To investigate the risk of Plasmodium falciparum infection in a cohort of naturally exposed individuals, we assessed the time to first P. falciparum infection by polymerase chain reaction analysis of dried blood spots and found no age-related differences in infection risk.
Keywords: preerythrocytic immunity, endemic population, Plasmodium falciparum, infection risk, malaria
Abstract
Background In experimental models of human and mouse malaria, sterilizing liver stage immunity that blocks progression of Plasmodium infection to the symptomatic blood stage can be readily demonstrated. However, it remains unclear whether individuals in malaria-endemic areas acquire such immunity.
Methods In Mali, 251 healthy children and adults aged 4–25 years who were free of blood-stage Plasmodium infection by polymerase chain reaction (PCR) were enrolled in a longitudinal study just prior to an intense 6-month malaria season. Subsequent clinical malaria episodes were detected by weekly active surveillance and self-referral. Asymptomatic P. falciparum infections were detected by blood-smear microscopy and PCR analysis of dried blood spots that had been collected every 2 weeks for 7 months.
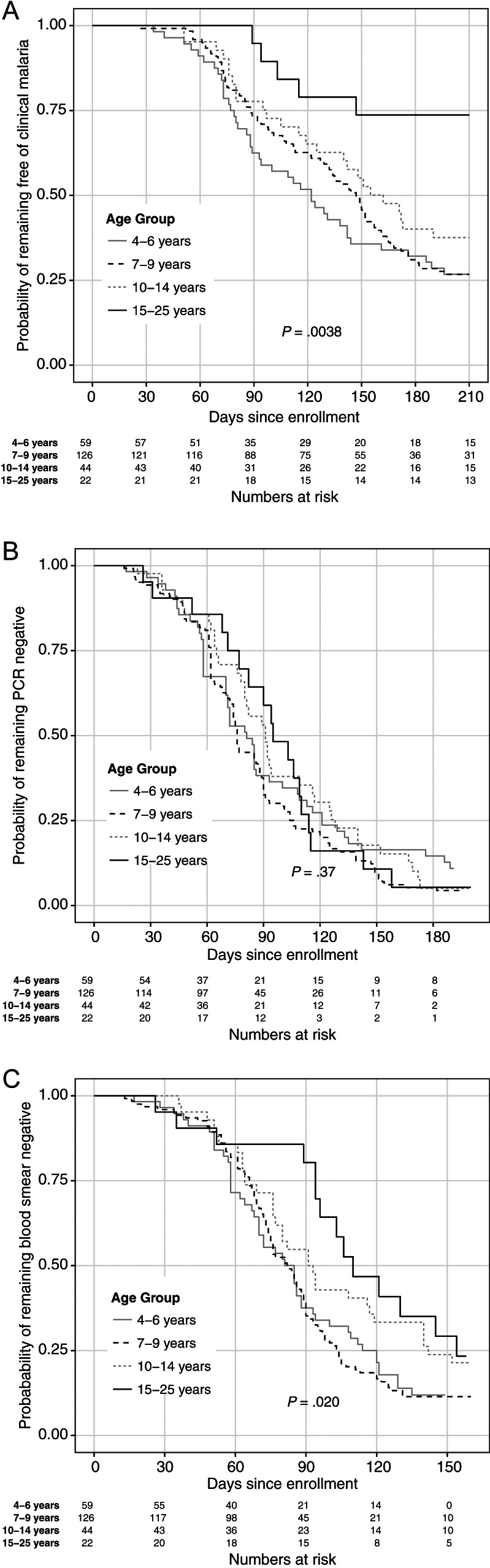
Results As expected, the risk of clinical malaria decreased with increasing age (log-rank test, P = .0038). However, analysis of PCR data showed no age-related differences in P. falciparum infection risk (log-rank test, P = .37).
Conclusions Despite years of exposure to intense P. falciparum transmission, there is no evidence of acquired, sterile immunity to P. falciparum infection in this population, even as clinical immunity to blood-stage malaria is clearly acquired. Understanding why repeated P. falciparum infections do not induce sterile protection may lead to insights for developing vaccines that target the liver stage in malaria-endemic populations.
