is the second most common hematologic cancer, with a higher incidence in elderly subjects: 26% are aged 65–74 years, and 37% are older than 75 years.1 In the future, the prevalence of myeloma is likely to increase due to the extended survival and the growing life expectancy of the general population. Until the late 1990s, the combination melphalan-prednisone (MP) had been the reference treatment for patients aged 65 years or over, and was associated with a median survival of 29–37 months.2 The introduction of novel agents, such as the proteasome inhibitor bortezomib and the immunomodulatory drugs (IMIDs) thalidomide and lenalidomide, has changed the treatment paradigm of patients aged 65 years or over. In 6 randomized trials, MP plus thalidomide (MPT) showed a progression-free survival (PFS) advantage in comparison with MP,3–9 while overall survival (OS) benefit has not been consistently reported. However, a recent meta-analysis has shown a significant improvement in PFS and OS.10 The incidence of any grade 3–4 non-hematologic adverse events (AEs) was approximately 50% and thalidomide discontinuation rate due to AEs varied from 33% to 45%, with higher incidence in patients aged 75 years or over. MP plus bortezomib (VMP) showed both a PFS and OS advantage in comparison with MP.11,12 In patients aged 75 years or over, the efficacy of VMP was less evident: the 3-year OS was shorter in comparison with patients aged under 75 years (55% vs. 74%). The incidence of any grade 3–4 AEs was 91% and bortezomib discontinuation rate due to AEs was 34%. Both VMP and MPT are now considered the new standards of care for newly diagnosed MM patients aged 65 years or over. Recently, the combination of bortezomib and thalidomide with or without alkylating agents has shown to be highly effective.13,14 The weekly administration of bortezomib reduced the incidence of AEs, especially peripheral neuropathy and gastrointestinal toxicity, without affecting efficacy.13,14 Nevertheless, outcome was mainly improved for patients aged under 75 years, while limited advantage was reported in patients aged 75 years or over.14 These data lead to the question as to whether less toxicity may translate into more efficacy, especially in unfit elderly patients.
In this meta-analysis of individual patient data from 4 randomized trials, we assessed the influence of age, organ damage, development of treatment-related AEs and drug discontinuation as predictive factors of outcome in elderly newly diagnosed MM patients receiving MP, MPT, VMP, or bortezomib and thalidomide combination (bortezomib, thalidomide and prednisone [VTP] or bortezomib, melphalan, prednisone and thalidomide [VMPT]).
Design and Methods
Study design and treatments
Patients with newly diagnosed MM, not eligible for high-dose therapy and autologous transplantation due to age (≥65 years) or co-existing co-morbidities, enrolled in the GISMM-2001 MP versus MPT, the HOVON 49 MP versus MPT, the GEM05MAS VMP versus VTP and the GIMEMA MM0305 VMP versus VMPT-VT phase III trials were included in this meta-analysis. Details on treatment regimens and results of these studies have been reported previously.5–7,13,14 All trials were approved by the institutional review board at each of the participating centers. All patients gave written informed consent before entering the study, which was performed in accordance with the Declaration of Helsinki. Trials were registered at ClinicalTrials.gov or controlled-trials.com (numbers NCT00232934, ISRCTN 90692740, NCT00443235 and NCT01063179).
Assessment
Individual patient data were collected for each patient enrolled in the 4 trials at each co-ordinating center. All data were sent to a central co-ordinating center, reviewed for consistency and completeness, and entered into a new database. The following data available across all studies were collected for each patient: baseline data [age, gender, creatinine value, International Staging System, (ISS)]; date of death or date the patient was last known to be alive; grade, type and date of AEs occurring during induction. AEs were graded according to National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0. OS was calculated from the time of symptomatic MM diagnosis until the date of death or the date the patient was last known to be alive.
Statistical analysis
Patients were analyzed on an intention-to-treat basis for all time-to-event end points. Times of observation were censored on October 31st 2010. For this non pre-planned analysis, outcome and AEs definitions were based on data common to all trials. Descriptive statistics were used to characterize the incidence of AEs and treatment discontinuation rate. In case of multiple grade 3–4 hematologic or grade 3–4 non-hematologic AEs, we considered only the worst and the first occurred. Cumulative incidence of AEs and treatment discontinuation were calculated considering death from any cause as competing event. The proportional hazard frailty model for the subdistribution was applied to evaluate the effect of baseline factors (age, gender, ISS, serum creatinine, treatment) on the cumulative incidence function of AEs and treatment discontinuation accounting for heterogeneity across studies.15 OS was estimated according to the Kaplan-Meier method and analyzed by univariate and multivariate Cox proportional hazards models. The following variables were assessed for potential association with OS: age at diagnosis (≥75 vs. <75 years), gender, ISS stages, baseline creatinine (≥2 vs. <2 mg/dL), treatment regimen (MP/MPT/VMP/VTP plus VMPT). As ISS stage and baseline creatinine data was not available for some patients, the category data missing were created, and all patients were included in the analysis. Grade 3–4 hematologic or grade 3–4 non-hematologic AEs during induction or drug discontinuation were included in the Cox regression models as time varying covariates. The inspection of Schoenfeld residuals suggested a violation of proportional hazard assumption for the hematologic AE, non-hematologic AE, and drug discontinuation variables. Thus, the Cox models were fitted dividing the post-event (hematologic AE, non-hematologic AE, drug discontinuation) period into two periods: the first six months after an event or after the start of follow up (for patients who did not experience an event), and after the six months to the final follow up. In order to account for clustering of patients within trials, Cox shared frailty models were estimated. Statistical analysis was performed by using STATA 11.0 (StataCorp LP, College Station, TX, USA).
