Abstract
Purpose:
To assess blood flow velocity in newly diagnosed indigenous black-skinned Africans with primary open angle glaucoma (POAG).
Materials and Methods:
Prospective case-control study at Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria on 50 newly diagnosed POAG patients (POAG group) and 50 control patients (control group). Ocular Doppler Color Imaging was performed on subjects in the supine position using 9 MHz linear array transducer of a Fukuda Denshi Ultrasound. The Peak Systolic Velocity (PSV) and End Diastolic Velocity (EDV) values were obtained by finding the average of two readings each for the ophthalmic artery (OA) and central retinal artery (CRA). Resistive Index (RI) was calculated as (PSV − EDV)/PSV. Data were analyzed and statistical significance was defined at P < 0.05.
Results:
The mean intraocular pressure (IOP) for the POAG group and control group was 28.1 ± 7.4 mmHg and 16.6 ± 2.0 mmHg, respectively (P < 0.001). The mean PSV for OA was 31.35 cm/s in POAG group and 37.61 cm/s for the control group (P < 0.001). The EDV for both OA and CRA were significantly lower in glaucoma patients as compared with the corresponding values in the control group (P < 0.001, both comparisons). The mean RI in the OA was 0.71 ± 0.05 and 0.63 ± 0.03 for the POAG and control group groups, respectively (P < 0.001). The increase in IOP in the POAG group was statistically significantly negatively correlated with PSV and EDV and positively correlated with RI for both OA and CRA.
Conclusion:
The outcomes of this study indicate that ocular blood flow alterations including reductions in PSV and EDV and increase in RI of the OA and CRA are present in black-skinned Africans with POAG.
Keywords: Black-skinned Africans, Case-Control Group, Case-Control, Glaucoma, Intraocular Pressure, Ocular Doppler, Resistive Index
INTRODUCTION
Glaucoma is a leading cause of blindness worldwide, accounting for 16.7% of blindness in Nigerian adults aged ≥40 years.1,2 Regional surveys in Osun state in southwestern Nigeria estimated that 11.1–14.3% of blindness was caused by glaucoma.3,4,5 Glaucoma is a disorder with common characteristics of optic nerve degeneration associated with typical visual field defect and, usually, associated with elevated intraocular pressure (IOP) greater than 21 mmHg.6 Glaucoma results from mechanical blockage of the flow of aqueous humor within the eye or limited egress of aqueous from the eye, leading to an increase in IOP and an excessive distending force that can result in excavation of the optic nerve (cupping) and loss of vision.7 The primary open angle variant is the most common type of glaucoma. Primary open angle glaucoma (POAG) runs a faster and more aggressive course and leads to blindness more rapidly in black-skinned individuals as compared to Caucasians.8
Glaucomatous optic nerve damage has been reported even in the absence of increased IOP. Growing evidence from clinical studies indicates that circulatory abnormalities can be involved in the pathogenesis of glaucomatous optic nerve disease and that vascular factors leading to ocular nerve ischemia may play a role.9–11 The introduction of orbital Color Doppler Imaging (CDI) in 1989 by Erickson et al.,11 presented the opportunity for assessment of orbital blood vessels. CDI is a non-invasive ultrasonic method for qualitative and quantitative assessment of blood flow velocity.12 It employs the simultaneous use of B-scan and Doppler imaging to locate and assess blood flow velocity in orbital blood vessels. Color Doppler Imaging is the most widely used imaging technology for investigating ocular blood flow in humans.13 Despite the known discrepancy in the course of the POAG between races, studies on ocular blood flow velocity on indigenous black-skinned individuals with POAG are lacking. This study was conducted to assess the velocity of blood flow in newly diagnosed indigenous black-skinned African patients with POAG in a Nigerian tertiary hospital.
MATERIALS AND METHODS
This is a prospective case-control group study conducted in the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria. Approval of the study protocol was granted by the institutional ethics committee. The study subjects included 50 consecutive newly diagnosed patients with POAG (POAG group) and 50 volunteers with normal IOP (10-21 mmHg) with no clinical evidence of glaucoma served as the control group. Cigarette smokers, diabetics, hypertensives, and patients on either β-blocker or sympathomimetic drugs were excluded from the study. Patients with cataract or corneal opacity precluding posterior segment assessment were also excluded.
