Abstract
Purpose:
While the effectiveness of teleophthalmology is generally accepted, its ability to diagnose glaucomatous eye disease remains relatively unknown. This study aimed to compare a web-based teleophthalmology assessment with clinical slit lamp examination to screen for glaucoma among diabetics in a rural African district.
Materials and Methods:
Three hundred and nine diabetic patients underwent both the clinical slit lamp examination by a comprehensive ophthalmologist and teleglaucoma (TG) assessment by a glaucoma subspecialist. Both assessments were compared for any focal glaucoma damage; for TG, the quality of photographs was assessed, and vertical cup-to-disk ratio (VCDR) was calculated in a semi-automated manner. In patients with VCDR > 0.7, the diagnostic precision of the Frequency Doubling Technology (FDT) C-20 screening program was assessed.
Results:
Of 309 TG assessment photos, 74 (24%) were deemed unreadable due to media opacities, patient cooperation, and unsatisfactory photographic technique. While the identification of individual optic nerve factors showed either fair or moderate agreement, the ability to diagnose glaucoma based on the overall assessment showed moderate agreement (Kappa [κ] statistic 0.55% and 95% confidence interval [CI]: 0.48-0.62). The use of FDT to detect glaucoma in the presence of disc damage (VCDR > 0.7) showed substantial agreement (κ statistic of 0.84 and 95% CI 0.79-0.90). A positive TG diagnosis of glaucoma carried a 77.5% positive predictive value, and a negative TG diagnosis carried an 82.2% negative predicative value relative to the clinical slit lamp examination.
Conclusion:
There was moderate agreement between the ability to diagnose glaucoma using TG relative to clinical slit lamp examination. Poor quality photographs can severely limit the ability of TG assessment to diagnose optic nerve damage and glaucoma. Although further work and validation is needed, the TG approach provides a novel, and promising method to diagnose glaucoma, a major cause of ocular morbidity throughout the world.
Keywords: Glaucoma, Optic Neuropathy, Slit Lamp Examination, Teleglaucoma, Teleophthalmology
INTRODUCTION
There are 60 million people with glaucomatous optic neuropathy resulting in 8.4 million cases of blindness from glaucoma world-wide. The burden of glaucoma is expected to increase significantly with an estimated 80 million people with glaucomatous optic neuropathy and 11.2 million blind by 2020.1 Epidemiology studies world-wide suggest that primary open angle glaucoma (POAG) disproportionately affects Africans, and this population accounts for the highest prevalence of all POAG cases.2 POAG in Africans may present at an earlier age, is associated with higher intraocular pressure (IOP), progresses more rapidly, and presents in late stages.2–4 Furthermore, cases of glaucoma in African populations present with increased severity and are more difficult to treat.1,5–7 In East, Central and Southern Africa, glaucoma affects an estimated 10,000 people per million and approximately 400 new cases of glaucoma per million are diagnosed yearly.3
Due to a limited number of trained health-care workers and limited diagnostic equipment, early diagnosis and follow-up treatment are especially challenging in many parts of Africa. Hence, innovation is required to optimally utilize the limited funding and other finite resources to address the burden of glaucoma and its related increase in morbidity.
Telemedicine refers to the evaluation and treatment of disease of patients at a distance. Teleophthalmology narrows the scope to eye diseases detected and managed from a distance. Teleglaucoma (TG), refers to the evaluation and treatment of glaucoma among patients at a distance.8 A TG program requires telemedical technologies that can provide two or three dimensional images of the nerve and online tools to grade glaucomatous cupping of the optic disc.9 With TG, ophthalmologists can evaluate stereo images sent via the internet using the prismatic viewers, red-green anaglyph spectacles or liquid crystal display shutter glasses.9 Although the majority of teleophthalmology to date has been on teleretinal applications, programs can and should be established to diagnose glaucomatous optic nerve disease for timely treatment.