Plasmodium falciparum malaria remains a major public health threat for which there is no licensed vaccine. In recent years, attention has shifted from the development of vaccines that would prevent or mitigate clinical disease caused by blood-stage infection to those that would induce sterile protection by targeting the preerythrocytic stages of infection [1]. Although sterilizing immunity to Plasmodium infection can be readily demonstrated in experimental models of both human and mouse malaria [2–4], evidence for naturally acquired, sterilizing immunity to P. falciparum in the endemic setting has been less clear. The high incidence of blood-stage reinfection after drug cure in adults would suggest that naturally exposed populations never fully achieve sterilizing immunity even after years of repeated and frequent infection [5], yet acquired immune responses specific for certain Plasmodium proteins expressed during the preerythrocytic stage of infection have been associated with reduced risk of P. falciparum infection in naturally exposed individuals [6]. Furthermore, the results of field studies directly addressing the relationship between age and risk of P. falciparum reinfection have been inconsistent, with several studies demonstrating reduced P. falciparum infection risk in older age groups [7–10], whereas others show no age-related differences in infection risk [11–14]. These prior field studies employed treatment-reinfection study designs, in which volunteers were cleared of any existing baseline parasitemia using standard antimalarial regimens prior to assessing reinfection risk. This approach could alter the subsequent risk of malaria and the acquisition of naturally acquired immunity [15, 16] and carries the risk of misclassifying recrudescent infections as new infections [17]. Additionally, in all prior studies that assessed P. falciparum infection risk between children and adults, blood-stage infection was determined by microscopic examination of thick blood smears, a relatively insensitive technique that can lead to misclassification of individuals with submicroscopic blood-stage infection as “sterilely” immune.
To our knowledge, there have been no prospective studies of P. falciparum infection risk in children and adults who were uninfected and non–drug treated at baseline. Here we took advantage of seasonal P. falciparum transmission in Mali to conduct an intensive longitudinal study of time to first P. falciparum infection in healthy children and adults who had not recently received antimalarial medications and who were free of Plasmodium infection by PCR prior to the 6-month malaria season. The aim was to test the hypothesis that immunity to P. falciparum infection is acquired through repeated infections in malaria-endemic areas.
METHODS
Study Design and Participants
From May to December 2011, an observational cohort study was conducted in Kalifabougou, Mali, a rural village of approximately 5000 inhabitants located 48 km northwest of Bamako in a region of Mali that typically experiences intense, seasonal P. falciparum transmission from July through December [18]. The study population had not participated in prior studies and is serviced by a single clinic and pharmacy that provides the only access to antimalarial drugs in the area. From an age-stratified, random sample of the entire village population, 547 healthy individuals aged 4–25 years were enrolled. The disproportionate sample size of each age group reflects the design of this ongoing study of malaria immunity, which focuses on older children as they transition from malaria susceptibility to immunity. Enrollment exclusion criteria were hemoglobin level <7 g/dL, axillary temperature ≥37.5°C, acute systemic illness, use of antimalarial or immunosuppressive medications in the past 30 days, and pregnancy. Clinical malaria episodes were detected prospectively by self-referral and weekly active clinical surveillance visits, which alternated between the study clinic and the participants’ homes. All individuals with signs and symptoms of malaria and any level of Plasmodium parasitemia detected by light microscopy were treated according to the National Malaria Control Program guidelines in Mali. The research definition of malaria was an axillary temperature of ≥37.5°C, ≥2500 asexual parasites/µL of blood, and no other cause of fever discernible by physical exam. During the scheduled clinic visits, blood was collected by finger prick every 2 weeks to prepare blood smears and dried blood spots on filter paper. Asymptomatic P. falciparum infections were detected by microscopic examination of blood smears and PCR analysis of blood spots at the end of the surveillance period. Individuals found to be PCR positive for Plasmodium infection at enrollment were excluded from this analysis.
Detection of P. falciparum Infection
For each participant, blood-smear microscopy and PCR were performed on blood samples in chronological order until the first P. falciparum infection was detected. Thick blood smears were stained with Giemsa and counted against 300 leukocytes, and P. falciparum densities were recorded as the number of asexual parasites per µL of whole blood based on a mean leukocyte count of 7500 cells per µL. Each smear was evaluated separately by at least 2 expert microscopists.
For PCR analysis, we adapted a previously described nested PCR technique to amplify parasite DNA directly from dried blood spots preserved on 903 Protein Saver filter paper (Whatman) using primers targeting the human Plasmodium species 18S ribosomal RNA gene [19]. Plasmodium positive samples were identified only as P. falciparum, P. malariae, or both (mixed infections) given the negligible incidence of other Plasmodium species in the study area (unpublished data). For the initial amplification, a 1-mm circular punch of dried blood on filter paper was added to a 20-µL reaction containing 1 µM rPLU5/rPLU6 primers, 1 × Phusion Blood PCR Buffer, and 0.4 µL Phusion Blood II DNA Polymerase (Finnzymes). The cycling protocol began with lysis of cells at 98°C for 5 minutes, followed by 30 cycles of amplification (98°C for 1 second, 61°C for 5 seconds, and 72°C for 30 seconds/kb) and a final extension at 72°C for 1 minute. For the second amplification, 1 µL of the PCR product from the first amplification was added to a reaction (25-µL final volume) containing either the 1 µM rFAL1/rFAL2 (for P. falciparum) or 1 µM rMAL1/rMAL2 (for P. malariae) primer sets (Supplementary Table 1), 0.2 mM dNTPs, 1 × GoTaq PCR Buffer, and 1.25 units GoTaq DNA Polymerase (Promega). The template DNA was denatured at 95°C for 2 minutes, followed by 30 cycles of amplification (95°C for 1 minute, 58°C for 1 minute, 72°C for 1 minute) and a final extension at 72°C for 5 minutes. Reactions were performed in 96-well PCR plates. Target band detection was performed using a DNA 5 K LabChip on the LabChip GX HT as per the manufacturer's recommended protocol (Caliper Lifesciences).
