Abstract
Background:
Pulmonary complications of diabetes mellitus (DM) have been poorly characterized. Some authors have reported normal pulmonary functions and even concluded that spirometry is not at all necessary in diabetic patients. Some studies have shown abnormal respiratory parameters in patients of DM. Moreover, the duration of DM and glycemic control have varied impact on the pulmonary functions.
Aims and Objectives:
The study was undertaken to analyze the pulmonary function parameters in diabetic patients and compare them with age and gender matched healthy subjects. We correlated forced vital capacity (FVC) and forced expiratory volume in 1 second (FEV1) in diabetic patients with duration of the disease and glycosylated hemoglobin (HbA1c).
Materials and Methods:
Pulmonary function tests (PFTs) were recorded in 60 type 2 diabetic male patients and 60 normal healthy male controls aged 40-60 years by using Helios 702 spirometer. The PFTs recorded were - FVC, FEV1, FEV1/FVC, FEF25, FEF50, FEF75, FEF25–75, FEF0.2–1.2, and peak expiratory flow rate (PEFR). HbA1c of all the patients was estimated by ion exchange resin method, which is a very standard method of estimation. PFTs of diabetic patients and controls were compared by applying Student′s unpaired t test. Associations between FVC and FEV1 and HbA1c and duration of illness in diabetic patients were analyzed by applying Pearson′s coefficient.
Results:
The PFTs were significantly decreased in diabetic patients compared with the healthy controls except FEV1/FVC. There was no correlation found between FVC and FEV1 and duration of illness as well as HbA1c.
Conclusion:
DM being a systemic disease, which also affects lungs causing restrictive type of ventilatory changes probably because of glycosylation of connective tissues, reduced pulmonary elastic recoil and inflammatory changes in lungs. We found glycemic levels and duration of disease are probably not the major determinants of lung pathology, which requires further research.
Keywords: Diabetes, glycemic control, pulmonary function
INTRODUCTION
The World Health Organization estimates that more than 180 million people worldwide have diabetes, and by 2030 it is expected that this number will have doubled.[1] There is an alarming increase in the incidence and prevalence of diabetes mellitus (DM) in Asian Indians.[2] Diabetes is a micro-macrovascular disorder with debilitating effects on many organs. Pulmonary complications of DM have been poorly characterized with conflicting results. The alveolar capillary network in the lung is a large micro-vascular unit and may be affected by microangiopathy.[3] However, because of its large reserve, substantial loss of the microvascular bed can be tolerated without developing dyspnoea. As a result, pulmonary diabetic micro-angiopathy may be under-recognized clinically. In DM pulmonary functions have been studied frequently in countries other than India,[4] while in our country there are few studies concerning these abnormalities and their relationship with glycosylated hemoglobin (HbA1c) and duration of the disease.
Reduced elastic recoil, reduced lung volume, diminished respiratory muscle performance, chronic low grade inflammation,[5,6] decrease in pulmonary diffusion capacity for carbon monoxide,[7] autonomic neuropathy involving respiratory muscles[8] are some of the important changes occurring in DM.
Despite the unclear nature, the relationship between DM and pulmonary function tests (PFTs) remains important because of potential epidemiological and clinical implications. The loss of pulmonary reserve may become clinically important.
Hence, we hypothesized that PFTs are affected in DM in Indian population and the changes may correlate with HbA1c and the duration of the disease.
Objectives
The study aims to evaluate the PFTs in type 2 DM patients and compare them with the age and gender matched healthy controls.
We also determine the co-relation of the HbA1c and duration of the disease with PFTs in type 2 DM patients.
MATERIALS AND METHODS
The study was carried out in collaboration with Diabetes Outpatient Department of Sassoon General Hospitals. The Institutional Ethics Committee approved the study protocol. Sixty male patients of type 2 DM diagnosed by the treating physician, of the age group 40–60 years taking oral hypoglycemics, were randomly selected from the Diabetes Outpatient Department.
Exclusion criteria
Patients having complaints of cough, sputum, or dyspnoea.
Smokers and patients with any cardio-respiratory illnesses or major diseases.
Sixty normal healthy males of the same age group and socioeconomic status from patient′s relatives were selected as control group. The controls were also thoroughly examined clinically. Those with cardio-respiratory, musculoskeletal, or endocrine diseases were excluded from the study. Fasting and postprandial blood glucose levels were measured by glucose oxidase method to rule out type 2 DM in them.