Results
Patients
A total of 1435 patients were analyzed: 332 did not receive novel agents (MP), 332 received MP plus thalidomide (MPT), 387 MP plus bortezomib (VMP), 384 MP plus thalidomide and bortezomib (VTP/VMPT). Baseline demographics and disease characteristics are listed in Table 1. Patients aged 75 years or over accounted for 36% of the entire study population, and were equally distributed in the 4 groups. Distribution of patients according to ISS staging was well balanced in all groups, except for a lower frequency of ISS III in the MP group. The proportion of patients with renal failure was higher in the MP and MPT groups due to less stringent inclusion/exclusion criteria of these protocols.
Table 1.
Baseline patients’ characteristics.
Outcome, adverse events and drug discontinuation
After a median follow up of 33 months (IQR 26–41 months), the median OS was 50 months (IQR 24-. months) and 64% of patients remained alive at three years. At the date of last follow up, 513 out of the 1435 patients (36%) died. The reasons for death were: 389 (76%) disease progression, and 124 (24%) toxic effects. The most frequent causes of toxicity-related death were infections (8%), cardiac complications (8%), second primary malignancies (2%) and venous thromboembolism (VTE) (2%). Early toxic mortality rate, occurring within the first three months from the start of treatment, was 2% (34 patients). The incidence of any grade 3–4 hematologic AEs was 563 of 1435 (39%) in the entire study population, with a lower incidence in patients receiving MPT (33%) compared with those receiving VMP (44%; P=0.002) or VTP/VMPT (43%; P=0.009). The most frequent hematologic AEs were neutropenia (27%), thrombocytopenia (10%) and anemia (3%).
The incidence of any grade 3–4 non-hematologic AEs was 412 of 1435 (29%) in the entire study population with a higher incidence in patients receiving MPT (43%) compared with those receiving VMP (24%) or VTP/VMPT (32%). The most frequent non-hematologic AEs were infections (10%), peripheral neuropathy (8%), cardiac (6%) and gastro-intestinal complications (5%). Rate of VTE was 3%. The cumulative incidences of all non-hematologic AEs and of relevant AEs (infections, cardiac and gastrointestinal AEs) increased over time reaching a plateau after six months of therapy in all treatment groups (Figure 1A and B). At six months, the cumulative incidence of all non-hematologic AEs was 6.4% (95%CI: 3.7–9.0) in the MP group, 23.8% (95%CI: 19.2–28.4) in the MPT group, 19.5% (95%CI: 15.5–23.5) in the VMP group and 25.2% (95%CI: 20.8–29.6) in the VTP/VMPT group. Similarly, the cumulative incidence of infections, cardiac and gastrointestinal AEs was 4.8% (95%CI: 2.5–7.2) in the MP group, 16.2% (95%CI: 12.2–20.2) in the MPT group, 12.1% (95%CI: 8.8–15.4) in the VMP group and 14.5% (95%CI: 11.0–18.1) in the VTP/VMPT group.
Figure 1.
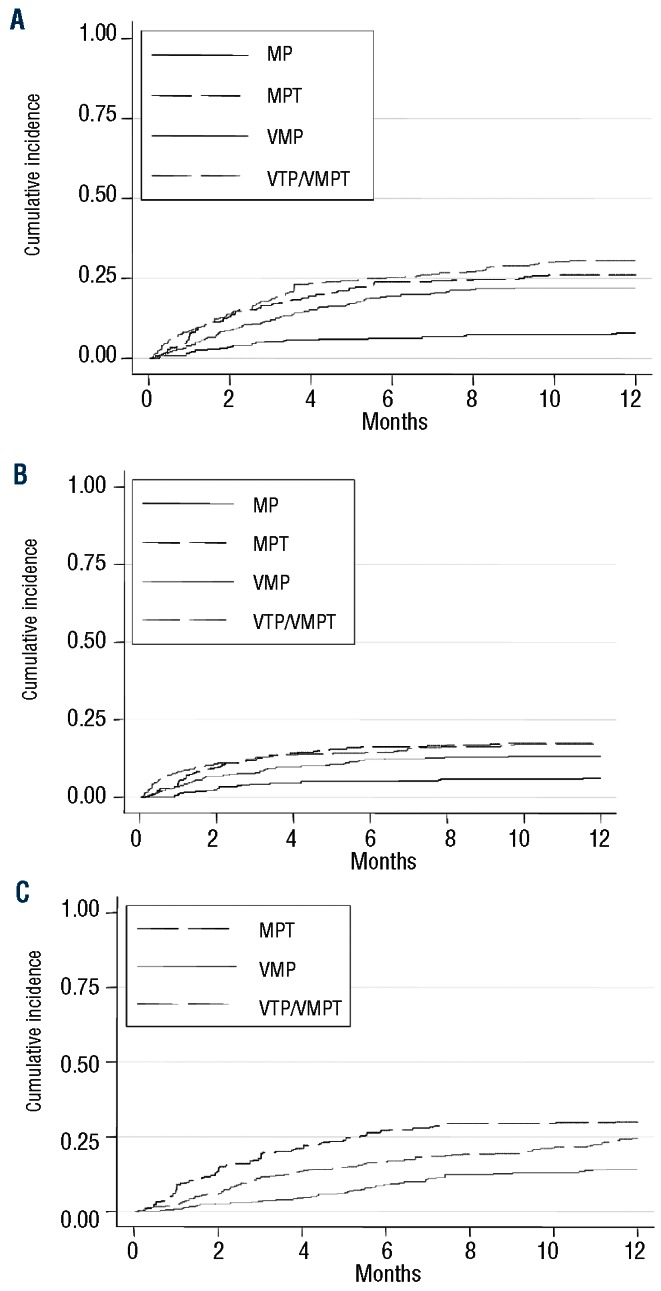
Cumulative incidence of grade 3–4 AEs and of drug discontinuation accounting for competing events (death and progressive disease). (A) Cumulative incidence of grade 3–4 non-hematologic toxicities. (B) Cumulative incidence of grade 3–4 infections, cardiac or gastrointestinal toxicities. (C) Cumulative incidence of drug discontinuation.