A study from South Africa demonstrated that TG was a cost-effective international development project. The connection to ophthalmologists from donor countries allowed South African practitioners to learn new procedures, improving ophthalmic clinical practice.10 TG has the potential to strengthen the capacity in the drive to build health-care infrastructure.10 The advantage lies in the potential to provide rural populations that have limited access to ophthalmic care, with better care through early diagnosis and prevention of vision related morbidity.8 Some have proposed that internet-based systems combined with TG diagnoses will form a new frame-work to treat both chronic and acute stage patients.11
Studies have evaluated the role of TG assessment. For example, a study assessing the feasibility of TG applications found that patients preferred the remote assessment due to the reduced travel, reduced cost and time savings.12 However, image quality was poorer with TG compared to a university-based clinical examination center.12 Another study evaluated the use of stereo digital images at primary care centers for review by specialists for glaucoma assessment and found general agreement in the vertical cup-to-disk ratio (VCDR) relative to conventional color stereo-pair slides.13 The study also found that some images could not be assessed and recommended that a larger study population be analyzed to determine the general overall effectiveness.13 While the effectiveness, practicality and usage of teleophthalmology techniques are generally accepted, the ability to diagnose glaucomatous ocular disease relative to the clinical slit lamp examination remains unknown.
The current study compares a web-based teleophthalmology technique (TG) to the clinical assessment by an ophthalmologist, for glaucoma screening among diabetic patients in a rural African district.
MATERIALS AND METHODS
Ethics approval
Ethics approval for this study was granted by the Aga Khan University Research Ethics Committee as part of the Faculty of Health Sciences Medical College in Nairobi, Kenya.
Patient selection
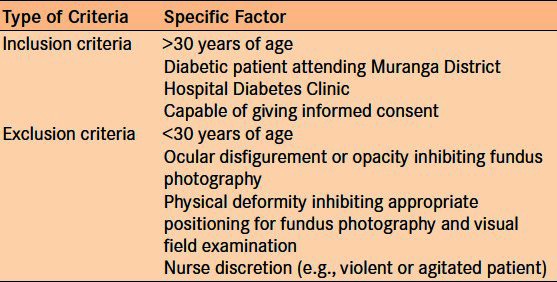
The study was carried out in the Muranga District Hospital diabetic and ophthalmology clinics. A precision-based method was used to calculate the appropriate sample size. For an expected sensitivity of 95% and lower limit of 85%, 93 glaucoma cases were needed. A previous study showed that 32% of diabetic patients in Kenya had POAG.14 Multiplying the two values together, 291 patients were recruited to the study. All patients above the age of 30 years who attended the Muranga Hospital Diabetic Clinic between August 2011 and October 2011 and were capable of giving consent were included in the study. Anyone under the age of 30 years, patients with ocular anatomy (natural or traumatic) inhibiting adequate fundus photography and those with physical deformities that inhibit proper positioning for fundus photography and visual field examination were excluded [Table 1]. Patients were also excluded as needed at the discretion of nurses in cases of potential for aggressive behavior or violence. Blurry fundus photos that were not conducive to TG assessment were excluded.
Table 1.
Inclusion and exclusion criteria
Patient prescreening
After obtaining informed consent, patients were escorted to an eye clinic where both a general medical and ocular history was performed by a trained Ophthalmic Assistant (OA) using a predetermined form [Appendix]. The OAs was trained on history taking prior to the study at the Aga Khan University Hospital, Nairobi. Initial ocular assessment conducted by the OA included visual acuity, external ocular exam with a pen torch, IOPs with Tonopen (Reichert Technologies, Depew, NY, USA), Frequency Doubling Technology (FDT) visual field screening perimetry (screening C-20 program), and fundus photography [Appendix]. A photograph of the visual field test printout was uploaded to the computer. Stereo images of fundus were acquired after dilating the pupils with 1% tropicamide. All fundus photography was taken by a Topcon 777 (Topcon Corp., Tokyo, Japan) three field fundus camera at 45°. Each 172 MB (24 bits/pixel) file was 16:1 lossy compressed to a 1.1 MB (1.5 bits/pixel) JPEG image. The OA uploaded the history form, the examination form, the color photographs and the visual fields onto a secure patient data website operated by Secure Diagnostic Imaging (SDI) based in Canada at the University of Alberta (www.teleophthalmology.com).14 The software, to date, has already been validated for diabetic retinopathy and is utilized for TG at the University of Alberta, Canada.15,16 An internet modem from Safaricom Limited was used to access the website. Patients were subsequently subjected to both slit lamp and teleophthalmology examination and results were compared.