Informed written consent was taken from patients as well as from controls. All the patients were handed a questionnaire that contained a detailed personal and medical history. PFTs of the patients as well as of the controls were performed with turbine flow sensor-based 702 Helios - Spirometer (Chandigarh, India) between 11 am and 12 pm. All the tests were conducted according to American Thoracic Society/European Respiratory Society (ATS/ERS guidelines) in a quiet room in sitting position by the trained personnel.[9] The controls and patients performed spirometry three times at the interval of 15 minutes and the best of the three was taken into account. Parameters recorded were - forced vital capacity (FVC) in liters, forced expiratory volume in 1 second (FEV1), FEV1/FVC in percentage (%), forced expiratory flow during 25% of FVC (FEF25), forced expiratory flow during 50% of FVC (FEF50), forced expiratory flow during 75% of FVC (FEF75), forced expiratory flow during 25–75% of FVC (FEF25–75), forced expiratory flow during 0.2–1.2 liters of FVC (FEF0.2–1.2), and peak expiratory flow rate (PEFR). For all these parameters percentage of predicted values for the respective age, height, and weight were taken into consideration.
Nearly 2 ml of venous blood was collected in ethylenediamine tetra acetic acid (EDTA) bulb in all the diabetic patients with aseptic precautions. HbA1c of all the patients was estimated by ion exchange resin method by the diagnostic glycohaemoglobin kits of Asritha Diatech as per the guidelines provided.[10]
All data were collected in a data collection form and then transferred to an Excel sheet by two independent data entry operators. Discrepant values were corrected by checking the data collection form. Clean data was then analyzed statistically.
PFTs of diabetic patients and controls were compared by applying Student′s unpaired ′t′ test. Correlations between FVC and FEV1 and HbA1c and duration of illness in diabetic patients were analyzed by applying Pearson′s coefficient. Statistical analysis was done by using SSPS version 11 and Graphic Prism Pad version 5 (Statistician, B.J. Medical College).
RESULTS
Table 1 depicts the physical characteristics of the normal controls as well as the patients of DM. Age, height, and weight of both the groups were comparable as statistically there was no difference between them (P > 0.05). Our study showed that all the pulmonary parameters, that is, FVC, FEV1, FEF25, FEF50, FEF75, FEF25–75, FEF0.2–1.2, and PEFR were significantly reduced except FEV1/FVC in patients of type 2 DM as compared with the healthy controls (P < 0.05). The ratio FEV1/FVC is almost equal in normal controls and diabetic patients (P > 0.05) [Table 2, Figure 2]. On correlating the FVC and FEV1 with duration of illness and HbA1c, we found that there was no significant correlation between them (P > 0.05) [Table 3, Figures 1 and 2].
Table 1.
Physical characteristics of subjects
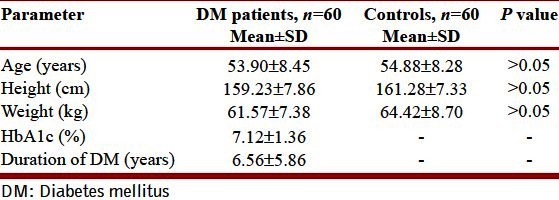
Table 2.
Comparison of PFTs in patients with type 2 DM and healthy controls
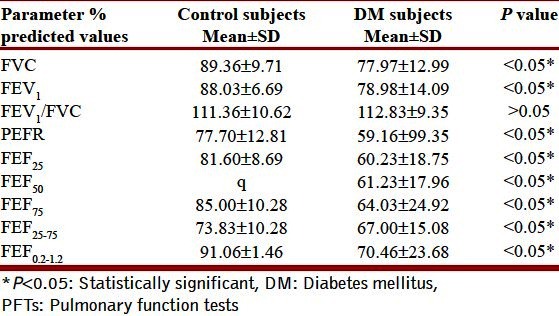
Figure 2.
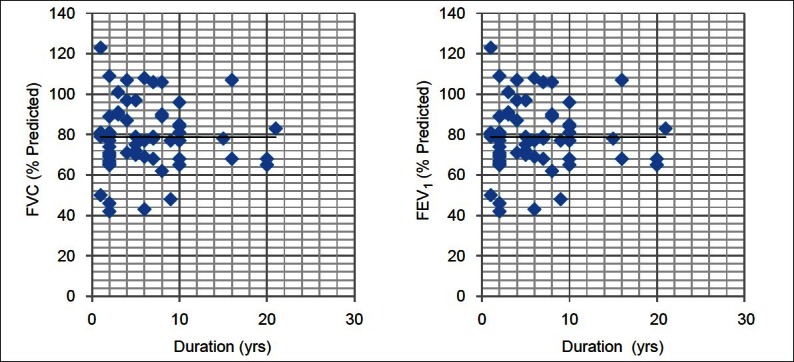
Correlation of PFTs with duration of diabetes. P > 0.05 - Statistically not significant
Table 3.