Among patients not receiving novel agents (MP), drug discontinuation for toxicity was reported in 4 of 332 patients (1%), mainly due to hematologic toxicity. Among patients receiving novel agents, drug discontinuation for toxicity was reported in 294 of 1103 patients (27%), with a higher incidence in patients receiving MPT (35%) compared with those receiving VMP (16%) or VTP/VMPT (29%). The main reasons for thalidomide discontinuation were peripheral neuropathy (9%), infections (4%), cardiac (4%) and hematologic toxicities (4%). The main reasons for bortezomib discontinuation were peripheral neuropathy (6%), hematologic AEs (4%), infections (2%) and cardiac AEs (3%). The cumulative incidences of drug discontinuation increased over time reaching a plateau after eight months of therapy in all treatment groups (Figure 1C). At six months, the cumulative incidence of drug discontinuation was 27.1% (95%CI: 22.3–32.0) in the MPT group, 9.0% (95%CI: 6.1–11.9) in the VMP group and 16.6% (95%CI: 12.8–20.3) in the VTP/VMPT group.
In a multivariable model, including several baseline characteristics, a higher risk to develop a grade 3–4 hematologic AE was detected for females (HR 1.32, 95%CI: 1.16–1.47; P<0.001), for patients with aggressive disease defined by ISS 3 (HR 1.49, 95%CI: 1.11–2.00; P=0.007) and for those with renal impairment (HR 1.51, 95%CI: 1.23–1.86; P<0.001). The risk of 3–4 non-hematologic AEs was increased by MPT treatment (HR 3.22, 95%CI: 1.20–8.64; P=0.02), by VTP/VMPT treatment (HR 3.03, 95%CI: 1.26–7.24; P=0.01) and by ISS 3 (HR 1.56, 95%CI: 1.09–2.23; P=0.01).
Impact of baseline patient conditions on outcome
The estimated 3-year OS was 68% in patients under 75 years and 57% in those aged 75 years or over (HR 1.44, 95%CI: 1.20–1.72; P<0.001). In patients aged 75 years or over the risk of death was higher in patients receiving VMP (HR 1.62, 95%CI: 1.04–2.52; P=0.03) and in those receiving VTP/VMPT (HR 3.02, 95%CI: 1.86–4.90; P<0.001) (Figure 2A).
Figure 2.
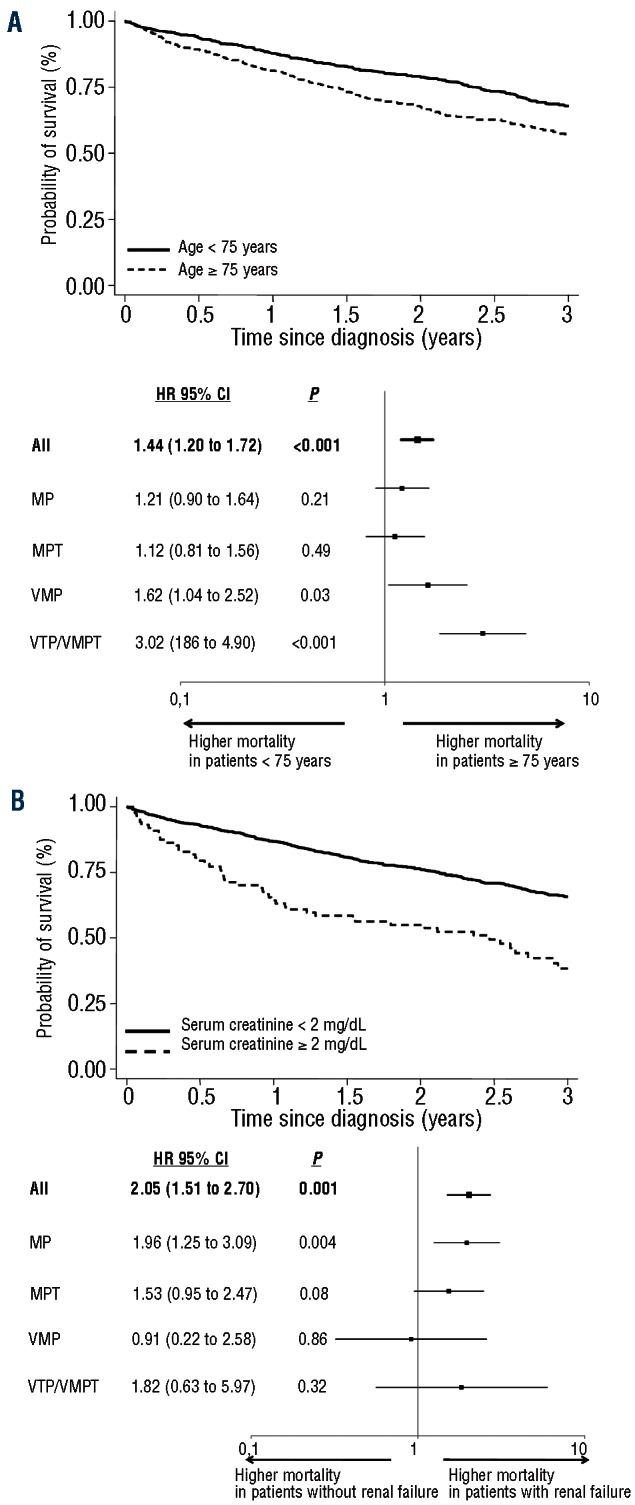
Overall survival according to baseline patient conditions. (A) Kaplan-Meier overall survival curves in patients younger or equal/older than 75 years in whole population and within the different treatment groups in the forest plot (results are shown, on a log10 scale, for analysis adjusted for gender, International Staging System and serum creatinine). Interaction test (heterogeneity) P=0.0041. (B) Overall survival in patients with or without renal failure (serum creatinine ≥ or < 2 mg/dL) in whole population and within the different treatment groups in the forest plot (results are shown, on a log10 scale, for analysis adjusted for gender, age and International Staging System. Interaction test (heterogeneity) P=0.65. Hazard ratios higher than 1 indicate a higher risk of death. The I bars represent 95% confidence intervals. MP: melphalan and prednisone; MPT: melphalan, prednisone and thalidomide; VMP: melphalan, prednisone and bortezomib; VTP/VMPT: bortezomib, thalidomide, prednisone/bortezomib, melphalan, prednisone, thalidomide.
