Abstract
Teleglaucoma is the application of telemedicine for glaucoma. We review and present the current literature on teleglaucoma; present our experience with teleglaucoma programs in Alberta, Canada and Western Australia; and discuss the challenges and opportunities in this emerging field. Teleglaucoma is a novel area that was first explored a little over a decade ago and early studies highlighted the technical challenges of delivering glaucoma care remotely. Advanced technologies have since emerged that show great promise in providing access to underserviced populations. Additionally, these technologies can improve the efficiency of healthcare systems burdened with an increasing number of patients with glaucoma, and a limited supply of ophthalmologists. Additional benefits of teleglaucoma systems include e-learning and e-research. Further work is needed to fully validate and study the cost and comparative effectiveness of this approach relative to traditional models of healthcare.
Keywords: Glaucoma, Teleglaucoma, Telemedicine, Teleophthalmology
INTRODUCTION
Spurred by the rapid rise in the use of information and communication technology, the telemedicine revolution has significantly enhanced the potential for equitable access to the expertise of an eye-care specialist, particularly in underserviced and rural areas.1,2 The American Telemedicine Association defines telemedicine as the use of medical information exchanged from one site to another via electronic communications to improve the health status of a patient.3 In keeping with this, we define teleglaucoma as the application of electronic technologies to detect and manage patients with or at risk of glaucoma. Important parameters in this type of delivery of healthcare include timeliness, quality, efficiency, and patient and provider acceptability. An ongoing concern of incorporating teleophthalmology into clinical care has been maintenance of proper standards and quality control. Thus it has become particularly important to be able to rigorously validate protocols similar to the model of diabetic retinopathy.4,5
Glaucomas represent a diverse group of diseases that have in common characteristic changes in the optic nerve neuroretinal rim tissue. An American Academy of Ophthalmology (AAO) ophthalmic technology assessment in 2007 reported that serial stereophotography of the optic nerve head is considered the reference standard of care for patients with glaucoma.6 A more recent assessment of the value of glaucoma tests emphasized that optic nerve examination as performed by a clinical exam or via stereo-disc photographs remains the foundation of glaucoma diagnosis.7 Other fundamental factors critical to diagnosis and management include integrating history, examination, as well as other structural and functional assessments. Considering that structural and functional assessment form the basis of diagnosis and monitoring progression, and that there are finite human, financial and other resources, a key question is how to optimize care for patients with glaucoma or at risk of developing glaucoma. This is an important consideration when one considers that common forms of glaucoma increase in prevalence with age and all projections indicate that the elderly population will expand considerably over the next decade. Optimal delivery of care is the impetus for teleglaucoma: providing access to those who do not currently receive it and efficiency to relieve an already burdened healthcare system in many countries with significant constraints on the supply of eye-care professionals.8
In this article, we review the available literature on teleglaucoma, share our experience with programs in Canada and Australia, and present opportunities and challenges for this emerging and evolving field.
LITERATURE TO DATE
The first studies published on teleglaucoma were in 1999. Yogesan et al.,9 and Li et al.,10 compared digital optic nerve assessments to slide film demonstrating strong and moderate agreement, respectively. Tuulonen et al.,11 evaluated access, efficiency and cost effectiveness for glaucoma care. They11 showed that the costs for telemedicine and conventional visits were equal; however, patients were extremely satisfied with their personal cost and time saving. However, in the study,11 image quality captured from video clips using frame grabbing technology was compromised compared with the digital imaging system at their university eye clinic. Similarly, a study by Labiris et al.,12 reported technical difficulties with video transmission or communication in 13/56 attempted telemedical glaucoma consultations via a mobile unit. A large study (N = 1729) from The Netherlands in 2004 assessed a shared care glaucoma screening model via image transmission, and reported satisfactory image quality in 89% of the cases, and a fairly good correlation between optometrists and trained in-hospital technicians in cases with suspicious findings (81%).13 This study13 was performed by scanning laser polarimetry to analyze the retinal nerve fiber layer (RNFL) and determine whether a patient had glaucoma. In 2008, Blαzquez et al.,14 found that patients had a very high acceptance rate for glaucoma screening via telemedicine. Blαzquez et al.,14 performed a large study that integrated results from Heidelberg Retinal Tomography (HRT), Frequency Doubling Technology (FDT) and intraocular pressures (IOPs) that were electronically transmitted to an ophthalmologist for interpretation. A study by Kumar et al.,15 found good correlation between digital cup-to-disc ratios and ophthalmoscopy for 399 eyes. The Kumar et al.,15 study also showed that IOP measurements did not enhance the sensitivity of a teleglaucoma screening program, which suggests IOP may be useful for telemonitoring but not telescreening.