Correlation of HbA1c and duration of DM with PFTs
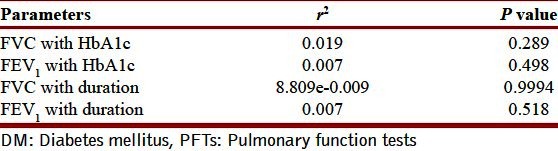
Figure 1.
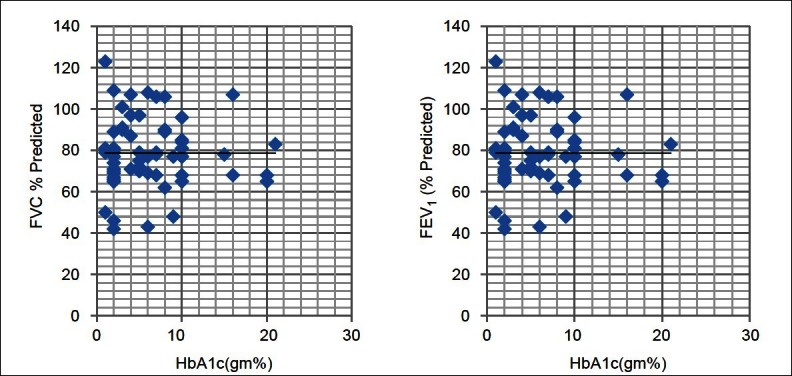
Correlation of HbA1c with PFTs. P > 0.05 - Statistically not significant
DISCUSSION
Our study showed that all the pulmonary parameters, that is, FVC, FEV1, FEF25, FEF50, FEF75, FEF25–75, FEF0.2–1.2, and PEFR were significantly reduced except FEV1/FVC in patients of type 2 DM as compared with the healthy controls. This is in accordance with previous studies.[11–15]
Some of the prospective and cross sectional studies have shown low vital capacity or restrictive pattern in type 2 DM.[16,17]
Meta-analysis by van den Borst, et al. showed that DM is associated with statistically significant, impaired pulmonary function in a restrictive pattern. Moreover, these results were irrespective of body mass index (BMI), smoking, diabetes duration, and HbA1c levels.[18]
Uchida, et al. found that there was decreased pulmonary diffusing capacity in patients with diabetes with perfusion defect on ventilation perfusion scintigrams.[19] It was not possible for us to analyze the pulmonary diffusing capacity because of practical difficulties.
Davis, et al. conducted a study in Western Australia in large number of patients of type 2 DM. They found that VC, FVC, FEV1, and PEFR decreased at an average of between 1.1% and 3.1% of predicted values/year in type 2 DM patients.[11]
Ehrlich, et al. showed that patients with type 2 DM were at increased risk of several pulmonary condition like - asthma, Chronic Obstructive Pulmonary Disease (COPD), fibrosis, and pneumonia.[20]
Few studies have mentioned that no significant differences were observed in patients of type 2 DM.[21–23] Probably the small sample size is the reason behind these findings.
Pathophysiology of reduced lung function is still an interesting research issue. Normal lung mechanics and gas exchange are influenced by the integrity of the pulmonary connective tissue and microvaculature. Acceleration of aging process in connective tissue cross links and presence of nonenzymatic glycosylation and modification of alveolar surfactant action causes reduction in PFTs.[3] There have been reports of histopathological changes in the diabetic patients. In the study by Weynand et al.,[24] it was found that alveolar epithelium, endothelium capillary, and basal laminaes were thickened in lungs on electron microscopy, when compared with the controls. In addition, the thickening of basal laminae was of the same magnitude in lung and kidney. Diabetic microangiopathy might be existing in the pulmonary vascular bed. Moreover, reduced pulmonary capillary blood volume was found, favoring the evidence of microangiopathy. This could lead to redistribution of the pulmonary circulation, resulting in well ventilated areas to become underperfused.[25]
The thorax and lungs are rich in collagen and elastin. Stiffening of thorax and lung parenchyma can occur because of nonenzymatic glycosytion of these structural compounds. This may lead to restrictive pattern.[3] In our studies, since the FVC/FEV1 ratio is statistically not significantly different in DM patients as compared with normal controls, other PFT values are lower in DM patients; this strongly suggests restrictive pattern in DM patients.
Studies have even shown diabetic polyneuropathy, which affects respiratory neuromuscular function and thus reducing pulmonary volumes.[26]
On correlating the FVC and FEV1 with duration of illness and HbA1c, we found that there was no significant correlation between them [Table 3, Figures 2 and 3].