The 3-year OS was 66% in patients without renal failure and 38% in those with renal failure (HR 2.02, 95%CI: 1.51–2.70; P<0.001). Renal failure did not increase the risk of death in patients receiving VMP (HR 0.91, 95%CI: 0.22–2.58; P=0.86) (Figure 2B).
Impact of treatment-related AEs and drug discontinuation on outcome
The development of grade 3–4 hematologic AEs did not increase the risk of death, both within the first six months from the occurrence of AE (HR 1.24, 95%CI: 0.88–1.73; P=0.21) and after this time point (HR 0.99, 95%CI: 0.80–1.23; P=0.94).
The development of grade 3–4 non-hematologic AEs increased the risk of death within the first six months from the occurrence of AEs (HR 1.82, 95%CI: 1.29–2.57; P=0.001) (Figure 3A), whereas after this time point the risk of death was similar to that of patients who did not experience non-hematologic AEs (HR 0.86, 95%CI: 0.67–1.10; P=0.22). The occurrence of grade 3–4 cardiac AEs (HR 3.19, 95%CI: 1.79–5.71; P<0.001), infections (HR 2.94, 95%CI: 1.93–4.46; P<0.001) or gastrointestinal AEs (HR 2.30, 95%CI: 1.14–4.67; P=0.02) were the most significant predictors of shorter OS. Pooled together, they increased the risk of death within the first six months (HR 2.53, 95%CI: 1.75–3.64; P<0.001) (Figure 3B), whereas after this time point the risk of death was similar to that of patients who did not experience this type of AEs (HR 0.86, 95%CI: 0.67–1.10; P=0.22). The increased risk of death associated with the development of cardiac AEs, infections and gastrointestinal AEs was similar in patients receiving MPT, VMP or VTP/VMPT. By contrast, the occurrence of VTE was not associated with shorter OS (HR 1.02, 95%CI: 0.32–3.19; P=0.97) and peripheral neuropathy was associated with improved OS (HR 0.27, 95%CI: 0.07–1.10; P=0.07).
Figure 3.
Impact of treatment-related complications on overall survival. (A) Impact of grade 3 to 4 non-hematologic toxicity in whole population and within the different treatment groups within the first 6 months after the occurrence of event in the forest plot (results are shown, on a log10 scale, for analysis adjusted for age, gender, International Staging System and serum creatinine). Interaction test (heterogeneity) P=0.79. (B) Impact of grade 3 to 4 infections, cardiac, or gastrointestinal adverse events in whole population and within the different treatment groups within the first 6 months after the occurrence of event in the forest plot (results are shown, on a log10 scale, for analysis adjusted for age, gender, International Staging System and serum creatinine). Interaction test (heterogeneity) P=0.52. (C) Impact of drug discontinuation due to grade 3–4 adverse events in whole population and within the different treatment groups within the first 6 months after the occurrence of event in the forest plot (results are shown, on a log10 scale, for analysis adjusted for age, gender, International Staging System and serum creatinine). Interaction test (heterogeneity) P=0.14. Hazard ratios higher than 1 indicate a higher risk of death. The I bars represent 95% confidence intervals. MP: melphalan and prednisone; MPT: melphalan, prednisone and thalidomide; VMP: melphalan, prednisone and bortezomib; VTP/VMPT: bortezomib, thalidomide, prednisone/bortezomib, melphalan, prednisone, thalidomide.