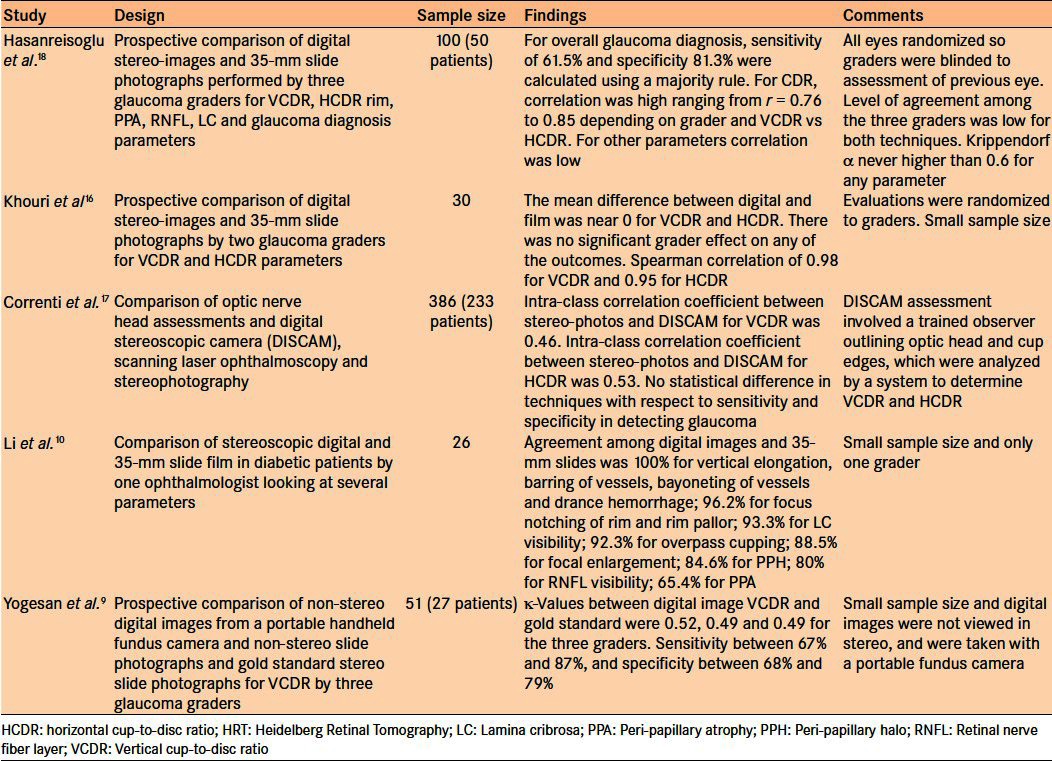
Despite the promising results of these early studies, controversy remains regarding the sensitivity and specificity of stereoscopic digital imaging of the optic nerve head compared with the gold standard of stereoscopic slide film photography. Several studies have compared digital optic nerve images to slide film with encouraging but mixed results when compared with the gold standard [Table 1].9,10,16–18 More conclusive validation studies are required, taking into consideration that correlation studies between digital images and the gold standard are dependent on the image quality obtained from the device as well as viewing techniques and resolution.
Table 1.
Summary of studies comparing assessment of optic nerves using digital images versus the gold standard
TELEGLAUCOMA PROGRAMS IN CANADA AND AUSTRALIA
We describe our experience at University of Alberta using a store and forward system as well as our experience in Western Australia using real-time videoconferencing for glaucoma diagnosis and management.
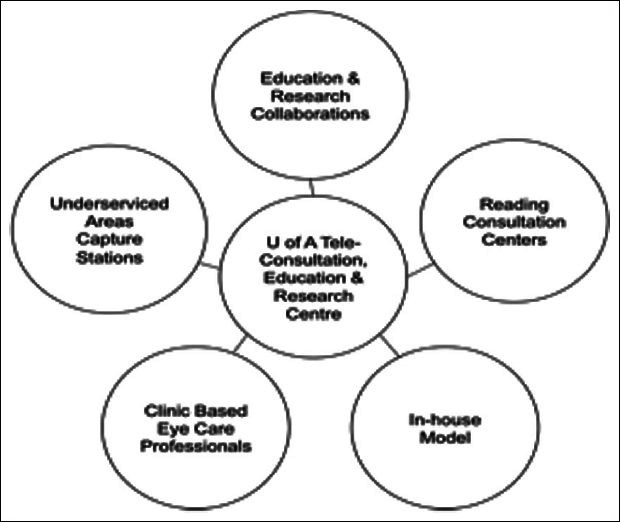
The teleglaucoma program at University of Alberta fits into a wider concept of collaborative care that integrates various eye-care professionals in order to provide streamlined, efficient management [Figure 1]. A key feature is of this program is stratification based on known glaucoma risk factors followed by management by the appropriate provider (optometrist or ophthalmologist). The Canadian Ophthalmological Society has recognized the importance of this concept with their committee's guidelines on inter-professional collaboration in glaucoma.19 It proposes that the solution to glaucoma care has to be patient-centered with the objective of improving access and quality of care; consideration must be given to geographic and ethnic differences, as well as the different needs of different types of patients, and consideration must also be given to all parties involved, including the various professionals involved in ocular care, patients, government and healthcare providers.
Figure 1.