There are certain studies showing no correlationship between HbA1c and PFTs.[7,14,22] They argued that HbA1c levels are indicators of glycemic control for a short period of 1–2 months, it was not adequate to conclude that the plasma glucose level was not related to decreased PFTs. While some studies have shown that the decline in PFTs was negatively correlated with HbA1c.[11,13]
There are studies that have reported no significant correlation between PFTs and duration of diseases,[22] while some of the studies have reported a strong negative correlation of PFTs with duration.[14,15] Since DM is a disease, which involves multiple organs randomly, the study of the effect of duration of the disease on them requires further research.
Several studies have analyzed the association between impaired lung function and death and found that a 10% decrease in FEV1 was associated with a 12% increase in all cause mortality in type 2 DM.[27]
Clinical implications
Pulmonary dysfunction should be regarded as a specific derangement induced by DM. Further studies may clarify whether this should be included as a long-term complication of diabetes. The role of strict glycemic control on pulmonary function in diabetic patients is another interesting aspect and needs further studies. The impairment in PFTs can lower the threshold for clinical manifestations of acute or chronic lung disease. Patients with DM admitted with pneumonia have increased risk of complications and mortality.[28]
Limitations and scope
It seems to be necessary to repeat PFTs and to assess the changes of pulmonary functions among the same subjects. Over a long observation course, the relationship between the plasma glucose concentration and the PFTs can be elucidated.
SUMMARY AND CONCLUSION
DM being a systemic disease, also affects lungs causing restrictive type of ventilatory changes probably because of glycosylation of connective tissues, reduced pulmonary elastic recoil, and inflammatory changes in lungs. We found that glycemic levels and duration of disease are probably not the major determinants of lung pathology, which requires further research.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.World Health Organization. Fact sheet: Diabetes No 312, November 2008. [Last accessed on 2009 Apr 14]. Available from: http://www.who.int/mediacentre/factsheets/fs312/en/index.html .
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes 1995 to 2025. Prevalence, numerical estimates and projections. Diabetes Care. 1998;21:1414–31. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Sandler M. Is the lung is target organ in diabetes mellitus? Arch Intern Med. 1990;150:1385–8. [PubMed] [Google Scholar]
- 4.Klein OL, Krishnan JA, Jlick S, Smith LJ. Systematic review of association between lung function and type 2 diabetes mellitus. Diabet Med. 2010;27:977–87. doi: 10.1111/j.1464-5491.2010.03073.x. [DOI] [PubMed] [Google Scholar]
- 5.Hamlin CR, Kohn RR, Luschin JH. Apparent accelerated aging of human collagen in diabetes mellitus. Diabetes. 1975;24:902–4. doi: 10.2337/diab.24.10.902. [DOI] [PubMed] [Google Scholar]
- 6.Fogarty AW, Jones S, Britton JR, Lewis SA, McKeever TM. Systemic inflammation and decline in lung function in a general population: A prospective study. Thorax. 2007;62:515–20. doi: 10.1136/thx.2006.066969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mori H, Okubo M, Okamura M, Yamane K, Kado S, Egusa G, et al. Abnormalities of pulmonary function in patients with non insulin dependent diabetes mellitus. Intern Med. 1992;31:189–93. doi: 10.2169/internalmedicine.31.189. [DOI] [PubMed] [Google Scholar]
- 8.Williams JG, Morris AI, Hayter RC, Ogilvie CM. Respiratory responses of diabetics to hypoxia, hypercapnia and exercise. Thorax. 1984;39:529–34. doi: 10.1136/thx.39.7.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardization of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 10.Nathan DM, Singer DE, Hurxthal K, Goodson JD. The clinical information value of the glycosylated haemoglobin assay. N Engl J Med. 1984;310:341–6. doi: 10.1056/NEJM198402093100602. [DOI] [PubMed] [Google Scholar]
