Abstract
Purpose
To study the spatial variances in ligand expression and angiogenic effect in response to the inflammatory response induced by b-FGF.
Methods
b-FGF micropellets (80ng) were implanted in the temporal side of the cornea of Balbc/c mice. On days 1, 3, and 7 blood (heme) and lymph-angiogenesis were observed by immunofluorescence staining of corneal flat mounts with LYVE-1 and CD31 to identify lymphatic and blood vessels, respectively. A second group of corneas were harvested for quantitative RT-PCR. Each cornea was divided in two different area defines as (i) pre-pellet area and (ii) opposite-pellet area. Expression of VEGF ligands were evaluated using Real-time PCR in each respective zone.
Results
Blood vessels grew into the cornea from the pre-pellet area while corneal lymphatic vessels grew from the opposite-pellet area toward the center of the cornea. VEGF-A was upregulated in the pre-pellet while VEGF-D expression was mostly observed in the opposite-pellet area. VEGF-C level increased simultaneously in both areas.
Conclusion
A single inducing factor, i.e., b-FGF, may simultaneously provoke heme-and lymph-angiogenesis in different locations of the cornea through differential expression of VEGF ligands. This distinctive spatial pattern should be considered while evaluating the corneal predilection for inflammation beyond that which is directly visible by slit lamp examination.
Keywords: Corneal angiogenesis, fibroblast growth factor, vascular endothelial growth factor
Introduction
The cornea is devoid of both blood and lymphatic vessels. However, many pathologic conditions of the cornea are associated with the ingrowth of new vessels from the pre-existing vasculature of the limbus with resultant adverse consequences 1–2. Several key angiogenic factors, including fibroblast growth factor (FGF),3 vascular endothelial growth factor (VEGF),4–6 platelet-derived growth factor (PDGF),7 and angiopoietin8–11 families regulate angiogenesis through multiple and overlapping molecular pathways in various physiological and pathological processes12. While each of these growth factors has been described to play a dominantly pro-hemangoigenic (e.g. VEGF-A13) or pro-lymphangiogenic (e.g. VEGF-C and VEGF-D13) function, their in vivo effect is more complex since the function of these factors is affected by the microenviroment in which they are expressed.
Investigation of the distinctive molecular and cellular mechanisms underlying the growth of corneal blood, as opposed to lymphatic, vessels has been particularly challenging as the majority of the pathologic conditions and animal models stimulate coincident growth of both lymphatic and blood vessels 14. Multiple groups have defined strategies to selectively induce blood or lymphatic growth in the cornea. One example is basic FGF (b-FGF) which was initiallycharacterized as an inducer of hemangiogenesis, while intrastromal micropellet implantation of very low doses of b-FGF (12.5 ng) was subsequently shown to be a selective lymphangiogenic stimulator. In addition, we and others have shown that systemic blockade of VEGFR315–16 and α5 integrin17 predominantly inhibit corneal lymphangiogenesis.
Very little is known about the relative contribution of blood versus lymphatic vessels to various pathological conditions of the cornea. This is relevant since pathological angiogenesis of the cornea, as appreciated in clinical cases, is highly heterogeneous, not just from eye to eye, but also within the same cornea. Here we study the spatial variances in ligand expression and angiogenic effects in response to the same inflammatory stimulus induced by b-FGF.
Material and methods
Animals
Male 6- to 8-week-old Balb/c mice were used in all experiments. Animals were anesthetized by intraperitoneal injection of ketamine (120 mg/kg) and xylazine (20mg/kg) before any surgery and were treated in accordance with ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Experiments described herein were conducted under institutional animal care and use committee approval.
Pellet Implantation
b-FGF micropellets (80 ng/pellet) were prepared as described previously. 18 To implant the pellets, half thickness linear incisions were made at the center of cornea using a disposable 30°microknife (F.S.T., Foster City, CA, USA). Lamellar pocket incisions were then made parallel to the corneal plane using a Von Graefe knife (F.S.T., Foster City, CA, USA) and advanced to the temporal limbus at the lateral canthal area. The pellets were positioned into the pocket 1.0 mm apart from the limbal vascular arcade in the temporal side, and tetracycline ophthalmic ointment was applied to the eye after pellet implantation.