In order to quantify submicroscopic blood-stage infections and to estimate the parasite liver-to-blood load (defined here as the parasite burden at the point of first detectable blood-stage infection), quantitative real-time PCR (qPCR) was performed on blood samples determined to be positive by nested PCR. Genomic DNA (gDNA) was extracted from a 3-mm circular punch of dried blood on filter paper as described elsewhere [20]. For each reaction, 2 µL of extracted template DNA was added to an 8-µL master mix containing 200 nM rFAL1/rFAL2 primers and 1 × Power SYBR Green PCR Master Mix (Applied Biosystems). Reactions were run in triplicate in 384-well MicroAmp optical PCR plates (Applied Biosystems). As a DNA extraction control, another set of triplicate reactions were run in tandem using human GAPDH primers in lieu of P. falciparum–specific primers (Supplementary Table 1) [21]. Each plate included a no DNA template control, human gDNA control, and purified P. falciparum gDNA control. Serial log-fold dilutions of DNA extracted from dried blood spots on filter paper of known parasite densities starting at approximately 100 000 parasites/µL were performed in triplicate to generate standard curves in which cycle threshold (Ct) values were plotted against log parasite density. Real-time PCRs were run on an Applied Biosystems 7900HT system using the manufacturer's recommended parameters. Amplification curves were evaluated with the ABI 7900 Sequence Detection System software, version 2.2 (Applied Biosystems).
Statistical Analyses
The Kaplan-Meier curve was used to estimate the probability of remaining free of clinical malaria and Plasmodium infection in the following age groups: 4–6 years, 7–9 years, 10–14 years, and 15–25 years. Individuals who were infected with P. malariae were censored at the time of infection. We used group and pairwise log-rank analyses to test the significance of differences in time to infection and time to first malaria episode between the age groups. For continuous outcomes, we compared the differences between group medians with the Kruskal-Wallis test. For binary outcomes, group comparisons were performed using Fisher exact test. Parasite densities determined by microscopy and calculated from Ct values were compared using Pearson correlation. Statistical significance was defined as a 2-tailed P value of ≤ .05. We performed all analyses in R version 2.13.2 (http://www.R-project.org) or GraphPad Prism version 5.0d (GraphPad software).
Ethical Approval
The Ethics Committee of the Faculty of Medicine, Pharmacy, and Dentistry at the University of Sciences, Technique, and Technology of Bamako, and the Institutional Review Board of the National Institute of Allergy and Infectious Diseases, National Institutes of Health, approved this study. Written informed consent was obtained from adult participants and from the parents or guardians of participating children. The study is registered in the ClinicalTrials.gov database (NCT01322581).
RESULTS
From 11 May to 31 May 2011, 547 healthy individuals aged 4–25 years were enrolled in this study. At the time of enrollment, just prior to the malaria season, 290 individuals (53.0%) were infected with P. falciparum and 36 were infected (6.6%) with P. malariae, as determined by PCR; 30 individuals (5.5%) had mixed infections with P. falciparum and P. malariae (Table 1). All subsequent analyses pertain to the 251 individuals who were found to be uninfected at enrollment.
Table 1.