Protocol #1: Slit lamp examination
Following pre-screening, each patient was seen by a comprehensive ophthalmologist based in the district. The ophthalmologist reviewed the history form, the examination form and the FDT print out and subsequently completed a dilated fundus exam to diagnose and grade glaucoma, diabetic retinopathy and age-related macular degeneration using the pre-determined form [Appendix]. Lens opacity was recorded based on the Lens Opacities Classification System III classification.17 During the slit lamp examination, a 90D lens was used to examine the optic nerve in order to record the VCDR and any other features of focal glaucomatous disc damage (notching, rim hemorrhage, peri-papillary atrophy).
Protocol #2: Remote teleophthalmology examination
After uploading the data onto the SDI website, a glaucoma specialist based in Nairobi, reviewed the history form, the examination form, the color photographs, and the visual fields. The specialist viewed the fundus pictures in 3D and manually outlined the rim edge and the cup edge. The software then automatically calculated the VCDR. The specialist also indicated any observed focal rim changes, including rim notching, hemorrhage, and peri-papillary atrophy.
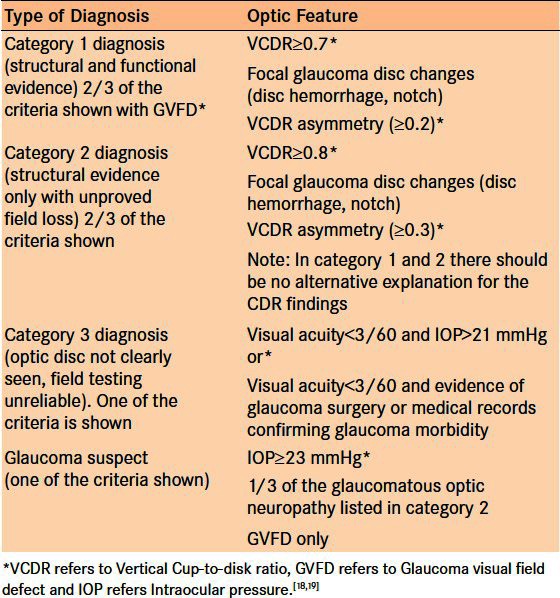
The case definition for glaucoma utilized a combination of the models proposed by Foster et al. and Jonasson et al. for cross-sectional epidemiological research in which the term glaucoma is reserved for people with established, visually significant optic nerve damage.18,19 As such, a positive glaucoma diagnosis in the TG analysis was based on a synthesis of history, nerve examination, IOP measurement, and FDT result [Table 2]. The models envisaged that cases of glaucoma would be classified according to three levels of evidence. For a summary of the criteria used to the diagnose glaucoma, please refer to Table 2.
Table 2.
Levels of glaucoma case definition
Blinding protocol
To ensure a fair comparison, the district comprehensive ophthalmologist doing the in-person fundus exam and glaucoma specialist doing the remote grading were blinded from each other's findings. Data obtained from the clinical examination, and the SDI program software was entered in to Microsoft Excel and SPSS 11.0 (IBM Corp, New York, NY, USA) for analysis and comparison.