The diagnosis of POAG was based on the presence of glaucomatous optic nerve head damage on funduscopy (Keeler direct ophthalmoscope), characteristic glaucomatous visual field defect (Octopus automated perimetry, Haag-Streit AG, Koeniz Switzerland), raised IOP >22 mmHg (Goldmann Applanation Tonometer), open and normal anterior chamber angles on gonioscopy (Goldmann 3-mirror lens), and absence of characteristic signs of other possible causes of open angle glaucoma on slit lamp examination (Haag-Streit AG, Koeniz Switzerland).
Ocular Doppler Color Imaging was conducted on all subjects in the supine position after the procedure was explained to them and consent was obtained. All subjects, in both groups, were examined between 12:00–4:00 PM. With eyes closed and gaze directed at the ceiling, a thick layer of acoustic gel was applied to the closed upper eyelid and a 9-MHz linear array transducer of a Fukuda Denshi Ultrasound (Fukuda Denshi Co., Ltd., Tokyo, Japan) was placed on the temporal part of the closed upper eyelid with the examiner's hand resting on the orbital margin to minimize pressure on the globe. The right eyes of both groups were used for the study. Pulsed Doppler Sonography was performed with the gain optimized such that color was seen within the vessels and there were no color artifacts, allowing the detection of low velocities. The sample volume depth was set at about 40 mm when imaging the ophthalmic artery (OA). The Doppler sample gate (≤2 mm) was then placed at the centre of the detected vessel to image the spectral pattern. As orbital vessels are frequently parallel to the ultrasound beam, an angle correction of ≤60° was used when needed and the color box steering was sometimes required to attain this. Low wall filter settings were used. To examine the OA, the sample volume was oriented nasally and superior to the optic nerve, OA is just lateral to and abuts the visible hyporeflective stripe representing the nerve, while the central retinal artery (CRA) was imaged in the shadow of the optic nerve, the sample volume was placed about 3 mm behind the surface of the optic disc.14 The Peak Systolic Velocity (PSV) and End Diastolic Velocity (EDV) values were obtained by taking the velocity reading at the peak of the spectral wave pattern and that at the wavetrough, respectively. Two readings of each artery were obtained and the average was taken, this was to minimize intraobserver error. Resistive index (RI) was calculated as the Porcelouts ratio for both OA and CRA as (PSV − EDV)/PSV.15
Data obtained were recorded and analyzed using with SPSS version 13 (IBM Inc., Armonk, NY, USA) and results are presented as range, mean, and standard deviations for POAG group and control group. Comparison of variables was performed with Students t-test and statistical significance was defined as p < 0.05.
RESULTS
The study sample consisted of 50 patients in the POAG group and 50 patients in the control group. The age range for the 50 cases was 16–88 years, while that for the control group was 18–85 years with a mean of 60.36 ± 17.68 and 60.12 ± 17.51 years, respectively. Twenty-eight (56%) of the glaucoma patients were females and 22 were males (44%). There was no statistically significant difference between group in age or gender. The mean IOP was 28.1 ± 7.4 mmHg for the POAG group and 16.6 ± 2.0 mmHg for the control group (P < 0.001).