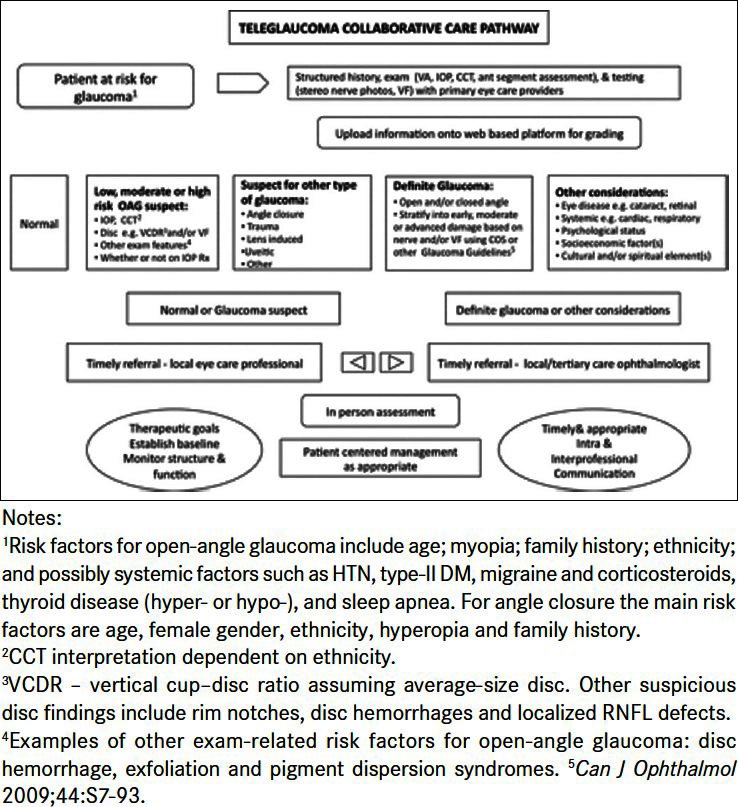
The teleglaucoma and collaborative care pathway from University of Alberta. Adapted from Kassam et al.36
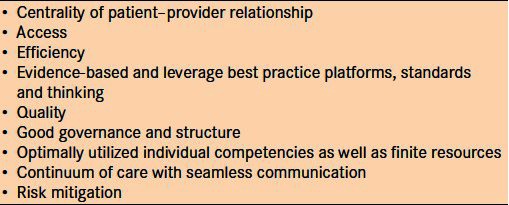
The principles outlined in Table 2 form the foundation of our teleglaucoma program along with the following four pillars: human resources (including proper governance and healthcare teamwork); adequate information technology support; appropriate provision of equipment; sound protocols; and best practices for quality e-consultation, e-learning and e-research.
Table 2.
Principles for the teleglaucoma program at University of Alberta
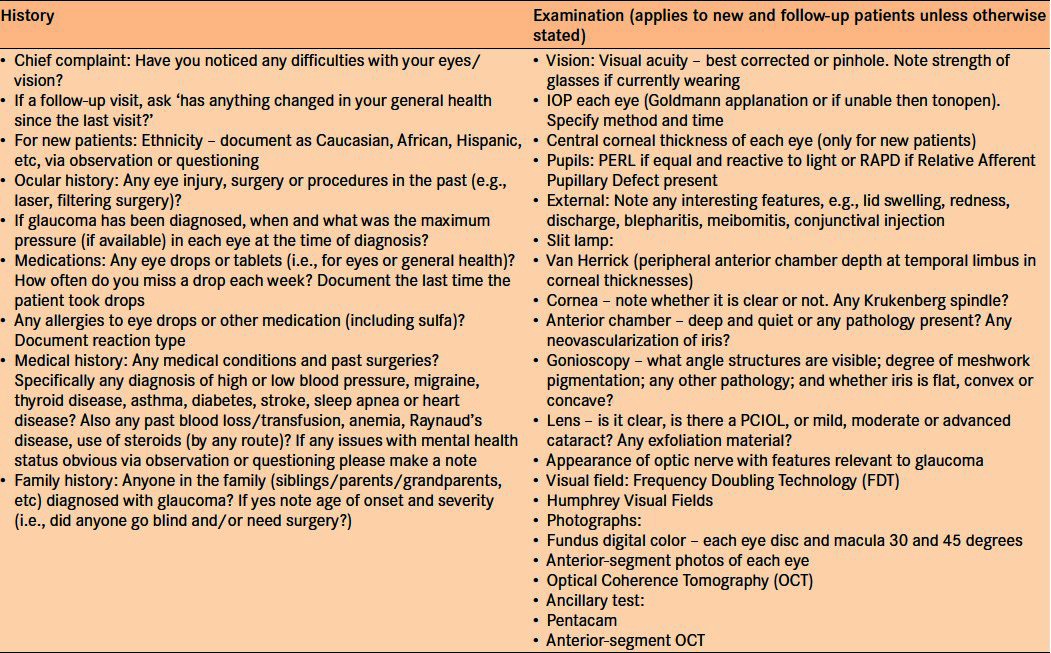
Key to teleglaucoma assessment is the information provided by a standardized ophthalmic and medical history [Table 3], slit-lamp examination, IOPs, visual fields, and standardized 30- or 45-degree fundus photographs. Grading occurs using cathode ray tube monitors, an emitter box (a device that facilitates stereoviewing), three-dimensional professional video cards and LCD shutter glasses. The digital photographs are compressed at a ratio of 16:1 and viewed at a resolution of 1024 × 1280 pixels. This viewing protocol has been validated for use in diabetic retinopathy screening.4 Once read, a PDF report is generated with management recommendations that are sent back to the referring eye-care provider and in-person follow-up is arranged as warranted.20 The report has a disclaimer that recognizes the limitations of the consult in assessing the secondary causes of glaucoma such as angle closure, pseudoexfoliation, pigment dispersion and uveitis. It also reinforces that clinical suspicion determines whether an in-person examination may be warranted. Lastly, the disclaimer describes the confidentiality of the teleglaucoma examination and reviews the research into this pilot program. This approach has been developed in order to ensure efficiency is appropriately balanced with quality.
Table 3.