Immunohistochemistry and Morphometry of HA and LA
After taking photographs under the slit lamp, five mice per group were sacrificed 1, 3, and 7 days after implantation, and freshly enucleated eyes were prepared into corneal flat mounts. Immunohistochemical staining was performed with FITC-conjugated CD31/PECAM-1 (rat-anti–mouse antibody; 1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Staining against LYVE-1 (lymphatic endothelium-specific hyaluronic acid receptor; goat-anti–rabbit antibody; 1:400; Abcam, Cambridge, MA) was also performed with purified antibody followed by rhodamine-conjugated secondary antibody. To quantify the level of blood vessel (CD31high/LYVE-1low) or lymphatic vessel (CD31low/LYVE-1high) formation low magnification (2X) micrographs were captured, and the area covered by each type of vessels was calculated (NIH ImageJ software 1.34; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD; available at http://rsb.info.nih.gov/ij/index.html) and expressed as the percentage of total corneal area (%).
Quantitative Real-Time PCR (qRT-PCR)
Corneas were harvested on days 1, 3, 7 post-implantation. In a group of animals, each cornea was divided based on the proximity to the pellet into 2 different areas: pre-pellet area (PA) or temporal cornea, and the opposite-pellet area (OA) or the nasal part of the cornea. RNA was isolated from either the whole or different regions of the cornea by RNeasy Micro Kit (Qiagen, Valencia, CA) and reverse transcribed (Superscript III Kit; Invitrogen Life Technologies, Carlsbad, CA). Real-time PCR was performed (TaqMan Universal PCR MasterMix; Applied Biosystems, Foster City, CA), and primers were preformulated for VEGF-A (assay ID Mm00437304_ml), VEGF-C (assay ID Mm00437313_ml), VEGF-D (assay ID Mm00438965_ml), (Applied Biosystems). PCR conditions were 2 minutes at 50°C, 10 minutes at 95°C, followed by 35 cycles of 15 seconds at 95°C and 60°C for 1 minute using an ABI PRISM 7900 HT (Applied Biosystems). The results were analyzed by comparative threshold cycle method. The relative expression level of each sample was expressed compare to 105 of GAPDH using Microsoft Office Excel 2003.
Statistical Analysis
Error bars displayed in the figures were calculated from the standard error of the mean (±SEM). Statistical analysis of blood and lymphatic vessel area was performed using a one-way ANOVA. Student’s t test was performed to compare the level of mRNA in qPCR. P < 0.05 was considered statistically significant.
Results
b-FGF induces corneal angiogenesis and up-regulates VEGF ligand expression
Hemangiogenic properties of b-FGF have long been studied. However its recently identified pro-lymphangiogenic properties have not been well-characterized. Using immunostaining for specific markers of blood and lymphatic vessel, we showed that implantation of b-FGF micropellets results in extensive corneal hemangiogenesis and lymphangiogenesis (Figure 1-B). As the pro-angiogenenic effects of b-FGF are believed to be mediated through VEGF19, we examined the expression levels of VEGF ligands at different time points following corneal implantation of the b-FGF micropellets. As shown in Figure 1-C, corneal mRNA expression of all three principal VEGF ligands, VEGF-A, VEGF-C and VEGF-D, were significantly up-regulated after b-FGF micropellet implantation compared to normal levels, implicating these VEGF species in b-FGF induced corneal neovascularization.
Figure 1.
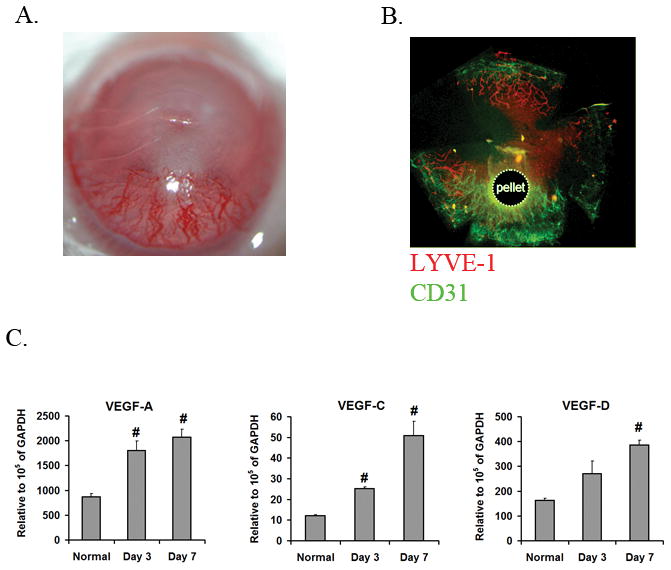
b-FGF induces both blood and lymphatic vessel growth. A. Representative photographs of a cornea at 7 days after implantation of b-FGF micropellet demonstrating extensive neovascularization. B. Immunostaining of a corneal flat mount at 7 days after b-FGF micropellet implantation with CD31 (green) and LYVE-1 (red) antibodies demonstrates the development of blood (CD31high/LYVE-1low) and lymphatic (CD31low/LYVE-1high) vessels. C. VEGF ligands mRNA expression levels were determined by qRT-PCR in corneas of normal and b-FGF micropellet implanted mice. Corneal expression of all VEGF ligands are increased 7 days after b-FGF micropellet implantation (#: P<0.01, compared to normal corneas).