Statistical analysis
TG and the clinical slit lamp examination were compared using predictive values. In order to analyze the reproducibility of measurement and diagnoses between the two assessments, the Κ statistic was calculated; in its determination, the unit of analysis was individual eyes. Because results from both eyes for both the TG and the clinical slit lamp protocols were used inducing some intra-patient clustering, the standard error was adjusted in the random effects model using the bootstrapping method described by Landis and Koch.20 In determining, the VCDR assessment Κ value, VCDR values within 0.1 were considered in full agreement. For all other analyses, exact agreement was used. Eyes with hazy media on the clinical exam or upgradeable photographs were excluded from the Κ analysis. Κ values above 0.81 illustrate almost perfect strength of agreement, beyond chance, between 0.61-0.80 illustrates substantial agreement, between 0.41-0.60 illustrates moderate agreement, between 0.21-0.40 illustrates fair agreement and between 0-0.20 illustrates slight agreement.20
RESULTS
Study population analysis
The study included 314 patients with a mean age 62 ± 11.6 years. Sixty percent of patients reported a history of hypertension 5% had heart diseases and 5% had asthma, 4% reported consumption of alcohol, and 1% had a history of smoking [Figure 1]. Six percent of the included patients had a history of prior eye surgery and 5% reported a family history of blindness [Table 3]. Four percent of patients reported previously diagnosed glaucoma, and 5% were already using the glaucoma medications. Three hundred and fourteen patients were clinically examined, and 308 patients underwent TG screening. Three hundred and six patients underwent both the clinical examination and TG assessment [Figure 2]. Out of 309 TG optic nerve photos, 74 (24%) were deemed ungradeable. Of the upgradeable cases, 39 were ungradeable due to media opacities: 22 cataracts, 10 corneal opacities, and 7 post-cataract posterior capsular opacities [Table 4]. The other 35 cases were ungradeable due to poor pupil dilation, unsatisfactory photography, and uncooperative patients.
Figure 1.
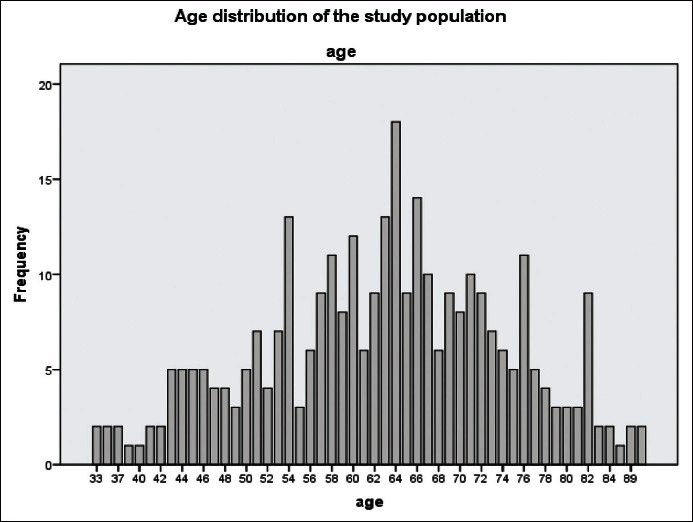
Age distribution of study population
Table 3.
Patient population overview
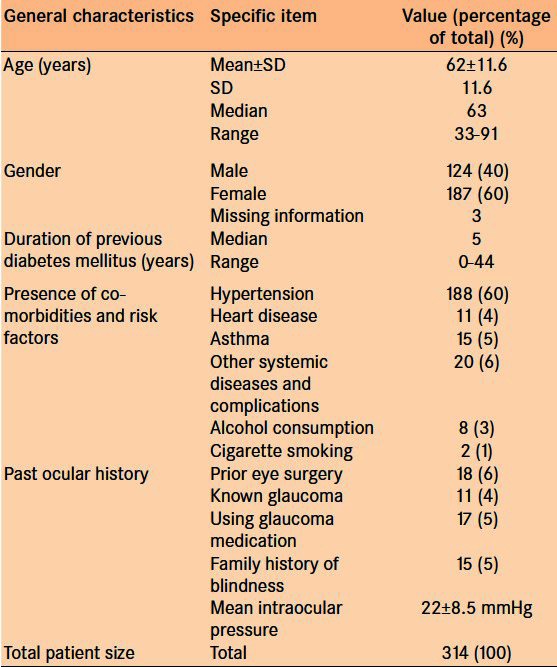
Figure 2.