Standardized history and testing protocol for teleglaucoma at University of Alberta. Adapted from Kassam et al.20
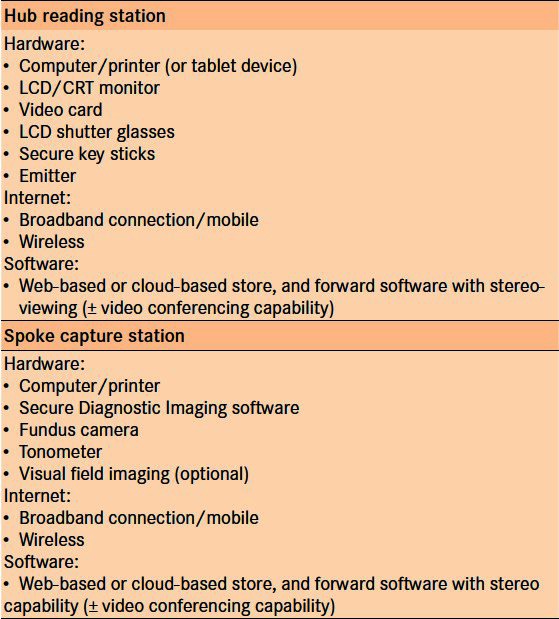
The Alberta program is comprised of two sub-programs: ‘Remote’ and ‘In-house’. Both these are store and forward teleglaucoma systems. The Remote program involves both diagnosis and management of glaucoma suspects remotely, with in-person exam arranged at the location and with a provider as appropriate. It operates as a ‘hub-and-spoke’ framework of delivery [Figure 2]. This involves web-based evaluation of information provided remotely by general ophthalmologists and optometrists (spokes), which is interpreted by a glaucoma specialist (at the hub) who follows up with recommendations. Fifteen optometrists and a comprehensive ophthalmologist that are properly equipped [Table 4], and whose staff has undergone training, have been recruited to the pilot program for teleconsultation. Standardized history [Table 3], slit-lamp examination, corneal pachymetry, fundus photographs, visual fields such as FDT or Humphrey Visual Fields, and any other available ancillary testing such as Optical Coherence Tomography (OCT) or HRT are uploaded to the proprietary software Secure Diagnostic Imaging server. The Secure Diagnostic Imaging software is made available to all optometrists in the pilot trial free of cost. One of four ophthalmologists, all glaucoma subspecialists, completes the consults. Interpretation requires special monitors and LCD shutter glasses for stereoscopic assessment of the nerve. The specialists review all of the information supplied within 10 days, including optic nerve assessment, and make appropriate recommendations for management of the patient. A standardized report is automatically generated and sent electronically to the referring optometrist or comprehensive ophthalmologist. Follow-up is arranged with the specialist if needed or repeat teleconsultation with the optometrist, if recommended. A copy of the report can also be mailed to the patient if they wish, with the hope of empowering the patient and improving health education. This approach is primarily intended for patients who live in underserviced areas, but could be utilized in semi-urban communities as well.20
Figure 2.
The ‘hub-and-spoke’ framework for delivery of teleglaucoma at University of Alberta
Table 4.
Components needed for ‘hub-and-spoke’ delivery of teleglaucoma
The In-house program runs at the Royal Alexandra Hospital, Edmonton, Canada (tertiary academic center), and at a private glaucoma group practice. Regular referrals that are received for glaucoma assessments are assessed for teleglaucoma suitability – typically patients who are likely suspects or may have early stages of glaucoma – and for those who do not fulfill any of the exclusion criteria listed in Table 5. If the patient is eligible, the appropriate anticipated tests and exams are ordered as per existing clinic protocols. The teleglaucoma program coordinator informs the patient and referring eye-care professional about the program, and if the patient is interested they are booked for the visit. Both the patient and referring optometrist are notified via a notification letter once the appointment is made. The visit involves a patient's encounter with an ophthalmic technician or nurse who performs a detailed history and slit-lamp examination, visual field testing, fundus photography and OCT [Figure 3]. The goal of the In-house teleglaucoma program is to provide access to glaucoma assessment within 1 month (typical access time is 3–4 months) and reduce cycle time (defined as the time from registration until departure, including testing, seeing the technician and physician) to less than 90 min (currently about 120 min for in-person exams).20 Periodically, complete evaluations are performed to assess the overall program, including outcomes of consultations, efficiency and effectiveness. This includes feedback from the patient, referring and consultation provider, and recommendations for improvement. Thus far, early data show potentially improved efficiency compared with conventional visits with time and cost-benefits.21 Medicare in Alberta currently reimburses teleconsultation in both the Remote and In-house programs at approximately two-thirds of a conventional in-person fee.
Table 5.
Exclusion criteria for teleglaucoma at University of Alberta20
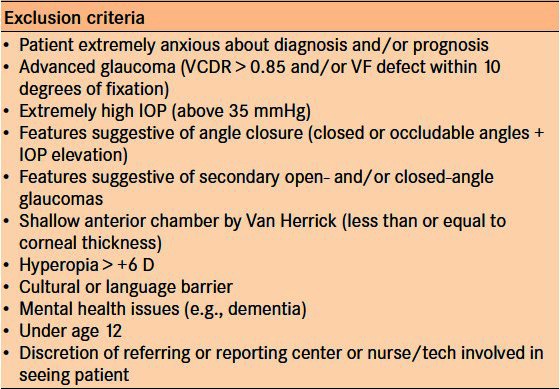
Figure 3.