b-FGF promotes a spatially distinct pattern of lymph- and hem-angiogenesis
As illustrated in Figure 2, we observed a robust hemangiogenic response in the limbal-peripheral cornea closest to the pellet (pre-pellet area [PA]), while lymphatics grew more primarily in the limbal-peripheral cornea opposite to the pellet (opposite area [OA]) toward the center. This distinct spatial pattern of blood and lymphatic growth was consistently observed at different time points after implantation of b-FGF micropellets. Quantification of the relative area of the corneas covered by each vessel type (Figure 3) showed that on the PA (92%) of the corneas are blood vessels, and only 8 % lymphatic vessels. In contrast on the OA there was nearly 3 times as much of the cornea covered by lymphatics (77%) as compared to blood vessels (23%).
Figure 2.
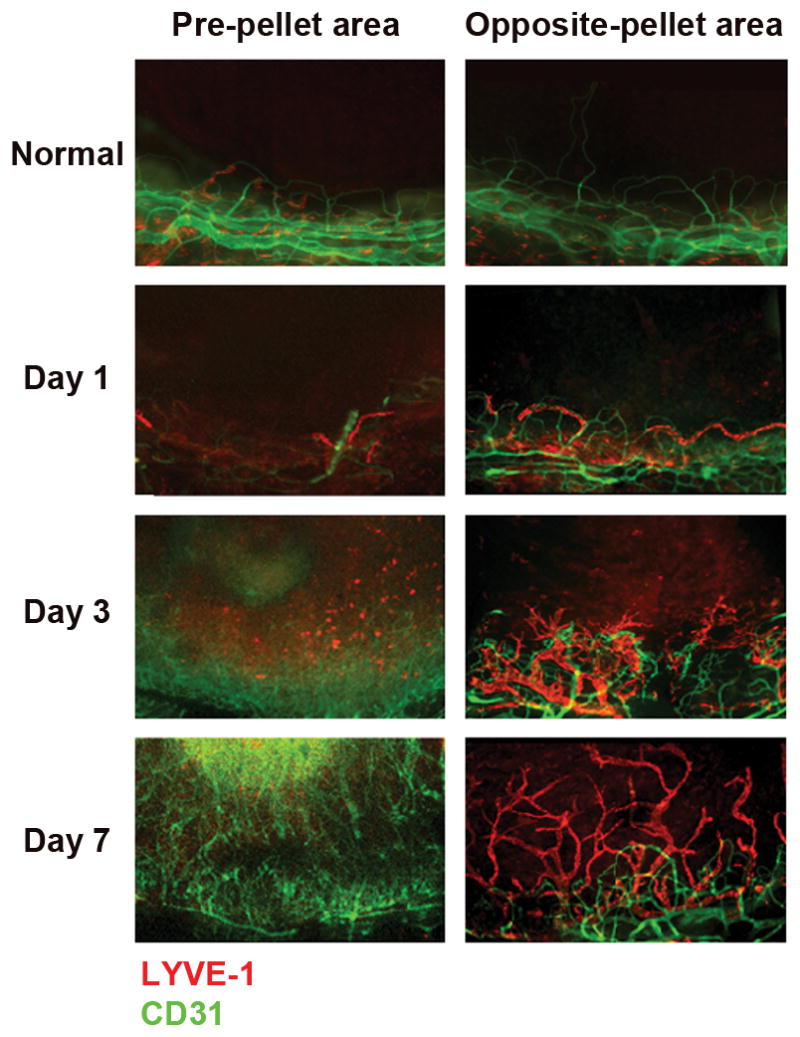
Corneal flat mounts staining demonstrates a spatially distinct pattern of hemangiogenesis- and lymphangiogenesis at 1, 3 and 7 days following implantation of b-FGF pellet (80 ng) into the corneal stroma. Extensive blood vessels growth was observed on the limbal-peripheral cornea closest to the pellet (pre-pellet area [PA]), while lymphatics grew more primarily in the limbal-peripheral cornea opposite to the pellet (opposite area [OA]).