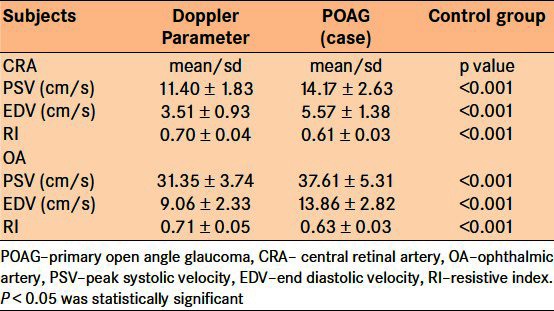
The range of PSV and EDV for both OA and CRA were faster in the control group as compared to the range in the POAG group (P < 0.001). The RI range of the CRA in POAG group was 0.62-0.80; this was higher than 0.53-0.66 RI range in the control group [Table 1]. The mean PSV (cm/s) in the CRA of the POAG group was 11.40 ± 1.83 as compared with 14.17 ± 2.63 in the control group. The mean PSV (cm/s) in OA of POAG group was 31.35 ± 3.74 as compared with the mean PSV of 37.61 ± 5.37 in control group [Table 2]. The mean EDV (cm/s) of 3.51 ± 0.9 obtained from the CRA of the POAG group was also significantly lower than the corresponding value of 5.57 ± 1.38 obtained from the control group. Mean EDV (cm/s) of 9.06 ± 2.33 in the OA of the POAG group was slower than the 13.86 ± 2.86 obtained for the OA of the control group. The degree of resistance to flow in the OA was significantly higher than that in the control group, as inferred from the mean RI values of 0.70 and 0.61 in POAG group and control group, respectively. The RI obtained from the CRA was also significantly higher when compared with the RI of the control group [Table 2].
Table 1.
Range of ocular blood flow velocity and resistive index in patients with primary open angle glaucoma and control group
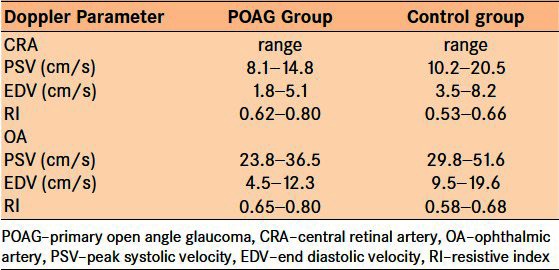
Table 2.
Mean ocular blood flow velocity and resistive index in the primary open angle glaucoma and control group
A high negative correlation (Pearson Correlation of −0.743) was recorded between the IOP and PSV of the OA in POAG group. A significant positive correlation between IOP and RI in the POAG group was found in OA and CRA. Similarly, the EDV values correlated negatively with the IOP values in the OA of POAG group [Figure 1]. Figure 2 shows a significant positive correlation between IOP and RI in the OA of POAG group. There was negative correlation of the values obtained for PSV and EDV in the CRA of POAG group in comparison with IOP values [Figures 3 and 4]. A high positive correlation (coefficient of +0.794) was found between RI of the CRA in the POAG group.
Figure 1.
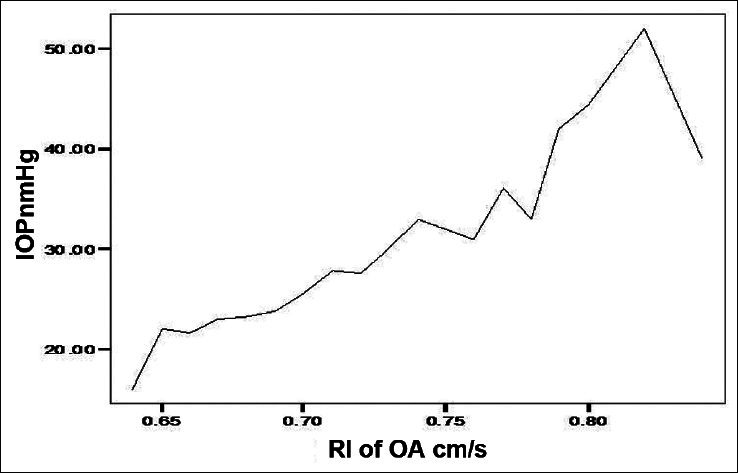
Relationship between end diastolic velocity (EDV) and intraocular pressure (IOP) in the ophthalmic artery (OA) of patients with primary open angle glaucoma (POAG)
Figure 2.