- 11.Davis WA, Knuiman M, Kendall P, Grange V, Davis TM. Glycemic exposure is associated with reduced pulmonary function in type 2 diabetes, the fremantle diabetes study. Diabetes Care. 2004;27:752–7. doi: 10.2337/diacare.27.3.752. [DOI] [PubMed] [Google Scholar]
- 12.Asanuma Y, Fujiya S, Ide H, Agishi Y. Characteristics of pulmonary function in patients with diabetes mellitus. Diabetes Res Clin Pract. 1985;1:95–101. doi: 10.1016/s0168-8227(85)80034-6. [DOI] [PubMed] [Google Scholar]
- 13.Lange P, Groth S, Kastrup J, Mortensen J, Appleyard M, Nyboe J, et al. Diabetes mellitus, plasma glucose and lung function in a cross sectional population study. Eur Respir J. 1989;2:14–9. [PubMed] [Google Scholar]
- 14.Barrett-Conor E, Frette C. NIDDM, impaired glucose tolerance, and pulmonary function in older adults. Diabetes Care. 1996;19:1441–4. doi: 10.2337/diacare.19.12.1441. [DOI] [PubMed] [Google Scholar]
- 15.Davis TM, Knuiman M, Kendall P, Vu H, Davis WA. Reduced pulmonary function and its association in type 2 diabetes: The fremantle diabetes study. Diabetes Res Clin Pract. 2000;50:153–9. doi: 10.1016/s0168-8227(00)00166-2. [DOI] [PubMed] [Google Scholar]
- 16.Engstrom GJ, Janzon L. Risk of developing diabetes is inversely related to lung function: A population based cohort study. Diabet Med. 2002;19:167–70. doi: 10.1046/j.1464-5491.2002.00652.x. [DOI] [PubMed] [Google Scholar]
- 17.Yeh HC, Punjabi NM, Wang NY, Pankow J, Duncan BB, Cox CE, et al. Cross sectional and prospective study of lung function in adults with diabetes mellitus. Diabetes. 2002;51:A242–3. doi: 10.2337/dc07-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borst BB, Gosker HR, Zeegers MP, Schols AM. Pulmonary function in Diabetes: A Metaanalysis. Chest. 2010;138:393–406. doi: 10.1378/chest.09-2622. [DOI] [PubMed] [Google Scholar]
- 19.Uchida K, Takahashi K, Aoki R, Ashitaka T. Ventilation-perfusion scintigram in diabetics. Ann Nucl Med. 1991;5:97–102. doi: 10.1007/BF03164621. [DOI] [PubMed] [Google Scholar]
- 20.Ehrlich SF, Quesenberry CP, Vanden Eeden SK, Shan J, Ferrara A. Patients diagnosed with diabetes are at increased risk for asthma, COPD, pulmonary fibrosis and pneumonia but not lung cancer. Diabetes Care. 2010;33:55–60. doi: 10.2337/dc09-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shan-ping J, Li-wen H, Yi-qun L, Guo-juan L, He-lin D, Yan L, et al. Pulmonary function in patients with diabetes mellitus. Chin J Pathophysiol. 2005;21:574–9. [Google Scholar]
- 22.Benbassat CA, Stern E, Kramer M, Lebzelter J, Blum I, Fink G. Pulmonary function in patients with diabetes mellitus. Am J Med Sci. 2001;322:127–32. doi: 10.1097/00000441-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 23.Sinha S, Guleria R, Misra A, Pandey RM, Yadav R, Tiwari S. Pulmonary functions in patients with type 2 diabetes mellitus and correlation with anthropometry and microvascular complications. Indian J Med Res. 2004;119:66–71. [PubMed] [Google Scholar]
- 24.Weynand B, Jonkheree A, Frans A, Rahier J. Diabetes mellitus induces a thickening of the pulmonary basal lamina. Respiration. 1999;66:14–9. doi: 10.1159/000029331. [DOI] [PubMed] [Google Scholar]
- 25.Sandler M, Bunn AE, Stewart RI. Cross section study of pulmonary function in patients with insulin-dependent diabetes mellitus. Am Rev Respir Dis. 1987;135:223–9. doi: 10.1164/arrd.1987.135.1.223. [DOI] [PubMed] [Google Scholar]
- 26.Kabitz HJ, Sonntag F, Walker D. Diabetic polyneuropathy is associated with respiratory muscle impairement in type 2 diabetes. Diabetologia. 2008;51:191–7. doi: 10.1007/s00125-007-0856-0. [DOI] [PubMed] [Google Scholar]
- 27.Knuiman MW, James AL, Diviniti ML, Ryan G, Bartholomew HC, Musk AW. Lung function, respiratory symptoms, and mortality: Results from the buselton health study. Ann Epidemiol. 1999;9:297–306. doi: 10.1016/s1047-2797(98)00066-0. [DOI] [PubMed] [Google Scholar]
- 28.Kornum JB, Thomsen RW, Riis A, Lervang HH, Schonheyder HC, Sorensen HT. Diabetes, glycemic control, and risk of hospitalization with pneumonia: A population based case control study. Diabetes Care. 2008;31:1541–5. doi: 10.2337/dc08-0138. [DOI] [PMC free article] [PubMed] [Google Scholar]