Drug discontinuation due to AEs was correlated with increased risk of death within the first six months (HR 1.67, 95%CI: 1.12–2.51; P=0.01) (Figure 3C), whereas after this time point the risk of death was similar to that of patients who did not discontinue treatment (HR 0.93, 95%CI: 0.70–1.23; P=0.61). In patients who discontinued treatment, the risk of death was higher among patients receiving VMP (HR 2.29, 95%CI: 0.88–5.95; P=0.09) or VTP/VMPT (HR 3.44, 95%CI: 1.64–7.23; P=0.001) (Figure 3C).
Multivariate analysis
A Cox proportional hazards regression model is shown in Figure 4. Advanced age (≥75 years) (HR 1.36, 95%CI: 1.14–1.63; P=0.001), renal failure (creatinine level ≥ 2 mg/dL) (HR 1.59, 95%CI: 1.18–2.16; P=0.003), and the occurrence of grade 3–4 non-hematologic AEs (HR 1.72, 95%CI: 1.19–2.47; P=0.004) (especially cardiac complications: HR 2.61, 95%CI: 1.49–4.60, P=0.001; and infections: HR 2.46, 95%CI: 1.58–3.82, P<0.001) as well as drug discontinuation due to toxicity (HR 1.61, 95%CI: 1.03–2.50; P=0.03) were the variables associated with a shorter OS, regardless of staging and treatment administered.
Figure 4.
Hazard ratios for deaths for any cause, according to presence (vs. absence) of risk factors, treated as time-dependent variables. Results are shown on a log10 scale, for analysis adjusted for treatment and International Staging System (multivariate analysis). Hazard ratios higher than 1 indicate a higher risk of death. The I bars represent 95% confidence intervals. AEs: Adverse Events. *Impact on overall survival within the first 6 months after the occurrence of the event.
Discussion
In this study, individual patient data from 1435 patients with newly diagnosed MM who received MP, either alone or in combination with thalidomide and/or bortezomib, were analyzed. In multivariate analysis, the advanced age (≥75 years), renal failure, occurrence of severe cardiac AEs, infections, gastrointestinal AEs and drug discontinuation were predictive of shorter survival. Several studies have analyzed the prognostic role of age and renal failure,16,25 but no study has evaluated the impact of AEs and drug discontinuation on long-term outcome. The strength of this analysis lies in the size and the similarity of the patient population, derived from 4 of the largest European randomized controlled trials. Furthermore, our study focused on the two reference treatment regimens for elderly MM patients: MPT and VMP.
In 10,549 patients with newly diagnosed MM, older patients had more adverse prognostic factors and shorter survival than the younger ones.26 In a recent meta-analysis of individual patient data from 1682 patients enrolled in 6 randomized trials comparing MP with MPT, as well as in the VISTA study comparing MP with VMP, and in the MM015 study comparing MP with MP plus lenalidomide with or without lenalidomide maintenance, survival was worse in patients aged 75 years or over.10,12,27 Life expectancy changes as one gets older: it is about 15 years in patients under 75 years of age and ten years in those who are older.28 Nevertheless, in our study, older patients had a higher risk of death when they were treated with a more aggressive treatment. Age 75 years or over increased the risk of death by 1.62-fold in patients receiving VMP, 3.02-fold in those receiving VTP/VMPT, but only marginally in patients receiving MP or MPT. Combination including both bortezomib and thalidomide should be used with caution in elderly patients. Some practical issues, such as costs and intravenous injection of bortezomib, together with our results further support the use of oral combinations including immunomodulatory drugs (IMIDs) in patients with more advanced age. In the future, the availability of new oral proteasome inhibitor or subcutaneous administration could eventually modify these indications.29,30
Aging is associated with a reduction in cardiac, renal, and gastric functions, hepatic mass, blood flow, and bone marrow status.31–35 All of these changes affect the pharmacokinetics and pharmacodynamics of drugs, increase toxicity, and decrease the clinical efficacy and the tolerability of treatments.