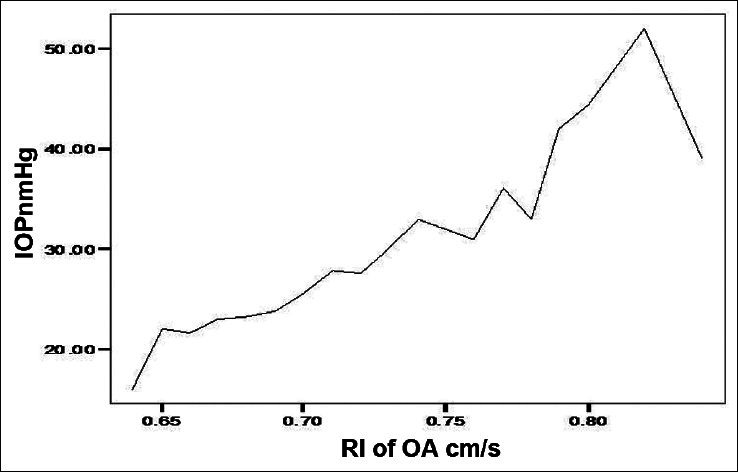
Resistive index (RI) and intraocular pressure (IOP) in ophthalmic artery (OA) of POAG
Figure 3.
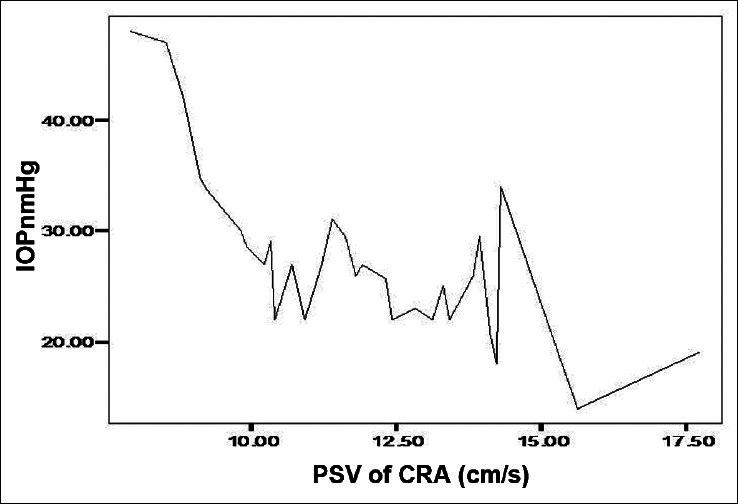
Relationship between peak systolic velocity (PDV) and intraocular pressure (IOP) in the central retina artery (CRA)
Figure 4.
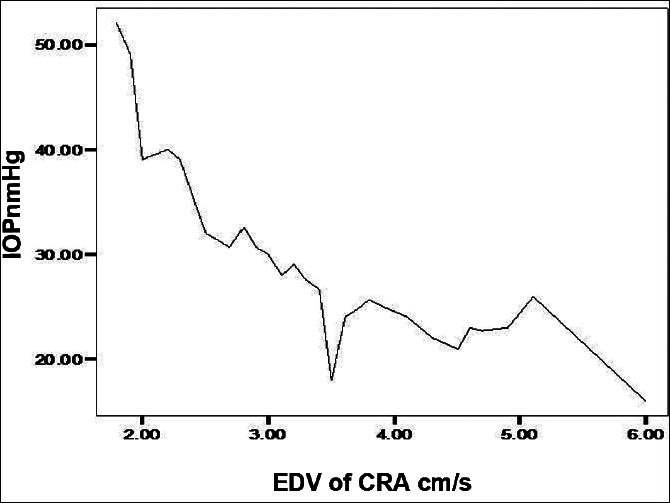
Relationship between end diastolic velocity (EDV) and intraocular pressure (IOP) in the central retina artery (CRA)
DISCUSSION
A primary problem in the optic nerve circulation resulting from localized organic changes in the blood vessels of the nerve with or without a low perfusion pressure is one of the likely mechanisms implicated in the pathogenesis of glaucomatous damage. The OA, central retina artery and the short posterior ciliary artery are important vessels in providing evidence of the influence of vascular factors in pathogenesis of glaucomatous optic neuropathy.6 Doppler velocimetry is a reproducible technique for evaluation of ocular vessels.16 The blood flow velocity in two of these arteries in glaucomatous and non glaucomatous eyes form the focus of the present study in indigenous Africans.