Figure 3.
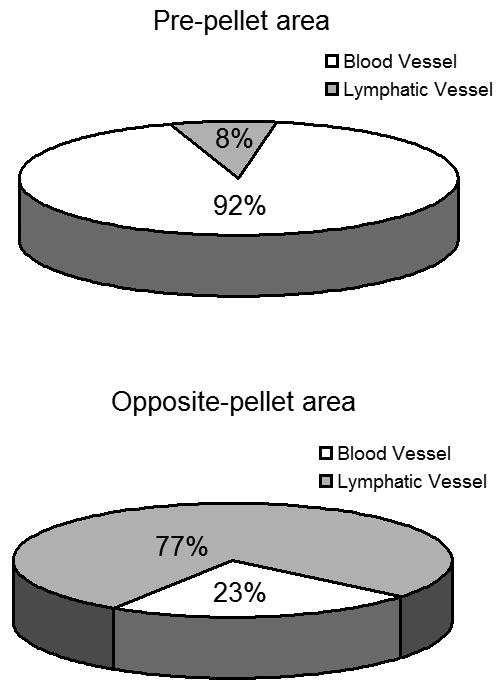
Percentage of the corneal area covered by blood and lymphatic vessels is significantly different in the pellet vs. opposite side of the cornea. Data are representative of 5 corneas.
b-FGF induces VEGF ligands in a spatially distinct pattern
Further to evaluate the relative expression of VEGF ligands in different areas of cornea by b-FGF microplellet we used qRT=PCR of VEGF ligands in PA and OA. Consistent with our in situ results on lymphatic and blood vessel growth induced by b-FGF micropellet, we observed that VEGF ligands were upregulated in a spatially distinct manner in the cornea (Figure 4A–C). VEGF-A and VEGF-C ligands were mainly upregulated in the PA, while in the OA there were significant increases in VEGF-D and VEGF-C, and to the lesser extent in VEGF-A compared to the normal state. This pattern of VEGF ligand upregulation was consistent with the ‘phenotype’ observed in the corneal immunofluorescent staining for lymphatic and blood vessel growth.
Figure 4.
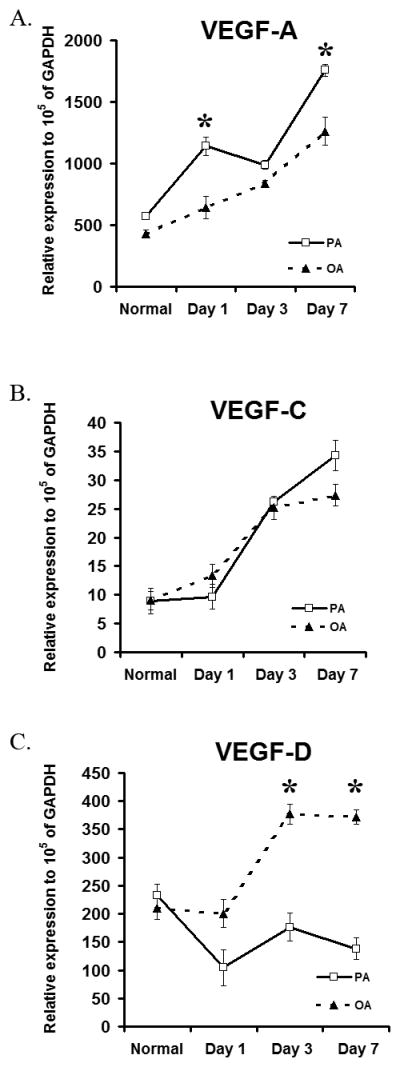
Spatial differences in mRNA expression of VEGF ligands in normal and b-FGF micropellet implanted mice were observed. A–C. The expression levels of VEGF- A increases most at pre-pellet area (PA) over time, while VEGF-D increased in opposite-pellet area (OA) of the pellet. VEGF-C shows a consistent pattern of up-regulation in all three areas over time. Graphs represent mean values (± SEM) (n=5) (*: P<0.01, PA compared to OA levels observed in the corneas in each time point).
Discussion
Angiogenesis is an intricate process that involves many cell types through a diverse group of growth factors and signaling pathways. FGF is a potent angiogenic factor19–22 which contributes to the pathogenesis of corneal inflammatory diseases through interactions with other angiogenic factors including VEGF. Here we evaluated the pattern of angiogenesis induced by FGF micropellet, and demonstrate that it results in a spatially distinct modulation of VEGF ligand expression and resultant growth of lymphatic versus blood vessels in the cornea.