Renal failure is a common feature of MM and has been associated with inferior survival.35–37 Thalidomide and bortezomib pharmacokinetics are not affected by renal impairment and no dose reduction is required. In the MPT meta-analysis, the benefit of MPT was not evident in patients with renal failure.13 By contrast, in the VISTA trial, the outcome of patients treated with VMP was not affected by renal impairment.23 Our data showed that creatinine 2 mg/dL or over increased the risk of death by 1.53-fold in patients receiving MPT and by 1.82-fold in those receiving VMPT/VTP, while it did not increase the risk of death in patients receiving VMP. Our results should be considered with caution, due to the small number of patients with creatinine 2 mg/dL or over; on the other hand they support the recent consensus statement on behalf of the International Myeloma Working Group that recommends VMP as first-line treatment in elderly patients with renal failure.38
Before the introduction of novel agents, early toxic death had reached 10% in newly diagnosed MM patients receiving conventional chemotherapy.37 Early mortality is often due to combined effect of active disease and co-morbidities. Our analysis showed that the use of novel agents decreased the early toxic death risk to 2%. This may be due to a quick reduction in tumor load. Nevertheless, the occurrence of grade 3–4 non-hematologic AEs is associated with a worse prognosis. The impact was different among the different types of toxicities. Infections, cardiac and gastrointestinal complications had the worst impact on survival, regardless of the type of therapy. Sub-clinical organ dysfunction may emerge as clinically relevant after the organ stress induced by MM treatment. These data further suggest the need for a detailed organ evaluation and geriatric assessment before starting any treatment in elderly subjects. In our study, VTE was not associated with shorter survival. Similar results were reported in a retrospective study on 668 newly diagnosed patients receiving chemotherapy with or without thalidomide39 and in a retrospective study on 353 relapsed or refractory patients receiving lenalidomide and high-dose dexamethasone.40 In our analysis, peripheral neuropathy was associated with improved survival. A sub-analysis of the VISTA trial showed a higher response rate and longer time-to-progression in patients who experienced peripheral neuropathy compared with those who did not.41–43 Responding patients are likely to continue therapy for a longer period of time than non-responding patients. They receive higher cumulative doses which may explain the higher frequency of peripheral neuropathy. Recently, an interaction between myeloma-related factors and the patient’s genetic background in the development of treatment-induced peripheral neuropathy has been shown.44
Drug discontinuation due to AEs was associated with a shorter survival, probably because of lower cumulative delivered-dose. The cumulative delivered-dose may be one of the factors affecting outcome. The outcome of patients aged 75 years or over receiving melphalan, prednisone and lenalidomide was worse than younger patients, due to the higher discontinuation rate and subsequent lower cumulative dose-intensity of both melphalan and lenalidomide.27 The once-weekly schedule of bortezomib significantly reduced the incidence of grade 3–4 hematologic and non-hematologic AEs and the rate of drug discontinuation without having a negative impact on outcome.13,45 Indeed, the cumulative delivered-dose of bortezomib was similar for both twice- and once-weekly schedules of bortezomib. This finding may underline the need to deliver the appropriate dose intensity in all patient sub-groups in order to avoid drug discontinuation and loss of efficacy. On the other hand, the inability to tolerate less drug may mirror a weaker resistance of the host to toxic injuries, which per se may be the main reason for shorter survival. In the future, a more precise definition of organ dysfunction (with appropriate co-morbidity scores) and frailty (with specific geriatric assessment) should become mandatory to immediately define a gentler, less dose-intense approach, instead of intervening later, after the occurrence of too many unacceptable toxicities. Patients with at least one risk factor such as age 75 years or over, cardiac, pulmonary, renal or hepatic organ dysfunction, frailty, or occurrence of grade 3–4 non-hematologic AEs during treatment, should be considered for a reduced dose-intensity treatment strategy.46
In conclusion, we showed that advanced age, renal failure, occurrence of infections, cardiac, or gastrointestinal grade 3–4 AEs have a significant negative impact on survival outcomes of elderly patients. Future trials focused on optimizing MM treatment regimens for both fit and unfit elderly adults are urgently needed to define tailored, ‘personalized’ therapy for this large patient population.
Acknowledgments
The authors thank the data managers Giulia Lupparelli, Federica Leotta and the editorial assistant Giorgio Schirripa
Footnotes
The online version of this paper has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial and other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Altekruse SF, Kosary CL, Krapcho M, Neyman N, Aminou R, Waldron W, et al. (eds). SEER Cancer Statistics Review, 1975–2007, National Cancer Institute; Bethesda, MD: Available from: http://seer.cancer.gov/csr/1975_2007/, based on November 2009 SEER data submission, posted to the SEER website 2010 [Google Scholar]
- 2.Palumbo A, Anderson K. Multiple Myeloma. N Engl J Med. 2011;364(11):1046–60 [DOI] [PubMed] [Google Scholar]
- 3.Facon T, Mary JY, Hulin C, Benboubker L, Attal M, Pegourie B, et al. Melphalan and prednisone plus thalidomide versus melphalan and prednisone alone or reduced-intensity autologous stem cell transplantation in elderly patients with multiple myeloma (IFM 99–06): a randomised trial. Lancet. 2007;370(9594):1209–18 [DOI] [PubMed] [Google Scholar]
- 4.Hulin C, Facon T, Rodon P, Pegourie B, Benboubker L, Doyen C, et al. Efficacy of melphalan and prednisone plus thalidomide in patients older than 75 years with newly diagnosed multiple myeloma: IFM 01/01 trial. J Clin Oncol. 2009;27(22):3664–70 [DOI] [PubMed] [Google Scholar]