Baseline Characteristics of All 547 Study Participants by Age Group
| Characteristic | Age Group |
All (N = 547) | |||
|---|---|---|---|---|---|
| 4–6 y (n = 90) | 7–9 y (n = 299) | 10–14 y (n = 111) | 15–25 y (n = 47) | ||
| Female sex | 43 (47.8) | 151 (50.5) | 48 (43.2) | 26 (55.3) | 268 (49.0) |
| Plasmodium falciparum | |||||
| Smear positive at enrollment | 18 (20.0) | 105 (35.1) | 29 (26.1) | 7 (14.9) | 159 (29.1) |
| PCR positive | 31 (34.4) | 168 (56.2) | 66 (59.5) | 25 (53.2) | 290 (53.0) |
| Plasmodium malariae | |||||
| Smear positive at enrollment | 0 (0.0) | 11 (3.7) | 0 (0.0) | 0 (0.0) | 11 (2.0) |
| PCR positive | 1 (1.1) | 26 (8.7) | 7 (6.3) | 2 (4.2) | 36 (6.6) |
| Mixed infections by PCR | 1 (1.1) | 21 (7.0) | 6 (5.4) | 2 (4.2) | 30 (5.5) |
| PCR negative for any Plasmodium species | 59 (65.5) | 126 (42.1) | 44 (39.6) | 22 (46.8) | 251 (45.9) |
Abbreviation: PCR, polymerase chain reaction.
As expected in an area of intense P. falciparum transmission, the risk of clinical malaria differed among age groups (a surrogate for cumulative P. falciparum exposure; Figure 1A; log-rank test, P = .0038), with increased median time to first malaria episode in older age groups (Table 2). In contrast, the risk of P. falciparum blood-stage infection as determined by PCR did not vary with age (Figure 1B; log-rank test, P = .37). However, adults (aged 15–25 years) demonstrated delayed time to infection compared to young children when the less sensitive method of blood-smear microscopy was used (Figure 1C and Table 2, log-rank test, P = .020; Table 3, pairwise log-rank test between adults and children aged 4–6 years, P = .020, and adults and children aged 7–9 years, P = .010). Of note, 92% individuals remained free of parasitemia for at least 30 days after enrollment, suggesting that subsequent blood-stage infection resulted from renewed P. falciparum transmission rather than recrudescence of blood-stage infections that were subpatent at enrollment. Table 2 provides a detailed summary of clinical malaria and P. falciparum infection risk by age group.
Figure 1.
Kaplan-Meier plots, stratified by age, for (A) time to first clinical malaria episode and time to first P. falciparum infection by (B) PCR and by (C) microscopy. Abbreviation: PCR, polymerase chain reaction.
Table 2.
Plasmodium falciparum Blood-Stage Infection and Malaria Episode Parameters by Age Group
| Parameter | Age Group |
All (N = 251) | P Value | |||
|---|---|---|---|---|---|---|
| 4–6 y (n = 59) | 7–9 y (n = 126) | 10–14 y (n = 44) | 15–25 y (n = 22) | |||
| Median days from enrollment to first malaria episode (95% CI) | 122 (93–161) | 147 (132–159) | 162 (125–NA) | NE | ND | .0038a |
| Median parasite density for first malaria episodes in parasites/µL (interquartile range) | 34 200 (15 250–53 100) | 23 900 (9240–54 900) | 17 400 (7600–39 600) | 9950 (7410–10 800) | 23 250 (9460–49 380) | .053b |
| Individuals with ≥1 malaria episodes (% of age group) | 41 (69) | 87 (69) | 25 (56) | 6 (27) | 159 (63) | .0015c |
| Median days to first P. falciparum infection (95% CI) | ||||||
| PCR | 81 (71–100) | 76 (74–88) | 91 (80–117) | 95 (82–115) | ND | .37a |
| Microscopy | 85 (70–94) | 82 (75–89) | 92 (77–140) | 110 (96–NA) | ND | .020a |
| Median calculated parasite density for first infections in parasites/µL (interquartile range)d | 7690 (43–324 00) | 2350 (30–39 500) | 1690 (540–37 400) | 561 (41–5730) | 2470 (38–48 200) | .090b |
| Individuals with ≥1 P. falciparum infections (% of age group) | 49 (83) | 114 (90) | 38 (86) | 18 (82) | 219 (87) | .37c |
Abbreviations: CI, confidence interval; NA, not available; ND, not done; NE, not estimated because 74% of individuals with complete follow-up remained free of malaria during the study period; PCR, polymerase chain reaction.
a Group log-rank test.
b Kruskal-Wallis rank sum test.
c Fisher exact test.
d Calculated from quantitative PCR cycle threshold values for PCR-positive first infections only; refer to Methods for derivations of calculated parasite density.
Table 3.
Pairwise Log-Rank Test P Values for Time to First Infection Between Age Groups by Parasite Detection Method
| Age Group | 4–6 y | 7–9 y | 10–14 y | 15–25 y |
|---|---|---|---|---|
| 4–6 y | .84 | .082 | .020 | |
| 7–9 y | .30 | .041 | .010 | |
| 10–14 y | .46 | .46 | .39 | |
| 15–25 y | .55 | .67 | .83 |
Matrix represents the P values of log-rank tests done between 2 age groups (noted by the row and column labels) for both time to first infection by polymerase chain reaction (lower left half) and time to first positive blood smear (upper right half).