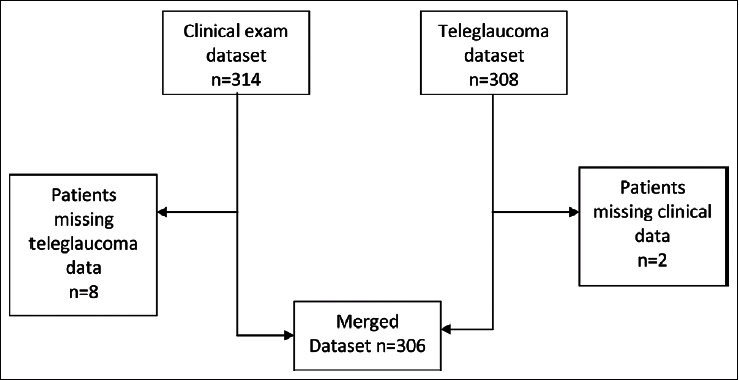
Merged data set compilation
Table 4.
Reasons for fundus photograph ungradability n = 37

Comparison of the VCDR grading of the optic nerve head
The VCDR Κ score of agreement (VCDR within ± 0.1) between the TG analysis and the clinical slit lamp examination was 0.55 with a 95% confidence interval (CI) between 0.50 and 0.61 indicating moderate agreement [Table 5].
Table 5.
Comparison of the agreement in diagnosis of clinical features between teleglaucoma and clinical slit lamp examination in the analysis of both patient eyes
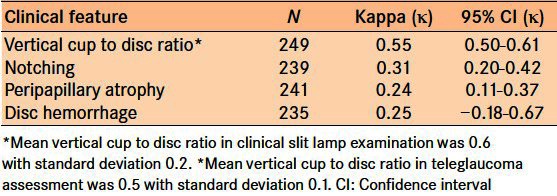
Comparison of the detection of focal glaucoma damage
Three types of focal glaucoma damage analysis were conducted: notching, peripapillary atrophy and disc hemorrhage. Agreement between the TG analysis and the clinical slit lamp examination for notching, peripapillary atrophy, and disc hemorrhage was 0.31, 0.24, and 0.25 respectively, indicating fair agreement for these features [Table 5].
Determination of the diagnostic precision for the FDT
In comparing the diagnostic precision of FDT to detect glaucoma in patients with glaucomatous disc damage, as shown with a VCDR > 0.7, the Κ score of agreement between the TG analysis and the clinical slit lamp examination was 0.84 indicating substantial agreement [Table 6].
Table 6.
Comparison of the agreement in diagnosis between teleglaucoma and clinical slit lamp examination in the analysis of both patient eyes
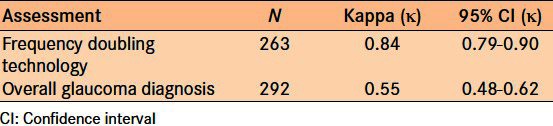
Comparison of the ability to diagnose glaucoma
In comparing the ability to diagnose glaucoma, where diagnosis was based on a synthesis of history, nerve examination, IOP measurement, and FDT results, the Κ score of agreement between the TG analysis and the clinical slit lamp examination was 0.55 with a 95% confidence interval between 0.48 and 0.62 indicating moderate agreement [Table 6].