Significantly slower mean blood flow velocities (e.g., PSV and EDV) were recorded in the OA and CRA of POAG patients as compared with the control group in this study. This finding is similar to a previous report by Rojanapongpun et al., who reported slower mean PSV and EDV of the OA in comparison with that in the control group in the same Asian population.17 They reported mean PSV and EDV of 43.86 ± 1.32 cm/s and 11.92 ± 0.44 cm/s respectively in the control group as compared to 39.04 ± 1.00 cm/s and 10.25 ± 0.33 cm/s, respectively, in glaucoma cases.17 Similarly, slower OA as well as central retinal artey PSV and EDV have been reported by different studies.6,12,18,19 Mokbel et al.,20 studied ocular color Doppler imaging of the CRA in Egyptian patients with POAG and found slower EDV in the POAG group as compared with the control group subjects in the same population.
RI has the advantage that its value does not depend on Doppler angle contrary to PSV and EDV; this is because RI is a ratio and hence its absolute value can be used for comparison of results among studies since values are constant irrespective of Doppler angle change.15 The increases in RI in both OA and CRA in glaucoma as compared to the control group have been previously reported. High RI implies an increased peripheral resistance to flow in causing direct impedance to blood flow in the retinal circulation. Butt et al.21 recorded increased RI seen in the CRA in glaucoma patients and suggested that it may have been caused by longstanding raised IOP that caused direct impedance to blood flow in the retinal circulation. This is consistent with our finding of positive correlation between IOP and RI in OA of POAG in the current study.
The alteration in ocular blood flow noted in this study, that is reduction in PSV and EDV as well as increase in RI of the OA and CRA in POAG in the tropical African population, is similar to previous reports in other Caucasian and Asian populations. Baseline ocular blood flow values in POAG patient may be useful in determining the severity of damage as well as monitor disease progress in addition to visual field analysis. This study is limited in that the measurement of ocular blood flow velocity was restricted to OA and CRAO alone, thus excluding the parameters in the posterior ciliary vessels. This study being a cross-sectional study on newly diagnosed POAG group yet to commence antiglaucoma medications could not assess the effect of lowering IOP on the blood flow parameters studied. Further studies are suggested in a similar population to overcome the above mentioned limitations.
ACKNOWLEDGMENT
We acknowledge the receipt of part funding by the Management of the Obafemi Awolowo University Teaching Hospital, Ile-Ife, Nigeria.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Resnikoff S, Pascolin D, Etya’ale D, Kocur I, Pararajasegaram R, Pokharel GP, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;84:844–51. [PMC free article] [PubMed] [Google Scholar]
- 2.Abdull MM, Sivasubramaniam S, Murthy GV, Gilbert C, Abubakar T, Ezelum C, et al. Causes of blindness and visual impairment in Nigeria: The Nigeria national blindness and visual impairment survey. Invest Ophthalmol Vis Sci. 2009;50:4114–20. doi: 10.1167/iovs.09-3507. [DOI] [PubMed] [Google Scholar]
- 3.Onakpoya OH, Adeoye AO, Akinsola FB, Adegbehingbe BO. Prevalence of blindness and visual impairment in Atakunmosa West Local Government area of southwestern Nigeria. Tanzan Health Res Bull. 2007;9:126–31. doi: 10.4314/thrb.v9i2.14315. [DOI] [PubMed] [Google Scholar]
- 4.Adeoti CO. Prevalence and causes of blindness in a tropical African population. West Afr J Med. 2004;23:249–52. doi: 10.4314/wajm.v23i3.28132. [DOI] [PubMed] [Google Scholar]