These data indicate that sterile immunity to P. falciparum infection is not acquired despite years of repeated exposures; however, it remains possible that partial liver-stage immunity is acquired, which might limit the number of parasites exiting the liver into the bloodstream. To explore this possibility further, we performed qPCR on dried blood spots that corresponded to the first PCR-detected infection of the study period. We correlated the calculated parasite densities (cPD) derived from qPCR Ct values with actual parasite densities from blood smears obtained simultaneously from the same individual (Supplementary Figure 1; Pearson r = .79, P < .0001). We also calculated parasite densities from relative qPCR values using the 2−ΔΔCt method [22] and a 5000 parasite/µL standard as the reference sample. However, this method demonstrated a weaker correlation with the microscopy data (Supplementary Figure 1; Pearson r = .33, P < .0001). Thus, we used cPD derived from absolute Ct in all subsequent analyses. Although the median cPD decreased with increasing age, this trend was not statistically significant (Table 2; Kruskal-Wallis χ2 = 6.48, P = .090). Parasite densities for first malaria episodes, as determined by microscopy, were approximately an order of magnitude greater than the cPD during the first PCR-positive infections and also decreased with increasing age (Table 2; Kruskal-Wallis χ2 = 7.68, P = .053).
DISCUSSION
In this longitudinal cohort study in Mali, we show that despite years of exposure to intense P. falciparum transmission there is no evidence of acquired sterile immunity to P. falciparum infection, even as clinical immunity to the blood stage of infection is clearly acquired. Although it is widely cited that sterile immunity to P. falciparum infection is seldom if ever acquired in endemic areas [23], the field studies that have addressed this question directly have generated conflicting results, with evidence both for [7–10] and against [11–14] natural acquisition of sterile protection with increasing age.
The present study was designed to address the limitations of prior studies that may have contributed to their conflicting results. For example, previous studies often employed a treatment-reinfection design in which individuals were treated with antimalarials at enrollment to clear blood-stage infection prior to assessing reinfection risk. Here we enrolled healthy, uninfected individuals at the end of the dry season and then assessed infection risk as the predictably intense malaria season ensued. This study design precluded the need for drug treatment and thus avoided the potential confounders inherent in treatment-reinfection studies—namely, recrudescence of subpatent infections [17] and the immunomodulatory effects of antimalarial drugs [15] and parasite killing in vivo. Intense P. falciparum transmission at the study site also decreased the probability of misclassifying unexposed individuals as sterilely immune. In addition, nearly all prior studies used microscopic examination of blood smears to determine blood-stage infection risk [7–13], a low-sensitivity method that could have led to the misclassification of individuals with submicroscopic blood-stage infections as sterilely immune. Indeed, in the present study we observed decreased infection risk with increasing age when microscopy was used to diagnose blood-stage infection (Figure 1C). However, when the more sensitive PCR assay was used, blood-stage infection risk did not vary with age (Figure 1B), consistent with treatment-reinfection studies conducted in Ghana [11, 12] and Kenya [13], which found no difference in P. falciparum infection risk between children and adults by microscopy. The observation that PCR-based detection negates observed delays in time to infection by microscopy has important implications for field trials of vaccines targeting the preerythrocytic stage of infection, which have traditionally employed microscopic detection of first parasitemia as an endpoint for evaluating vaccine efficacy [24].
Although the present study fails to provide evidence in support of the hypothesis that sterile immunity is acquired through natural P. falciparum infection, we cannot rule out the possibility of partial acquired immunity to sporozoites or the liver stage, which could decrease the “fitness” or number of parasites exiting the liver into the blood. Consistent with this notion, we observed a trend toward lower blood-stage parasite densities by qPCR during the first PCR positive blood-stage infection of the study period. However, it is equally if not more probable that the decreased parasite density with increasing age is due to the acquisition of blood-stage immunity (eg, antibodies), which is known to rapidly suppress parasite numbers in the blood [25]. The identification of biomarkers that reflect the magnitude of the liver-to-blood parasite inocula would help distinguish between these 2 possibilities. It is also possible that the inclusion of older adults (>25 years of age) in this study would have revealed evidence of acquired immunity to P. falciparum infection that is only acquired after several decades of exposure, but this seems unlikely because immune function generally declines in late adulthood [26].