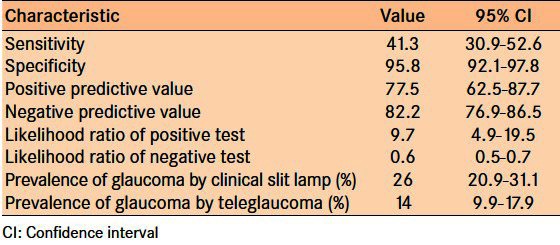
Relative to clinical slit lamp examination, TG has a 41.3% (95% CI: 30.9-52.6) sensitivity for diagnosing glaucoma and 89.6% (95% CI: 92.1-97.8) specificity for diagnosing glaucoma; a diagnosis of glaucoma using TG assessment has a 77.5.8% (95% CI: 62.5-87.7) positive predictive value and a 82.2% (95% CI: 76.9-86.5) negative predicative value [Table 7]. The likelihood ratio of a positive diagnosis of glaucoma with TG relative to clinical slit lamp examination was 9.7 (95% CI: 4.9-19.5), and the likelihood ratio of a negative diagnosis was 0.6 (95% CI: 0.5-0.7) [Table 7]. The prevalence of glaucoma analyzed by clinical slit lamp examination was 26%; the prevalence of glaucoma by TG assessment was 14%.
Table 7.
Teleglaucoma ability to diagnose glaucoma relative to the clinical slit lamp examination
DISCUSSION
When comparing the ability to diagnose glaucoma between the clinical slit lamp examination and TG assessment, results from this study showed moderate agreement. A positive TG diagnosis of glaucoma carried a 77.5% positive predictive value, and a negative TG diagnosis carried an 82.2% negative predicative value both the relative to clinical examination. In comparing TG analysis with the clinical slit lamp examination, VCDR results showed moderate agreement while other diagnostic features including the identification of notching, peripapillary atrophy, and disc hemorrhage, showed fair agreement. In comparing the use of the FDT to detect glaucoma in the presence of VCDR > 0.7, results showed substantial agreement.
Limitations and implications for future study
Out of 309 photos taken, 74 (24%) were deemed unreadable inhibiting assessment and follow-up. This value illustrates that while a TG approach may increase patient access to glaucoma care, some screening examinations may prove ineffective due to challenges with patient cooperation, media opacities, and technology. Poor quality information including blurry photography can severely limit the effectiveness of TG assessment.
In cases when an appropriate clarity of photography was achieved, there still exists some concern surrounding false negatives indicating that a patient with glaucoma normally diagnosed in the clinical slit lamp examination could be missed in TG assessment. The discrepancy in agreement between the TG analysis and the slit lamp examination may also be the result of differences in physician training and variations in the grading approach. The ophthalmologist seeing the patient in person was a general ophthalmologist whereas, the ophthalmologist grading virtual data was a glaucoma specialist.
In the TG arm, the VCDR was electronically generated by a software application that has not yet been validated. While the SDI software permits a semi-automated calculation of the VCDR, the grader still outlines the disc and cup edge; as such, the calculation is likely different from the manual estimation of the VCDR of the uploaded images with stereo viewer by a glaucoma specialist. Qualitative reports from glaucoma specialists suggest that the lower VCDR presentations could be overrated, and the higher VCDR presentations could be underrated by automatic software based analysis. Future study should seek to validate this semi-automated SDI-based VCDR tool relative to assessment of the nerve by a glaucoma specialist without using the tool.
While this study shows moderate agreement between the clinical examination and TG assessment, further research is still needed. We suggest that future studies should not only involve a larger patient population in the analysis, but that the same ophthalmologist completing the slit lamp examination be one grading images virtually. By using the same ophthalmologist, with some time lapse to reduce recall bias, inter-observer variation and the effect of variable training can be eliminated.
Finally, our population was enrolled from a diabetic clinic and therefore, does not provide true prevalence of glaucoma in the population. Future investigation comparing TG assessment and the clinical slit lamp examination could be conducted in population-based studies in order to determine the power of TG at detecting cases.
Relation to the literature
As discussed above, while the effectiveness, practicality, and usage of teleophthalmology techniques are generally accepted, there exists a dearth of knowledge surrounding its ability to diagnose glaucomatous ocular disease in particular. We were unable to find other studies to compare our findings. Our study has helped shed light upon the ability for teleophthalmology applications specifically within the field of glaucoma and in the assessment of the optic nerve damage.