Accumulating evidence is challenging the original characterization of angiogenic phenotypes through receptor-ligand specific interactions. Angiogenesis is not interrupted in the double knockout of FGF1 and FGF2, indicating extensive redundancy of the ligand system 23–25. On the other hand, while exogenous FGF2 is able to induce vascular structure formation in normal embryos, VEGF-R2 −/− embryos fail to develop vessels in the presence of b-FGF 26. These findings support the idea that FGF may act upstream to VEGFs. Supporting this concept is our finding that all VEGF ligand are over-expressed in the presence of b-FGF stimulation in the cornea.
The novel finding in this study is the observed high lymphangiogenic response noted following b-FGF stimulation in the cornea on side opposite to where the FGF pellet is implanted. More importantly, we observed differential pattern of VEGF stimulation by b-FGF micropellet in the various parts of the cornea, with high levels of VEGF-D expression on the OA.
Our findings have several interesting implications for our assessment of immunity in the setting of corneal inflammatory angiogenesis. First, given that the majority of cases of corneal angiogenesis are sectoral rather than diffuse, it is provocative to hypothesize that ample lymphatics may well be present in areas of the cornea directly opposite from the clinically visible blood vessels and adjacent area of stromal inflammation. Given that corneal lymphatics which cannot be seen directly by slit lamp examination, play a crucial role in the induction and amplification of alloreactive28 and autoimmune29 responses to corneal antigens, these findings would suggest that continued induction of immune reactivity could be ongoing away from the site of inflammation even in a setting where the therapy has led to blood vessel regression. Put another way, inflammatory changes that induce even sectoral corneal angiogenesis likely lead to more ‘global’ changes in corneal immunity. This concept is indirectly supported by the definition of risk factors for rejecting a corneal graft, which induces as little as 1–2 quadrants of neovascularization in the host bed30. Additionally, given the development of in vivo imaging technologies that allow for imaging and monitoring of corneal lymphatics31 our data imply that the area likely requiring our focus for assessment of lymphatics also included the area opposite from the site of inflammation.
Here we show for the first time that both processes of blood and lymphatic growth can potentially happen at the same time but in different locations in the cornea in response to differential expression of VEGF ligands, suggesting more global changes in corneal immunity and predilection for inflammation beyond that which is directly visible by slit lamp examination.
Acknowledgments
Grant support: This work was supported by the NIH (EY-12963), and a Research to Prevent Blindness Lew R. Wasserman Award.
References
- 1.Dana R. Angiogenesis and lymphangiogenesis-implications for corneal immunity. Semin Ophthalmol. 2006;21(1):19.22. doi: 10.1080/08820530500509358. [DOI] [PubMed] [Google Scholar]
- 2.Cursiefen C. Immune privilege and angiogenic privilege of the cornea. Chem Immunol Allergy. 2007;92:50.7. doi: 10.1159/000099253. [DOI] [PubMed] [Google Scholar]
- 3.Slavin J. Fibroblast growth factors: at the heart of angiogenesis. Cell Biol Int. 1995;19:431.44. doi: 10.1006/cbir.1995.1087. [DOI] [PubMed] [Google Scholar]
- 4.Ferrara N, Davis-Smyth The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4.25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 5.Alitalo K, Carmeliset P. Molecular mechanism of lymphangiogenesis in health and disease. Cancer Cell. 2002;1:219.27. doi: 10.1016/s1535-6108(02)00051-x. [DOI] [PubMed] [Google Scholar]
- 6.Bjorndahl MA, Cao R, Burton JB, et al. Vascular endothelial growth factor-a promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005;65:9261.8. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- 7.Cao R, Bjorndahl MA, Religa P, et al. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333.45. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- 8.Thurston G. Role of angiopoietins and Tie receptor tyrosine kinases in angiogenesis and lymphangiogenesis. Cell Tissue Res. 2003;314:61.8. doi: 10.1007/s00441-003-0749-6. [DOI] [PubMed] [Google Scholar]
- 9.Veikkola T, Alitalo K. Dual role of Ang2 in postnatal angiogenesis and lymphangiogenesis. Dev Cell. 2002;3:302.4. doi: 10.1016/s1534-5807(02)00231-9. [DOI] [PubMed] [Google Scholar]