- 5.Adeoye A. Survey of blindness in rural communities of south-western Nigeria. Trop Med Int Health. 1996;1:672–6. doi: 10.1111/j.1365-3156.1996.tb00093.x. [DOI] [PubMed] [Google Scholar]
- 6.Sharma NC, Bangiya D. Comparative study of ocular blood flow parameters by colour doppler imaging in healthy and glaucomatous eye. Indian J Radiol Imaging. 2006;16:679–82. [Google Scholar]
- 7.Catalano RA, Belin M, Eames FA, Litoff D. Ocular emergencies. Philadelphia: WB sanders; 1992. Glaucoma Emergencies; pp. 261–187. [Google Scholar]
- 8.Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt JC, et al. Racial difference in the cause-specific prevalence of blindness in East Baltimore. N Engl J Med. 1991;325:1412–7. doi: 10.1056/NEJM199111143252004. [DOI] [PubMed] [Google Scholar]
- 9.Fechtner RD, Weinreb RN. Mechanisms of optic nerve damage in primary open angle glaucoma. Surv Ophthalmol. 1994;39:23–42. doi: 10.1016/s0039-6257(05)80042-6. [DOI] [PubMed] [Google Scholar]
- 10.Drance SM. The vascular factors in glaucoma. In: Bucci MG, editor. Glaucoma: Decision Making in Therapy. New York: Springer-Verlag; 1996. pp. 31–35. [Google Scholar]
- 11.Erickson SJ, Hendrick LE, Massaro BM, Harris GJ, Lewandowski MF, Foley WD, et al. Colour Dopper flow imaging of the normal and abnormal orbit. Radiology. 1989;173:511–6. doi: 10.1148/radiology.173.2.2678264. [DOI] [PubMed] [Google Scholar]
- 12.Lieb WE, Cohen SM, Merton DA, Shields JA, Mitchell DG, Goldberg BB. Colour Doppler Imaging of the eye and orbit. Technique and normal vascular anatomy. Arch Opthalmol. 1991;109:527–31. doi: 10.1001/archopht.1991.01080040095036. [DOI] [PubMed] [Google Scholar]
- 13.Wienreb RN, Harris A, editors. Section II: Clinical Measurement of Ocular Blood Flow. Amsterdam: Kugler Publications; 2009. Ocular Blood Flow in Glaucoma: The 6th consensus report of the World Glaucoma Association; pp. 21–2. [Google Scholar]
- 14.Zion IB, Harris A, Siesky B, Shulman S, McCranor L, Garzozi HJ. Pulsatile ocular blood flow: Relationship with flow velocities in vessels supplying the retina and choroids. Br J Ophthamol. 2007;91:882–4. doi: 10.1136/bjo.2006.108340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pourcelot L. Applications cliniques de l’examinen Doppler transcutane. INSERM. 1974;34:213–40. [Google Scholar]
- 16.de Oliveira CA, de Sá RA, Velarde LG, Marchiori E, Netto HC, Ville Y. Doppler velocimetry of the ophthalmic artery in normal pregnancy: Reference values. J Ultrasound Med. 2009;28:563–9. doi: 10.7863/jum.2009.28.5.563. [DOI] [PubMed] [Google Scholar]
- 17.Rojanapongpun P, Drance SM, Morrison BJ. Ophthalmic artery flow velocity in glaucomatous and normal subjects. Br J Ophthalmol. 1993;77:25–9. doi: 10.1136/bjo.77.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerr J, Nelson P, O’Brien C. A comparison of ocular blood flow in untreated primary open-angle glaucoma and ocular hypertension. Am J Ophthalmol. 1998;126:42–51. doi: 10.1016/s0002-9394(98)00074-9. [DOI] [PubMed] [Google Scholar]
- 19.Akarsu C, Bilgili MY. Colour Doppler imaging in ocular hypertension and open-angle glaucoma. Graefes Arch Clin Exp Ophthalmol. 2004;242:125–9. doi: 10.1007/s00417-003-0809-3. [DOI] [PubMed] [Google Scholar]
- 20.Mokbel TH, Shahin MM, El-Said EM, Abd EL-Ghaffar WM. Potential Diagnostic value of fluorescein angiography and color doppler imaginh in primary open angle glaucoma. Eur J Ophthalmol. 2009;19:957–62. doi: 10.1177/112067210901900610. [DOI] [PubMed] [Google Scholar]
- 21.Butt Z, O’Brien C, Mckillop G, Aspinall P, Allan P. Colour Doppler Imaging in untreated high-and normal-pressure open-angle glaucoma. Invest Ophthalmol Vis Sci. 1997;38:690–6. [PubMed] [Google Scholar]