In striking contrast to the results of this study, sterile protection has been demonstrated in human experimental models in which malaria-naive adults were exposed to bites of P. falciparum-infected mosquitoes that were either irradiated [2] or administered during receipt of prophylactic chloroquine [3]. Likewise, the most advanced malaria vaccine candidate RTS,S, which targets a surface protein on sporozoites termed circumsporozoite protein, was shown to induce sterile protection from infection in approximately 50% of malaria-naive adults challenged with P. falciparum approximately 3 weeks after the last immunization, although only 44% of protected subjects remained protected upon rechallenge 5 months later [27]. Importantly, in African infants, RTS,S has shown only approximately 30% efficacy in reducing clinical episodes of malaria [28] and does not provide durable protection from infection [29]. The mechanism by which RTS,S, a vaccine that targets the sporozoite and liver stages, confers partial protection against blood-stage disease remains largely unexplained. It is possible that RTS,S induces protection against clinical malaria by temporarily reducing the number of merozoites emerging from the liver. This may lead to prolonged exposure to subclinical levels of blood-stage parasites, which in turn allows boosting of naturally acquired blood-stage immunity [29].
Given the increased focus on the development of vaccines that aim to induce sterile protection by targeting the sporozoite and liver stage of infection, studies are needed to understand why sterile immunity is not induced through natural infection in endemic areas and the extent to which this apparent refractoriness might interfere with vaccination. Possible explanations for the lack of naturally acquired sterile immunity in this population include extensive genetic diversity of preerythrocytic antigens in endemic areas [30], low sporozoite inoculums from infected mosquitoes [6], or dysregulation of potential immune effectors such as antibody or CD8 T cells [31]. MHC class I restriction of the most effective preerythrocytic T-cell epitopes may severely limit the frequency of responders in populations with diverse HLA haplotypes [32]. In addition, malnutrition and chronic coinfections [33–35] may contribute further to the immune dysregulation observed in malaria-endemic areas [36].
Indeed, studies that identify the mechanisms underlying refractoriness to the natural acquisition of sterile immunity may prove more important for preerythrocytic vaccine development than studies that have attempted to link various immune responses with what is presumed to be naturally acquired sterile protection [6]. Unless biomarkers reflective of the liver stage parasite burden are identified that can allow us to disentangle the relative contributions of effective liver-stage immunity from early blood-stage immunity in controlling initial parasitemia, studies in endemic settings may not be informative for preerythrocytic vaccine development.
In this study, 14 individuals (5.6%) with complete follow-up remained uninfected throughout the transmission season, and 15 individuals (6.0%) experienced a delay in time to infection ≥150 days; however, there were no associations between age and either absence or delay of infection. One possible explanation is that uninfected individuals had less exposure to infective mosquito bites, perhaps due to insecticide-treated bednet use or other behavioral factors. Although biweekly parasitological surveillance reduced the likelihood of missing infections, another possibility is that “uninfected” individuals maintained parasitemia below the detection threshold of our PCR assay. This raises an important point regarding assay sensitivity. Although more sensitive than microscopic detection, the limit of detection for the PCR assays used in this study was approximately 1 parasite/µL of blood, which is comparable to methods that use DNA extracted from small punches of dried blood on filter paper [37] but less sensitive than PCR-based detection assays that achieve sensitivities of 0.02 parasites/µL using genetic material obtained from 50 µL of blood [38]. Had we interrogated larger quantities of blood per sample, we may have detected infections earlier and more often and may have seen a larger difference between median time to PCR and blood smear positivity (Table 2). It is also conceivable that there are age-independent host genetic determinants of infection risk that have yet to be identified. Several host genetic determinants have been associated with protection from clinical malaria [39]. However, to our knowledge, sickle cell trait, which is present in approximately 10% of our study participants (unpublished data), is the only genetic factor that has been associated with delayed or reduced risk of P. falciparum infection [7, 40], but this may simply be a reflection of subpatent blood-stage infection.
In summary, despite years of exposure to intense malaria transmission, there is no evidence for the acquisition of sterile immunity to P. falciparum infection in individuals living in endemic areas. Understanding why repeated P. falciparum infections do not induce sterile protection may lead to insights for developing vaccines that target the liver stage in individuals living in malaria-endemic areas.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments.
We thank the study participants and research support staff in Kalifabougou and Bamako, Mali, as well as Dr Susan Pierce and Dr Louis Miller for helpful discussions.
Author contributions.
T. M. T., S. L., S. D., K. K., A. O., O. K. D., B. T., and P. D. C. conceived and designed the study and experiments. T. M. T., S. L., C. H., and P. D. C. analyzed the data. K. K., A. O., D. D., S. N., J. A., A. B., Y. K., M. N., C. D., and A. T. managed the collection of clinical data and biospecimens. S. L., B. A., Y. K., D. D., J. A., and A. T. performed the assays. T. M. T. and P. D. C. wrote the manuscript.
Financial support.