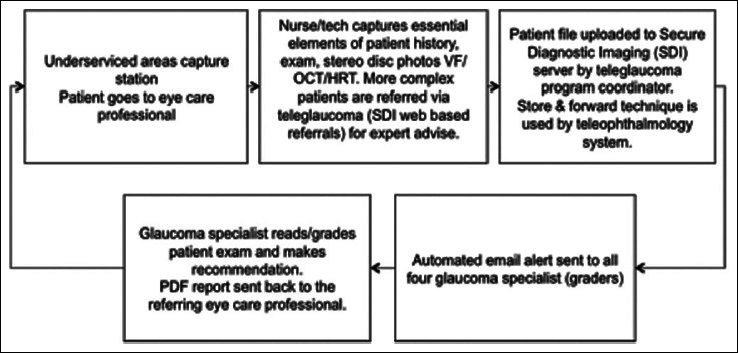
The In-house teleglaucoma program patient flow at University of Alberta
In Australia, there has been a fee for online consultations from Medicare since July 2011; however, this covers only real-time consultations and is the main difference between the Canadian and Australian programs. The patient is required to visit a primary care provider or a medical clinic to connect to an ophthalmologist for diagnostic advice. The consultation could take place over an internet-based communication service such as Skype. The ophthalmologist receives high-resolution images and other data using a store and forward system (EHR) and then speaks to the patient and the primary care provider to provide disease management and recommendations. In Western Australia, a proprietary web-based system called Remote-I is being utilized to connect primary care providers to ophthalmologists in rural and remote locations. The system combines video conferencing and store and forward technology such that the ophthalmologists providing telemedicine consultation can meet the requirements to charge a fee for service from Medicare. Ophthalmologists are able to connect to Remote-I using a tablet device such as an iPad in order to provide glaucoma consultations remotely at their convenience. Video conferencing and the ability to view high-resolution retinal images are also possible through the system. Additionally, tools are available, which can assist in clinical decision-making such as automated estimation of cup-to-disc ratio and rim width. It can also compare images of optic nerves at different time points to alert the clinician of possible disease progression.
LEARNING AND PERSPECTIVES
To date, our program has been very well received by ophthalmologists, glaucoma specialists, optometrists, ophthalmic nurses and technicians in Northern Alberta. The program can be run with focus on glaucoma or potentially as part of a broader teleophthalmology approach to detection of other eye disorders (e.g., with diabetic retinopathy, macular degeneration).22 One of the key success factors for both programs is reimbursement for service as this is an important component of physician adoption. However, in Australia the store and forward technology is not included in this Medicare item. The ophthalmologist must speak to the patient who is with a primary care provider and then review images and other data using EHR, which places constraints on refining and improving the processes. Both the Australian and Canadian programs leverage existing teleophthalmology platforms and serve as two examples of how teleglaucoma can be delivered, but many other possibilities exist. Replication of these or similar models in other settings is highly dependent on the environment of the healthcare system in which the program is being initiated.
Any telemedicine approach comes with medico-legal risks and must be closely considered when implementing a teleglaucoma program. Fortunately, the Canadian Medical Protective Association has guidelines on collaborative care and e-health. Furthermore, guidance is offered by the American Telemedicine Association for telehealth practice related to diabetic retinopathy and the Digital Imaging and Communications in Medicine (DICOM) Standard.23–25 Important considerations include that each party involved in a shared care model must have adequate medico-legal coverage, there must be timely and clear communication between providers, well-developed standards for care, and confidentiality of patient information and backup of data. These guidelines are crucial to the development and progression of teleglaucoma programs. Our programs are developed within the context of the larger evidence-based Canadian Glaucoma Guidelines and AAO Preferred Practice Patterns for Glaucoma.26,27
There are a number of limitations to teleglaucoma. First, telemedicine strategies may put the patient–physician relationship at risk. This must be carefully considered and ultimately communication and empathy will shift heavily onto front-line staff collecting diagnostic information (technicians, photographers, nurses, optometrists, etc). Additionally, reporting information back to the patient in a timely manner, and continuously assessing and responding to patient feedback will be important. Hence, staff will require ongoing training in these areas. Second, as with any consultation in medicine, there are sensitive issues surrounding patient ownership and scope of practice, thus a collaborative stakeholder approach is important. A third limitation of teleglaucoma is gonioscopic assessment to obtain information about the angle. In some cases, referring ophthalmologists and optometrists provide gonioscopy information. In our In-house program, we employed anterior segment imaging with OCT or Pentacam, which can assist in determining iris configuration and whether the angle is open. However, both these modalities do not provide direct information about the angle structures (such as presence of angle pigment or neovascularization) and they do not replace compression gonioscopy. Finally, the software and user interface design is a critical component of the efficiency of completing teleconsultation. Consultation time could be reduced significantly by using drop-down menus and multiple screens to display all investigations concurrently.
FUTURE DIRECTIONS
As more centers begin to utilize telemedicine approaches for glaucoma diagnosis and management, a comprehensive set of standards and best practices should be created similar to those created by the American Telemedicine Association for diabetic retinopathy. There are still several questions that need to be addressed prior to determining the exact utility of teleglaucoma. For example, what is the optimal image compression ratio for the optic nerve? Can stereo-imaging be performed without stereo-glasses? How much value does stereoscopic imaging add to the diagnosis? Can our models be replicated and validated in other healthcare settings? Could teleglaucoma be used to diagnose and manage acute presentations? To date, existing data suggest that IOP plays little role in glaucoma screening, but its role in telemonitoring remains to be determined. A collaborative research group for projects related to teleglaucoma would be a worthwhile initiative to share knowledge, skills, approaches and design research to improve teleglaucoma delivery and determine best practices.