- 5.Palumbo A, Bringhen S, Caravita T, Merla E, Capparella V, Callea V, et al. Oral melphalan and prednisone chemotherapy plus thalidomide compared with melphalan and prednisone alone in elderly patients with multiple myeloma: randomised controlled trial. Lancet. 2006;367(9513):825–31 [DOI] [PubMed] [Google Scholar]
- 6.Palumbo A, Bringhen S, Liberati AM, Caravita T, Falcone A, Callea V, et al. Oral melphalan, prednisone, and thalidomide in elderly patients with multiple myeloma: updated results of a randomized controlled trial. Blood. 2008:112(8):3107–14 [DOI] [PubMed] [Google Scholar]
- 7.Wijermans P, Schaafsma M, Termorshuizen F, Ammerlaan R, Wittebol S, Sinnige H, et al. Phase III study of the value of thalidomide added to melphalan plus prednisone in elderly patients with newly diagnosed multiple myeloma: the HOVON 49 Study. J Clin Oncol. 2010;28(19):3160–6 [DOI] [PubMed] [Google Scholar]
- 8.Waage A, Gimsing P, Fayers P, Ammerlaan R, Wittebol S, Sinnige H, et al. Melphalan and prednisone plus thalidomide or placebo in elderly patients with multiple myeloma. Blood. 2010;116(9):1405–12 [DOI] [PubMed] [Google Scholar]
- 9.Beksac M, Haznedar R, Firatli-Tuglular T, Ozdogu H, Aydogdu I, Konuk N, et al. Addition of thalidomide to oral melphalan/prednisone in patients with multiple myeloma not eligible for transplantation: results of a randomized trial from the Turkish Myeloma Study Group. Eur J Haematol. 2010;86(1):16–22 [DOI] [PubMed] [Google Scholar]
- 10.Fayers PM, Palumbo A, Hulin C, Ozdogu H, Aydogdu I, Konuk N, et al. Thalidomide for previously untreated elderly patients with multiple myeloma: meta-analysis of 1685 individual patient data from 6 randomized clinical trials. Blood. 2011;118(5):1239–47 [DOI] [PubMed] [Google Scholar]
- 11.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–17 [DOI] [PubMed] [Google Scholar]
- 12.Mateos MV, Richardson PG, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, et al. Bortezomib plus melphalan and prednisone compared with melphalan and prednisone in previously untreated multiple myeloma: updated follow-up and impact of subsequent therapy in the phase III VISTA trial. J Clin Oncol. 2010;28(13):2259–66 [DOI] [PubMed] [Google Scholar]
- 13.Mateos MV, Oriol A, Martínez-López J, Gutiérrez N, Teruel AI, de Paz R, et al. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934–41 [DOI] [PubMed] [Google Scholar]
- 14.Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, et al. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–9 [DOI] [PubMed] [Google Scholar]
- 15.Katsahian S, Resche-Rigon M, Chevret S, Porcher R. Analysing multicentre competing risk data with mixed proportional hazards model for the subdistribution. Stat Med. 2006;25(24):4267–78 [DOI] [PubMed] [Google Scholar]
- 16.Siegel DS, Desikan KR, Mehta J, Singhal S, Fassas A, Munshi N, et al. Age is not a prognostic variable with autotransplants for multiple myeloma. Blood. 1999;93(1):51–4 [PubMed] [Google Scholar]
- 17.Lenhoff S, Hjorth M, Westin J, Brinch L, Bäckström B, Carlson K, et al. Impact of age on survival after intensive therapy for multiple myeloma: a population-based study by the Nordic Myeloma Study Group. Br J Haematol. 2006;133(4):389–96 [DOI] [PubMed] [Google Scholar]
- 18.Clark AD, Shetty A, Soutar R. Renal failure and multiple myeloma: pathogenesis and treatment of renal failure and management of underlying myeloma. Blood Rev. 1999;13 (2):79–90 [DOI] [PubMed] [Google Scholar]
- 19.Dimopoulos MA, Roussou M, Gavriatopoulou M, Zagouri F, Migkou M, Matsouka C, et al. Reversibility of renal impairment in patients with multiple myeloma treated with bortezomib-based regimens: identification of predictive factors. Clin Lymphoma Myeloma. 2009;9(4):302–6 [DOI] [PubMed] [Google Scholar]
- 20.Chanan-Khan AA, Kaufman JL, Mehta J, Richardson PG, Miller KC, Lonial S, et al. Activity and safety of bortezomib in multiple myeloma patients with advanced renal failure: a multicenter retrospective study. Blood. 2007;109(6):2604–6 [DOI] [PubMed] [Google Scholar]
- 21.Ludwig H, Drach J, Graf H, Lang A, Meran JG. Reversal of acute renal failure by bortezomib-based chemotherapy in patients with multiple myeloma. Haematologica. 2007;92 (10):1411–4 [DOI] [PubMed] [Google Scholar]
- 22.Morabito F, Gentile M, Ciolli S, Petrucci MT, Galimberti S, Mele G, et al. Safety and efficacy of bortezomib-based regimens for multiple myeloma patients with renal impairment: a retrospective study of Italian Myeloma Network GIMEMA. Eur J Haematol. 2010;84(3):223–8 [DOI] [PubMed] [Google Scholar]
- 23.Dimopoulos MA, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kastritis E, et al. VMP (Bortezomib, Melphalan, and Prednisone) is active and well tolerated in newly diagnosed patients with multiple myeloma with moderately impaired renal function, and results in reversal of renal impairment: cohort analysis of the phase III VISTA study. J Clin Oncol. 2009;27(36): 6086–93 [DOI] [PubMed] [Google Scholar]
- 24.Tosi P, Zamagni E, Cellini C, Cangini D, Tacchetti P, Tura S, et al. Thalidomide alone or in combination with dexamethasone in patients with advanced, relapsed or refractory multiple myeloma and renal failure. Eur J Haematol. 2004;73(2):98–103 [DOI] [PubMed] [Google Scholar]
- 25.Fakhouri F, Guerraoui H, Presne C, Peltier J, Delarue R, Muret P, et al. Thalidomide in patients with multiple myeloma and renal failure. Br J Haematol. 2004;125(1):96–7 [DOI] [PubMed] [Google Scholar]
- 26.Ludwig H, Durie BG, Bolejack V, Turesson I, Kyle RA, Blade J, et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: an analysis of 10 549 patients from the International Myeloma Working Group. Blood. 2008;111(8):4039–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Palumbo A, Hajek R, Delforge M, Kropff M, Petrucci MT, Catalano J, et al. Continuous lenalidomide treatment for newly diagnosed multiple myeloma. N Engl J Med. 2012;366 (19):1759–69 [DOI] [PubMed] [Google Scholar]
- 28.Ssa.gov. The Official Website of the U.S. Social Security Administration. [last reviewed or modified, October 10, 2012]. Available from: http://www.ssa.gov/OACT/STATS/table4c6.html.