- 10.Tammela T, Saaristo A, Lohela M, et al. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005;105:4642.8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- 11.Morisada T, Oike Y, Yamada Y, et al. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649.56. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y, Zhong W. Tumor-derived lymphangiogenic factors and lymphatic metastasis. Biomed Pharmacother. 2007;61(9):534.9. doi: 10.1016/j.biopha.2007.08.009. [DOI] [PubMed] [Google Scholar]
- 13.Rossant J, Hirashima M. Vascular development and patterning: making the right choices. Curr Opin Genet Dev. 2003;13(4):408.12. doi: 10.1016/s0959-437x(03)00080-7. [DOI] [PubMed] [Google Scholar]
- 14.Cursiefen C, Maruyama K, Jackson DG, et al. Time course of angiogenesis and lymphangiogenesis after brief corneal inflammation. Cornea. 2006;25(4):443.7. doi: 10.1097/01.ico.0000183485.85636.ff. [DOI] [PubMed] [Google Scholar]
- 15.Bock F, Onderka J, Dietrich T, et al. Blockade of VEGFR3-signalling specifically inhibits lymphangiogenesis in inflammatory corneal neovascularisation. Graefes Arch Clin Exp Ophthalmol. 2008;246(1):115.9. doi: 10.1007/s00417-007-0683-5. [DOI] [PubMed] [Google Scholar]
- 16.Chung ES, Saban DR, Chauhan SK, et al. Regulation of blood vessel versus lymphatic vessel growth in the cornea. Invest Ophthalmol Vis Sci. 2009;50(4):1613.8. doi: 10.1167/iovs.08-2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dietrich T, Onderka J, Bock F, et al. Inhibition of inflammatory lymphangiogenesis by integrin alpha5 blockade. Am J Pathol. 2007;171(1):361.72. doi: 10.2353/ajpath.2007.060896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kenyon BM, Voest EE, Chen CC, et al. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625.32. [PubMed] [Google Scholar]
- 19.Murakami M, Simons M. Fibroblast growth factor regulation of neovascularization. Curr Opin Hematol. 2008;15:215.20. doi: 10.1097/MOH.0b013e3282f97d98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomas KA, Riley MC, Lemmon SK, et al. Brain fibroblast growth factor: nonidentity with myelin basic protein fragments. J Biol Chem. 1980;255(12):5517.20. [PubMed] [Google Scholar]
- 21.Itoh N. The Fgf families in humans, mice, and zebrafish: their evolutional processes and roles in development, metabolism, and disease. Biol Pharm Bull. 2007;10:1819.25. doi: 10.1248/bpb.30.1819. [DOI] [PubMed] [Google Scholar]
- 22.Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16(2):139.49. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Ortega S, Ittmann M, Tsang SH, et al. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc Natl Acad Sci U S A. 1998;95(10):5672.7. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou M, Sutliff RL, Paul RJ, et al. Fibroblast growth factor 2 control of vascular tone. Nat Med. 1998;2:201.7. doi: 10.1038/nm0298-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller DL, Ortega S, Bashayan O, et al. Compensation by fibroblast growth factor 1 (FGF1) does not account for the mild phenotypic defects observed in FGF2 null mice. Mol Cell Biol. 2000;20(6):2260.8. doi: 10.1128/mcb.20.6.2260-2268.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnusson P, Rolny C, Jakobsson L, et al. Deregulation of Flk-1/vascular endothelial growth factor receptor-2 in fibroblast growth factor receptor-1-deficient vascular stem cell development. J Cell Sci. 2004;117:1513.23. doi: 10.1242/jcs.00999. [DOI] [PubMed] [Google Scholar]
- 27.Chang LK, Garcia-Cardena G, Farnebo F, et al. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci USA. 2004;101:11658.63. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen L, Hamrah P, Cursiefen C, et al. Vascular endothelial growth factor receptore-3 mediates induction of corneal alloimmunity. Nat Med. 2004;10(8):813.5. doi: 10.1038/nm1078. [DOI] [PubMed] [Google Scholar]
- 29.Goyal S, Chauhan SK, El Annan J, et al. Evidence of corneal lymphangiogenesis in dry eye disease: a potential link to adaptive immunity? Arch Ophthalmology. 2010;128(7):819.24. doi: 10.1001/archophthalmol.2010.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hill JC. High risk corneal grafting. Br J Ophthalmol. 2002;86(9):945. doi: 10.1136/bjo.86.9.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peedo BB, Fagerholm P, Traneus-rockert C, et al. Cellular-level characterization of lymph vesses in live, unlabeled corneas by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. 2010;51(2):830.5. doi: 10.1167/iovs.09-4407. [DOI] [PubMed] [Google Scholar]