As with many areas of telemedicine, teleglaucoma can eventually evolve to be most useful in serving the poorest populations in the developing world. Sub-Saharan Africa and South Asia, among other regions, have a significant burden of glaucoma. Additionally, access to care is challenging as much of the population resides in rural areas and there can be geographic barriers to reaching subspecialists. Similar to our model, spoke stations strategically positioned in remote areas could be created, with images and diagnostic information being digitally transferred to the urban centers with follow-up and intervention as needed. Crucial to this is strong institutional capacity and local empowerment, as well as training subspecialty experts for these developing regions.28
Teleglaucoma also carries with it the potential for e-learning and e-research by utilizing the large volume of captured disc images. These images could be used to teach fellows, residents and medical students, and may provide important epidemiological information to better understand the disease process in rural areas worldwide. In the developing world particularly, acceptability is dependent on the ability to use teleglaucoma in low-resource settings. This in turn requires equipment innovation that is low-cost and has multiple functions (e.g., one device to take pictures of the anterior segment, including the angle, as well as the fundus). Along similar lines, advances are needed in diagnostic equipment use, automated analysis,29 and determining how progression of the optic nerve will be displayed and identified. Standardized grading such as rim-to-disc ratios or a disc damage likelihood scale may prove to be increasingly powerful tools in monitoring progression.30 Many novel research applications may develop with teleglaucoma, including support for conducting multicenter trials.31
The increased prevalence of mobile phones and the internet worldwide is being used in innovative ways to improve patient health.32 There are many ways this can be integrated into glaucoma care such as special applications or text reminders for patients to take glaucoma medications and online visual field testing.33 Although we should be mindful of ensuring high standards and quality, this should be balanced with the significant potential for patient empowerment using these technologies. This was highlighted by a recent case in which a glaucoma patients who lived a 5-h drive from the academic center e-mailed one of us (LRP) a picture of his eye (with an infected bleb) from his cellular device [Figure 4] asking if it was a cause for concern.
Figure 4.
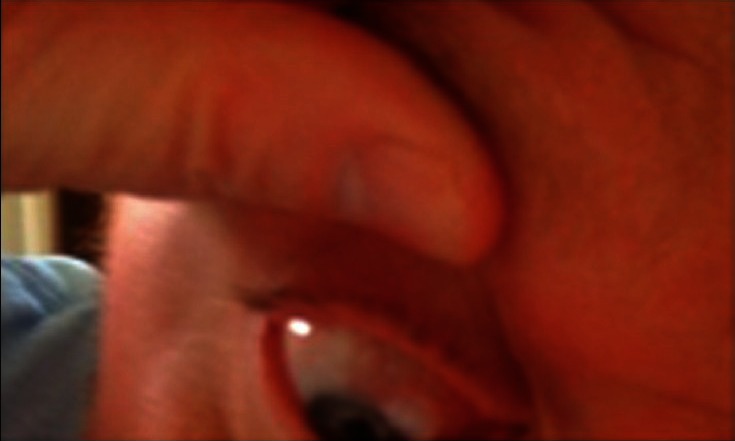
Picture taken with a cellular device by a patient e-mailed to his physician
Another crucial area for further research is health economics, cost effectiveness and comparative effectiveness when delivering this type of care. A systematic review of economic evaluations in telemedicine highlighted the diversity in economic evaluations and methodology, making these types of analyses challenging.34 In ophthalmology specifically, it does appear that the telemedicine approach in vitreoretinal diseases is cost-effective.35 Intimately linked to cost effectiveness and the potential success of teleglaucoma is determination of reimbursement models for providers when utilizing the service. In countries such as the United States, Canada and Australia, public healthcare programs are willing to reimburse for teleglaucoma and teleretinal screening programs given the potential cost savings, although this can vary by state/provincial governments and have strict requirements (e.g., live conferencing). In resource-poor settings, it will be more challenging to involve the government and implementation will greatly depend on innovative business models for paying patients as well as cheaper IT startup equipment.
CONCLUSION
We believe teleglaucoma could transform how glaucoma is diagnosed and managed both in the developing and developed world. The literature to date has demonstrated some successes but highlights that delivery of quality teleglaucoma care is deeply intertwined with the quality of available technology and systems. Our experiences in Canada and Australia are part of a collaborative approach to care. These programs have shown great promise in optimizing processes to provide comprehensive teleglaucoma service; however, technical and psychosocial limitations remain. Furthermore, general acceptability by patients and providers is dependent on the healthcare system and the population being serviced, and it may not work well in all settings. Significant research is warranted to validate teleglaucoma assessments compared with the gold standard. Additionally, evaluation of cost efficiency compared with traditional models of healthcare delivery is required. We encourage other studies that explore this emerging area that has the potential to improve patient access and increase efficiency for glaucoma-related care.
ACKNOWLEDGMENTS
Pfizer Canada Inc. provided a startup grant for the teleglaucoma program at University of Alberta. No funding was received for the writing of this paper.