CONCLUSIONS
In conclusion, virtual grading of patient history combined with structure and function information to diagnose glaucoma produces moderate agreement with in the person clinical examination. Independent assessment of the VCDR showed moderate agreement while assessment of notching, peripapillary atrophy, and disc hemorrhage produced fair agreement. Furthermore, the use of FDT C-20 screening protocol to detect glaucoma in the presence of glaucomatous disc damage with a ratio of greater than 0.7 proved to have substantial agreement and can be used in screening and diagnosis. We also conclude that poor quality information including blurry fundus photography can severely limit the ability of TG assessment to diagnose optic nerve damage and glaucoma. Although further validation is needed, the TG approach provides a novel and promising method to diagnose glaucoma, a major cause of ocular morbidity throughout the world.
ACKNOWLEDGMENTS
A grant for this study was obtained from the Aga Khan University Research council with supplement from Pfizer Canada. The SDI software was provided at no charge by the directors of the company. Special thanks are given to Abshir Moalin who was invaluable in the software set up and support throughout the study. We also thank the Ophthalmic Assistants Florence Mutisya and Beatrice Nyaga who took the photographs, filled the history and examination forms and uploaded all information to the website. We thank Anne Kinyanjui, the Muranga District Hospital Ophthalmic Nurse, for booking patients and assisting in the pre-exam work up. We appreciate all patients who participated in the study as well as the administration of Muranga District hospital for allowing us to use their facility. Finally, we thank Dr. Michael Gichangi of the Kenya Division of Ophthalmic Services, for facilitating the collaboration with the Muranga District Hospital Eye Clinic.
Appendix
Footnotes
Source of Support: Nil
Conflict of Interest: None.
REFERENCES
- 1.Quigley HA, Broman AT. The number of people with glaucoma worldwide in 2010 and 2020. [Accessed 2012 Nov 27];Br J Ophthalmol. 2006 90:262–7. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cook C, Foster P. Epidemiology of glaucoma: What's new. [Accessed 2012 Nov 27];Can J Ophthalmol. 2012 47:223–6. doi: 10.1016/j.jcjo.2012.02.003. [DOI] [PubMed] [Google Scholar]
- 3.Cook C, Cockburn N, van der Merwe J, Ehrlich R. Cataract and glaucoma case detection for Vision 2020 programs in Africa: An evaluation of 6 possible screening tests. [Accessed 2012 Nov 27];J Glaucoma. 2009 18:557–62. doi: 10.1097/IJG.0b013e318193c15b. [DOI] [PubMed] [Google Scholar]
- 4.Cedrone C, Mancino R, Cerulli A, Cesareo M, Nucci C. Epidemiology of Primary Glaucoma: Prevalence, Incidence, and Blinding Effects. Progress in Brain Research. 2008;173:3014. doi: 10.1016/S0079-6123(08)01101-1. [DOI] [PubMed] [Google Scholar]
- 5.Rotchford AP, Johnson GJ. Glaucoma in Zulus: A population-based cross-sectional survey in a rural district in South Africa. [Accessed 2012 Nov 27];Arch Ophthalmol. 2002 120:471–8. doi: 10.1001/archopht.120.4.471. [DOI] [PubMed] [Google Scholar]
- 6.Buhrmann RR, Quigley HA, Barron Y, West SK, Oliva MS, Mmbaga BB. Prevalence of glaucoma in a rural East African population. [Accessed 2012 Nov 27];Invest Ophthalmol Vis Sci. 2000 41:40–8. [PubMed] [Google Scholar]