- 29.Gupta N, Liu G, Berg D, Kalebic T, Gomez-Navarro J. Clinical Pharmacokinetics of Intravenous and Oral MLN9708, An Investigational Proteasome Inhibitor: An Analysis of Data From Four Phase 1 Monotherapy Studies. Blood 2010;16 (Abstract 1813). [Google Scholar]
- 30.Bianchi G, Ramakrishnan VG, Kimlinger T, Haug J, Rajkumar SV, Kumar S. Investigational Agent MLN2238/MLN9708, a Specific, Orally Available, Small Molecule Proteasome Inhibitor, Shows Promising In Vitro Activity Against Multiple Myeloma Cell Lines. Blood 2010;116(Abstract 3014). [Google Scholar]
- 31.Vestal RE. Aging and pharmacology. Cancer. 1997;80(7):1302–10 [DOI] [PubMed] [Google Scholar]
- 32.Baker SD, Grochow LB. Pharmacology of cancer chemotherapy in the older person. Clin Geriatr Med. 1997;13(1):169–83 [PubMed] [Google Scholar]
- 33.Yuen GJ. Altered pharmacokinetics in the elderly. Clin Geriatr Med. 1990;6(2):257–67 [PubMed] [Google Scholar]
- 34.Sotaniemi EA, Arranto AJ, Pelkonen O, Pasanen Ml. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin Pharmacol Ther. 1997;61(3):331–9 [DOI] [PubMed] [Google Scholar]
- 35.Abbott KC, Agodoa LY. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: patient characteristics and survival. Clin Nephrol. 2001;56(3):207–10 [PubMed] [Google Scholar]
- 36.Rayner HC, Haynes AP, Thompson JR, Russell N, Fletcher J. Perspectives in multiple myeloma: survival, prognostic factors and disease complications in a single centre between 1975 and 1988. Q J Med. 1991;79 (290):517–25 [PubMed] [Google Scholar]
- 37.Augustson BM, Begum G, Dunn JA, Barth NJ, Davies F, Morgan G, et al. Early mortality after diagnosis of multiple myeloma: analysis of patients entered onto the United Kingdom Medical Research Council trials between 1980 and 2002--Medical Research Council Adult Leukaemia Working Party. J Clin Oncol. 2005;23(36):9219–26 [DOI] [PubMed] [Google Scholar]
- 38.Dimopoulos MA, Terpos E, Chanan-Khan A, Leung N, Ludwig H, Jagannath S, et al. Renal impairment in patients with multiple myeloma: a consensus statement on behalf of the International Myeloma Working Group. J Clin Oncol. 2010;28(33):4976–84 [DOI] [PubMed] [Google Scholar]
- 39.Zangari M, Barlogie B, Cavallo F, Bolejack V, Fink L, Tricot G. Effect on survival of treatment-associated venous thromboembolism in newly diagnosed multiple myeloma patients. Blood Coagul Fibrinolysis. 2007;18 (7):595–8 [DOI] [PubMed] [Google Scholar]
- 40.Zangari M, Tricot G, Polavaram L, Zhan F, Finlayson A, Knight R, et al. Survival effect of venous thromboembolism in patients with multiple myeloma treated with lenalidomide and high-dose dexamethasone. J Clin Oncol. 2010;28(1):132–5 [DOI] [PubMed] [Google Scholar]
- 41.Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, et al. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31 [DOI] [PubMed] [Google Scholar]
- 42.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, et al. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–20 [DOI] [PubMed] [Google Scholar]
- 43.Richardson PG, Sonneveld P, Schuster MW, Stadtmauer EA, Facon T, Harousseau JL, et al. Reversibility of symptomatic peripheral neuropathy with bortezomib in the phase III APEX trial in relapsed multiple myeloma: impact of a dose-modification guideline. Br J Haematol. 2009;144(6):895–903 [DOI] [PubMed] [Google Scholar]
- 44.Broyl A, Corthals SL, Jongen JL, van der Holt B, Kuiper R, de Knegt Y, et al. Mechanisms of peripheral neuropathy associated with bortezomib and vincristine in patients with newly diagnosed multiple myeloma: a prospective analysis of data from the HOVON-65/GMMG-HD4 trial. Lancet Oncol. 2010;11(11):1057–65 [DOI] [PubMed] [Google Scholar]
- 45.Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, et al. Efficacy and safety of once weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23): 4745–53 [DOI] [PubMed] [Google Scholar]
- 46.Palumbo A, Bringhen S, Ludwig H, Dimopoulos MA, Bladé J, Mateos MV, et al. Personalized therapy in multiple myeloma according to patient age and vulnerability: a report of the European Myeloma Network (EMN). Blood. 2011;118(17):4519–29 [DOI] [PubMed] [Google Scholar]