Conceptualization of the paper: FK, KY, ES, LRP, KFD; preparation, review and approval of the paper: FK, KY, ES, LRP, KFD.
No IRB approval was sought for the writing of this paper.
The authors thank Samreen Amin, the teleglaucoma program coordinator; Matthew Tennant from Secure Diagnostic Imaging Inc; Marianne Edwards, Michael Dorey and Ordan Lehmann of the teleglaucoma consultation team; and Abshir Moalin, the teleophthalmology program coordinator.
Footnotes
Source of Support: Pfizer Canada Inc. provided a startup grant for the teleglaucoma program at University of Alberta. No funding was received for the writing of this paper
Conflict of Interest: None declared.
REFERENCES
- 1.Prathiba V, Rema M. Teleophthalmology: A model for eye care delivery in rural and underserved areas of India. Int J Family Med. 2011;2011:683267. doi: 10.1155/2011/683267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Nathoo N, Rudnisky CJ, Tennant MT. Improving access to eye care: Teleophthalmology in Alberta, Canada. J Diabetes Sci Technol. 2009;1(3):289–96. doi: 10.1177/193229680900300209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Telemedicine Association. Telemedicine Defined. [Last accessed on 2011 Mar 27]. Available from: http://www.americantelemed.org/i4a/pages/index.cfm?pageid=3333 .
- 4.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. Ophthalmology. 2007;114:1748–54. doi: 10.1016/j.ophtha.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Rudnisky CJ, Hinz BJ, Tennant MT, de Leon AR, Greve MD. High-resolution stereoscopic digital fundus photography versus contact lens biomicroscopy for the detection of clinically significant macular edema. Ophthalmology. 2002;109:267–74. doi: 10.1016/s0161-6420(01)00933-2. [DOI] [PubMed] [Google Scholar]
- 6.Lin SC, Singh K, Jampel HD, Hodapp EA, Smith SD, Francis BA, et al. Optic nerve head and retinal nerve fiber layer analysis: A report by the American Academy of Ophthalmology. Ophthalmology. 2007;114:1937–49. doi: 10.1016/j.ophtha.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lieberman MF, Congdon NG, He M. The value of tests in the diagnosis and management of glaucoma. Am J Ophthalmol. 2011;152:889–99. doi: 10.1016/j.ajo.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 8.Resnikoff S, Felch W, Gauthier TM, Spivey B. The number of ophthalmologists in practice and training worldwide: A growing gap despite more than 200,000 practitioners. Br J Ophthalmol. 2012;96:783–7. doi: 10.1136/bjophthalmol-2011-301378. [DOI] [PubMed] [Google Scholar]
- 9.Yogesan K, Constable IJ, Barry CJ, Eikelboom RH, Morgan W, Tay-Kearney ML, et al. Evaluation of a portable fundus camera for use in the teleophthalmologic diagnosis of glaucoma. J Glaucoma. 1999;8:297–301. [PubMed] [Google Scholar]
- 10.Li HK, Tang RA, Oschner K, Koplos C, Grady J, Crump WJ. Telemedicine screening of glaucoma. Telemed J. 1999;5:283–90. doi: 10.1089/107830299312032. [DOI] [PubMed] [Google Scholar]
- 11.Tuulonen A, Ohinmaa T, Alanko HI, Hyytinen P, Juutinen A, Toppinen E. The application of teleophthalmology in examining patients with glaucoma: A pilot study. J Glaucoma. 1999;8:367–73. [PubMed] [Google Scholar]
- 12.Labiris G, Fanariotis M, Christoulakis C, Petounis A, Kitsos G, Aspiotis M, et al. Tele-ophthalmology and conventional ophthalmology using a mobile medical unit in remote Greece. J Telemed Telecare. 2003;9:296–9. doi: 10.1258/135763303769211337. [DOI] [PubMed] [Google Scholar]
- 13.de Mul M, de Bont AA, Reus NJ, Lemij HG, Berg M. Improving the quality of eye care with tele-ophthalmology: Shared-care glaucoma screening. J Telemed Telecare. 2004;10:331–6. doi: 10.1258/1357633042602107. [DOI] [PubMed] [Google Scholar]
- 14.Blázquez F, Sebastián MA, Antón A. Detection of glaucoma using SisGlaTel: Acceptability and satisfaction among participants, and problems detected. Arch Soc Esp Oftalmol. 2008;83:533–8. doi: 10.4321/s0365-66912008000900005. [DOI] [PubMed] [Google Scholar]
- 15.Kumar S, Giubilato A, Morgan W, Jitskaia L, Barry C, Bulsara M, et al. Glaucoma screening: Analysis of conventional and telemedicine-friendly devices. Clin Experiment Ophthalmol. 2007;35:237–43. doi: 10.1111/j.1442-9071.2007.01457.x. [DOI] [PubMed] [Google Scholar]
- 16.Khouri AS, Szirth B, Realini T, Fechtner RD. Comparison of digital and film stereo photography of the optic nerve in the evaluation of patients with glaucoma. Telemed J E Health. 2006;12:632–8. doi: 10.1089/tmj.2006.12.632. [DOI] [PubMed] [Google Scholar]