This work was supported by the Division of Intramural Research, National Institute of Allergy and Infectious Diseases, National Institutes of Health.
Potential conflicts of interest.
All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33:247–54. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 3.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 4.Nussenzweig RS, Vanderberg J, Most H, Orton C. Protective immunity produced by the injection of x-irradiated sporozoites of Plasmodium berghei. Nature. 1967;216:160–2. doi: 10.1038/216160a0. [DOI] [PubMed] [Google Scholar]
- 5.Doolan DL, Dobano C, Baird JK. Acquired immunity to malaria. Clin Microbiol Rev. 2009;22:13–36. doi: 10.1128/CMR.00025-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Offeddu V, Thathy V, Marsh K, Matuschewski K. Naturally acquired immune responses against Plasmodium falciparum sporozoites and liver infection. Int J Parasitol. 2012;42:535–48. doi: 10.1016/j.ijpara.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Sokhna CS, Rogier C, Dieye A, Trape JF. Host factors affecting the delay of reappearance of Plasmodium falciparum after radical treatment among a semi-immune population exposed to intense perennial transmission. Am J Trop Med Hyg. 2000;62:266–70. doi: 10.4269/ajtmh.2000.62.266. [DOI] [PubMed] [Google Scholar]
- 8.Sokhna CS, Faye FBK, Spiegel A, Dieng H, Trape JF. Rapid reappearance of Plasmodium falciparum after drug treatment among Senegalese adults exposed to moderate seasonal transmission. Am J Trop Med Hyg. 2001;65:167–70. doi: 10.4269/ajtmh.2001.65.167. [DOI] [PubMed] [Google Scholar]
- 9.Dent AE, Bergmann-Leitner ES, Wilson DW, et al. Antibody-mediated growth inhibition of Plasmodium falciparum: relationship to age and protection from parasitemia in Kenyan children and adults. PLoS One. 2008;3:e3557. doi: 10.1371/journal.pone.0003557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tall A, Sokhna C, Perraut R, et al. Assessment of the relative success of sporozoite inoculations in individuals exposed to moderate seasonal transmission. Malaria J. 2009;8:161. doi: 10.1186/1475-2875-8-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Owusu-Agyei S, Koram KA, Baird JK, et al. Incidence of symptomatic and asymptomatic Plasmodium falciparum infection following curative therapy in adult residents of northern Ghana. Am J Trop Med Hyg. 2001;65:197–203. doi: 10.4269/ajtmh.2001.65.197. [DOI] [PubMed] [Google Scholar]
- 12.Baird JK, Owusu Agyei S, Utz GC, et al. Seasonal malaria attack rates in infants and young children in northern Ghana. Am J Trop Med Hyg. 2002;66:280–6. doi: 10.4269/ajtmh.2002.66.280. [DOI] [PubMed] [Google Scholar]
- 13.John CC, Koech DK, Sumba PO, Ouma JH. Risk of Plasmodium falciparum infection during a malaria epidemic in highland Kenya, 1997. Acta Trop. 2004;92:55–61. doi: 10.1016/j.actatropica.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 14.Michon P, Cole-Tobian JL, Dabod E, et al. The risk of malarial infections and disease in Papua New Guinean children. Am J Trop Med Hyg. 2007;76:997–1008. [PMC free article] [PubMed] [Google Scholar]
- 15.Coulibaly D, Diallo DA, Thera MA, et al. Impact of preseason treatment on incidence of falciparum malaria and parasite density at a site for testing malaria vaccines in Bandiagara, Mali. Am J Trop Med Hyg. 2002;67:604–10. doi: 10.4269/ajtmh.2002.67.604. [DOI] [PubMed] [Google Scholar]
- 16.Liljander A, Bejon P, Mwacharo J, et al. Clearance of asymptomatic P. falciparum infections interacts with the number of clones to predict the risk of subsequent malaria in Kenyan children. PLoS One. 2011;6:e16940. doi: 10.1371/journal.pone.0016940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cattamanchi A, Kyabayinze D, Hubbard A, Rosenthal PJ, Dorsey G. Distinguishing recrudescence from reinfection in a longitudinal antimalarial drug efficacy study: comparison of results based on genotyping of msp-1, msp-2, and glurp. Am J Trop Med Hyg. 2003;68:133–9. [PubMed] [Google Scholar]
- 18.Crompton PD, Traore B, Kayentao K, et al. Sickle cell trait is associated with a delayed onset of malaria: implications for time-to-event analysis in clinical studies of malaria. J Infect Dis. 2008;198:1265–75. doi: 10.1086/592224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 20.Bereczky S, Martensson A, Gil JP, Farnert A. Short report: Rapid DNA extraction from archive blood spots on filter paper for genotyping of Plasmodium falciparum. Am J Trop Med Hyg. 2005;72:249–51. [PubMed] [Google Scholar]