- 7.Wadhwa SD, Higginbotham EJ. Ethnic differences in glaucoma: Prevalence, management, and outcome. [Accessed 2012 Nov 19];Curr Opin Ophthalmol. 2005 16:101–6. doi: 10.1097/01.icu.0000156137.28193.48. [DOI] [PubMed] [Google Scholar]
- 8.Kassam F, Amin S, Sogbesan E, Damji KF. The use of teleglaucoma at the University of Alberta. [Accessed 2012 Nov 19];J Telemed Telecare. 2012 18:367–73. doi: 10.1258/jtt.2012.120313. [DOI] [PubMed] [Google Scholar]
- 9.Pasquale LR. Telemedical Strategies for Glaucoma: Advances in Technology are Helping Ophthalmologists Expand their Diagnostic and Therapeutic Boundaries. Glaucoma Today. 2009 Jul-Aug;:23–6. [Google Scholar]
- 10.Johnston K, Kennedy C, Murdoch I, Taylor P, Cook C. The cost-effectiveness of technology transfer using telemedicine. [Accessed 2012 Nov 19];Health Policy Plan. 2004 19:302–9. doi: 10.1093/heapol/czh035. [DOI] [PubMed] [Google Scholar]
- 11.Go K, Kashiwagi K, Tanabe N. Deployment of Remote-Control Slit Lamp Microscopes and Its Effect On Local Medical Service. Proceedings of Society of Instrument and Control Engineers Annual Conference. 2010:1929–32. [Google Scholar]
- 12.Tuulonen A, Ohinmaa T, Alanko HI, Hyytinen P, Juutinen A, Toppinen E. The application of teleophthalmology in examining patients with glaucoma: A pilot study. [Accessed 2012 Nov 19];J Glaucoma. 1999 8:367–73. [PubMed] [Google Scholar]
- 13.Li HK, Tang RA, Oschner K, Koplos C, Grady J, Crump WJ. Telemedicine screening of glaucoma. [Accessed 2012 Nov 19];Telemed J. 1999 5:283–90. doi: 10.1089/107830299312032. [DOI] [PubMed] [Google Scholar]
- 14.Gichuhi S, Kollmann K, Choksey PV. The prevalence of primary open angle glaucoma in black diabetics. East Afr J Ophthalmol. 2000;10:24–25. [Google Scholar]
- 15.Rudnisky CJ, Tennant MT, Weis E, Ting A, Hinz BJ, Greve MD. Web-based grading of compressed stereoscopic digital photography versus standard slide film photography for the diagnosis of diabetic retinopathy. [Accessed 2012 Nov 19];Ophthalmology. 2007 114:1748–54. doi: 10.1016/j.ophtha.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 16.Tennant MT, Greve MD, Rudnisky CJ, Hillson TR, Hinz BJ. Identification of diabetic retinopathy by stereoscopic digital imaging via teleophthalmology: A comparison to slide film. [Accessed 2012 Nov 19];Can J Ophthalmol. 2001 36:187–96. doi: 10.1016/s0008-4182(01)80039-9. [DOI] [PubMed] [Google Scholar]
- 17.Chylack LT, Jr, Wolfe JK, Singer DM, Leske MC, Bullimore MA, Bailey IL, et al. The Lens Opacities Classification System III. The Longitudinal Study of Cataract Study Group. [Accessed 2012 Nov 27];Arch Ophthalmol. 1993 111:831–6. doi: 10.1001/archopht.1993.01090060119035. [DOI] [PubMed] [Google Scholar]
- 18.Foster PJ, Buhrmann R, Quigley HA, Johnson GJ. The definition and classification of glaucoma in prevalence surveys. [Accessed 2012 Nov 27];Br J Ophthalmol. 2002 86:238–42. doi: 10.1136/bjo.86.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jonasson F, Damji KF, Arnarsson A, Sverrisson T, Wang L, Sasaki H, et al. Prevalence of open-angle glaucoma in Iceland: Reykjavik Eye Study. [Accessed 2012 Nov 27];Eye (Lond) 2003 17:747–53. doi: 10.1038/sj.eye.6700374. [DOI] [PubMed] [Google Scholar]
- 20.Landis JR, Koch GG. The measurement of observer agreement for categorical data. [Accessed 2012 Nov 27];Biometrics. 1977 33:159–74. [PubMed] [Google Scholar]