- 17.Correnti AJ, Wollstein G, Price LL, Schuman JS. Comparison of optic nerve head assessment with a digital stereoscopic camera (discam), scanning laser ophthalmoscopy, and stereophotography. Ophthalmology. 2003;110:1499–505. doi: 10.1016/S0161-6420(03)00496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hasanreisoglu M, Priel E, Naveh L, Lusky M, Weinberger D, Benjamini Y, et al. Digital versus film stereo-photography for assessment of the optic nerve head in glaucoma and glaucoma suspect patients. J Glaucoma. 2013 Mar;22(3):238–42. doi: 10.1097/IJG.0b013e31823298da. [DOI] [PubMed] [Google Scholar]
- 19.Canadian Glaucoma Society Committee on Interprofessional Collaboration in Glaucoma Care. Model of interprofessional collaboration in the care of glaucoma patients and glaucoma suspects. Can J Ophthalmol. 2011;46(6 Suppl):S1–21. doi: 10.1016/j.jcjo.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Kassam F, Amin S, Sogbesan E, Damji KF. The use of teleglaucoma at the University of Alberta. J Telemed Telecare. 2012;18:367–73. doi: 10.1258/jtt.2012.120313. [DOI] [PubMed] [Google Scholar]
- 21.Sogbesan E, Rudnisky C, Tennant M, Damji KF. Abstract presented at: Canadian Ophthalmological Society Annual Meeting. Quebec City: 2010. Jun 26, Time and cost–benefit analysis of a collaborative web-based stereoscopic teleglaucoma care model. [Google Scholar]
- 22.Asefzadeh B, Pasquale LR Ocular Telehealth Team. Detection of glaucoma-like optic discs in a diabetes teleretinal program. Optometry. 2008;79:560. doi: 10.1016/j.optm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 23.The Canadian Medical Protective Association. CMPA assistance in legal matters arising from telehealth: Technology makes location of physician less relevant. 2006. Mar, [Last revised on: 2008 August, 2009 March]. Available from: http://www.cmpa-acpm.ca/cmpapd04/docs/member_assistance/pdf/com_is0661-e.pdf .
- 24.American Telemedicine Association. Telehealth Practice Recommendations for Diabetic Retinopathy. 2011. Feb, Available from: http://www.americantelemed.org/files/public/standards/DiabeticRetinopathy_withCOVER.pdf . [DOI] [PubMed]
- 25.Digital Imaging and Communications in Medicine. The DICOM Standard. [Last accessed on]. Available from: http://medical.nema.org/standard.html .
- 26.Canadian Ophthalmological Society Glaucoma Clinical Practice Guideline Expert Committee. Canadian Ophthalmological Society evidence-based clinical practice guidelines for the management of glaucoma in the adult eye. Can J Ophthalmol. 2009;44:S7–93. doi: 10.3129/cjo44s1. [DOI] [PubMed] [Google Scholar]
- 27.American Academy of Ophthalmology. Primary open-angle glaucoma suspect PPP. [Last accessed on]. Available from: http://one.aao.org/CE/PracticeGuidelines/PPP.aspx?sid=ca9ec1b5-2567-4e85-96f6-b6540e5ac5a1 .
- 28.Kassam F, Damji KF, Kiage D, Carruthers C, Kollmann KH. The Sandwich fellowship: A subspecialty training model for the developing world. Acad Med. 2009;84:1152–60. doi: 10.1097/ACM.0b013e3181acf95c. [DOI] [PubMed] [Google Scholar]
- 29.Khouri AS, Szirth BC, Shahid KS, Fechtner RD. Software-assisted optic nerve assessment for glaucoma tele-screening. Telemed J E Health. 2008;14:261–5. doi: 10.1089/tmj.2007.0049. [DOI] [PubMed] [Google Scholar]
- 30.Spaeth GL, Henderer J, Liu C, Kesen M, Altangerel U, Bayer A, et al. The disc damage likelihood scale: Reproducibility of a new method of estimating the amount of optic nerve damage caused by glaucoma. Trans Am Ophthalmol Soc. 2002;100:181–5. [PMC free article] [PubMed] [Google Scholar]
- 31.Kennedy C, Kirwan J, Cook C, Roux P, Stulting A, Murdoch I. Telemedicine techniques can be used to facilitate the conduct of multicentre trials. J Telemed Telecare. 2000;6:343–7. doi: 10.1258/1357633001936030. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. mHealth: New Horizons for Health through Mobile Technologies. [Last accessed on]. Available from: http://whqlibdoc.who.int/publications/2011/9789241564250_eng.pdf .
- 33.Ianchulev T, Pham P, Makarov V, Francis B, Minckler D. Peristat: A computer-based perimetry self-test for cost-effective population screening of glaucoma. Curr Eye Res. 2005;30:1–6. doi: 10.1080/02713680490522399. [DOI] [PubMed] [Google Scholar]
- 34.Bergmo TS. Can economic evaluation in telemedicine be trusted? A systematic review of the literature. Cost Eff Resour Alloc. 2009;7:18. doi: 10.1186/1478-7547-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Au A, Gupta O. The economics of telemedicine for vitreoretinal diseases. Curr Opin Ophthalmol. 2011;22:194–8. doi: 10.1097/ICU.0b013e3283459508. [DOI] [PubMed] [Google Scholar]
- 36.Kassam F, Sogbesan E, Boucher S, Rudnisky CJ, Prince W, Leinweber G, et al. Collaborative care and teleglaucoma: A novel approach to delivering glaucoma services in Northern Alberta, Canada. Clin Exp Optom. doi: 10.1111/cxo.12065. [DOI] [PubMed] [Google Scholar]