- 21.Taylor SM, Juliano JJ, Trottman PA, et al. High-throughput pooling and real-time PCR-based strategy for malaria detection. J Clin Microbiol. 2010;48:512–9. doi: 10.1128/JCM.01800-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Langhorne J, Ndungu FM, Sponaas AM, Marsh K. Immunity to malaria: more questions than answers. Nat Immunol. 2008;9:725–32. doi: 10.1038/ni.f.205. [DOI] [PubMed] [Google Scholar]
- 24.Abdulla S, Oberholzer R, Juma O, et al. Safety and immunogenicity of RTS,S/AS02D malaria vaccine in infants. N Engl J Med. 2008;359:2533–44. doi: 10.1056/NEJMoa0807773. [DOI] [PubMed] [Google Scholar]
- 25.Cohen S, Mc GI, Carrington S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961;192:733–7. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- 26.Haynes L, Swain SL. Aged-related shifts in T cell homeostasis lead to intrinsic T cell defects. Semin Immunol. 2012;24:350–5. doi: 10.1016/j.smim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kester KE, Cummings JF, Ofori-Anyinam O, et al. Randomized, double-blind, phase 2a trial of falciparum malaria vaccines RTS,S/AS01B and RTS,S/AS02A in malaria-naive adults: safety, efficacy, and immunologic associates of protection. J Infect Dis. 2009;200:337–46. doi: 10.1086/600120. [DOI] [PubMed] [Google Scholar]
- 28.The RTS SCTP. A Phase 3 Trial of RTS,S/AS01 Malaria Vaccine in African Infants. N Engl J Med. 2012;367:2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guinovart C, Aponte JJ, Sacarlal J, et al. Insights into long-lasting protection induced by RTS,S/AS02A malaria vaccine: further results from a phase IIb trial in Mozambican children. PLoS One. 2009;4:e5165. doi: 10.1371/journal.pone.0005165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry AE, Schultz L, Buckee CO, Reeder JC. Contrasting population structures of the genes encoding ten leading vaccine-candidate antigens of the human malaria parasite, Plasmodium falciparum. PLoS One. 2009;4:e8497. doi: 10.1371/journal.pone.0008497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Butler NS, Moebius J, Pewe LL, et al. Therapeutic blockade of PD-L1 and LAG-3 rapidly clears established blood-stage Plasmodium infection. Nat Immunol. 2012;13:188–95. doi: 10.1038/ni.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zevering Y, Houghten RA, Frazer IH, Good MF. Major population differences in T cell response to a malaria sporozoite vaccine candidate. Int immunol. 1990;2:945–55. doi: 10.1093/intimm/2.10.945. [DOI] [PubMed] [Google Scholar]
- 33.Schaible UE, Kaufmann SH. Malnutrition and infection: complex mechanisms and global impacts. PLoS Med. 2007;4:e115. doi: 10.1371/journal.pmed.0040115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartgers FC, Yazdanbakhsh M. Co-infection of helminths and malaria: modulation of the immune responses to malaria. Parasite Immunol. 2006;28:497–506. doi: 10.1111/j.1365-3024.2006.00901.x. [DOI] [PubMed] [Google Scholar]
- 35.Flateau C, Le Loup G, Pialoux G. Consequences of HIV infection on malaria and therapeutic implications: a systematic review. Lancet Infect Dis. 2011;11:541–56. doi: 10.1016/S1473-3099(11)70031-7. [DOI] [PubMed] [Google Scholar]
- 36.Good MF, Doolan DL. Malaria vaccine design: immunological considerations. Immunity. 2010;33:555–66. doi: 10.1016/j.immuni.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Mangold KA, Manson RU, Koay ES, et al. Real-time PCR for detection and identification of Plasmodium spp. J Clin Microbiol. 2005;43:2435–40. doi: 10.1128/JCM.43.5.2435-2440.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murphy SC, Prentice JL, Williamson K, et al. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg. 2012;86:383–94. doi: 10.4269/ajtmh.2012.10-0658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Driss A, Hibbert JM, Wilson NO, Iqbal SA, Adamkiewicz TV, Stiles JK. Genetic polymorphisms linked to susceptibility to malaria. Malaria J. 2011;10:271. doi: 10.1186/1475-2875-10-271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gong L, Maiteki-Sebuguzi C, Rosenthal PJ, et al. Evidence for both innate and acquired mechanisms of protection from Plasmodium falciparum in children with sickle cell trait. Blood. 2012;119:3808–14. doi: 10.1182/blood-2011-08-371062. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.