Abstract
Fat embolism and fat embolism syndrome (FES) are well-known complications of long bone fracture and surgery involving manipulation of skeletal elements. Many non-traumatic causes of FES have been suggested but they constitute only a small portion. FES presents with classical symptoms of petechiae, hypoxemia, central nervous system symptoms along with other features such as tachycardia and pyrexia. Diagnosis of FES relies on clinical judgment rather than objective findings such as emboli present in the retinal vessels on fundoscopy, fat globules present in urine and sputum, a sudden inexplicable drop in hematocrit or platelet values, increasing erythrocyte sedimentation rate.
Keywords: Alveolar hemorrhage, fat embolism syndrome, bone fractures
INTRODUCTION
Fat embolism and Fat embolism syndrome (FES) are rare but life threatening complications associated with fracture and surgical manipulation of skeletal elements. None of the proposed diagnostic criteria have good diagnostic value as FES may present atypically such as, diffuse alveolar haemorrhage. Clinical suspicion is the cornerstone in early diagnosis and treatment of FES.
CASE REPORT
A 25-year-old man was referred to us three days post road traffic accident. He was found to have open fracture shaft of left femur and fracture condyle of left humerus. There was no associated history of loss of consciousness, vomiting, convulsions or any bleed. Intra medullary nailing was done for fracture femur under general anesthesia in a peripheral hospital. Intra-operative and immediate post-operative period were uneventful. Within next 18 hours he developed altered sensorium and became restless. He was hypotensive and shortly became breathless and hypoxic. He was intubated and mechanically ventilated. Endotracheal bleed was detected post intubation. He was transferred to our center for further management. On evaluation in emergency department, he was found to be drowsy, restless, hypotensive, and there were signs of pulmonary hemorrhage in the form of blood stained endotracheal secretion with hypoxia. There were no obvious petechial rashes apart from small hemorrhagic spots in bilateral conjunctiva. Initial resuscitation was done and he was started on noradrenalin infusion in view of hypotension. Fiberoptic bronchoscopy showed blood in trachea and bilateral tracheobronchial tree. Bronchoalveolar lavage fluid was hemorrhagic suggestive of alveolar hemorrhage. Initial laboratory parameters showed: Hemoglobin 8.4 gm/dl, total leucocyte count 11300/cc, platelet count 80000/cc. Renal functions were deranged with serum urea 38 mg/dl and serum creatinine 1.2 mg/dl. His liver function tests showed serum billirubin 2.1 mg/dl (direct 1.0 mg/dl), SGOT/SGPT 235 and 114 IU/L. His serum lactate level was 2.6 mmol/dl. Initial arterial blood gas analysis showed, pH 7.37, PaCO2 63.5 mm Hg, PaO2 70.8, SaO2 93% with FiO2 of 1. X-ray chest was consistent with bilateral diffuse alveolar opacities consistent with ARDS. Other investigations include Serum d-Dimer 4.3 (Normal 0.063–0.245 mg/dl), Anti-CCP antibodies 0.43 (Normal < 5 RU/ml), ANA (Immunofluroscence) negative at 1:100 dilutions, PR-3ANCA (ELISA) 1.5 IU/ml (Normal < 6 u/ml). Bronchoalveolar lavage fluid showed numerous debris laden alveolar macrophages and Pearl′s stain was suggestive of intracellular positivity. Urine examination for fat globules was negative. His serum creatinine phosphokinase was 691IU/L. EEG showed delta-theta coma pattern suggestive of modest encephalopathy.
On presentation there was evidence of subcutaneous emphysema with X-ray chest showing signs of pneumomediastenum. Initial high resolution computed tomography (HRCT) chest scan showed confluencing air space opacity with ground glass densities and interlobar septal thickening involving bilateral lung fields suggestive of acute respiratory distress syndrome (ARDS) [Figure 1]. He was electively ventilated for next three days, intercostal drain was placed in view of pneumomediastenum. Subcutaneous emphysema gradually subsided and elective tracheostomy was done on 7th day of admission. Repeat HRCT chest on day 6 revealed patchy ill defined air space opacity in bilateral lower lobes with ground glassing in bilateral upper and middle lobes. Right pneumothorax, pneumo mediastenum, excessive subcutnaneous emphysema. ARDS changes in bilateral lung fields were significantly reduced [Figure 2]. His neurological status gradually improved over next 5 days and was put on T piece on 12th day, which he tolerated well. He underwent fixation of fracture humerus condyle, which was uneventful. He was decannulated on 15th day of admission and discharged on 18th day.
Figure 1.
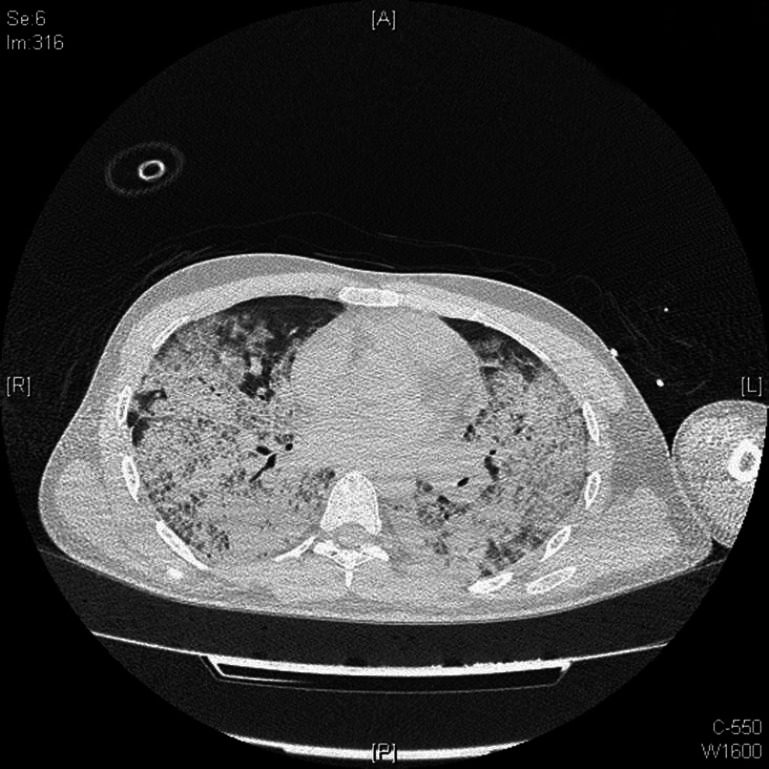
Computer tomography of chest showing bilateral alveolar hemorrhage
Figure 2.

CT of chest showing recovering acute respiratory distress syndrome and pnemothoarax, pneumomediastenum with intercostal chest tube insitu
DISCUSSION
Original paper describing fat embolism dates from 1873. Fat embolism is defined as a blockage of blood vessels by intravascular fat globules ranging from 10–40 μm in diameter.[1]
Fat embolism syndrome (FES) comprises a defined set of clinical pattern and is a serious consequence of fat embolism. Though the major cause of FES is skeletal fracture associated with trauma, small percentage (5%) of cases do have atraumatic etiology. These atraumatic causes include bone marrow transplantation, pancreatitis, sickle cell disease, burns, prolonged high-dose corticosteroid therapy, diabetes mellitus, hepatic trauma, liposuction, lipectomy, external cardiac compression, gas gangrene, decompression sickness, and lipid infusions etc.[2,3] Perioperative incidence of FES is between 3.5 to 5% in surgeries involving early fixation of fractures.[4]
Age is considered to be a determining factor in the development of FES: Young men with fractures are at increased risk.[5,6] Our patient was a 25 year old male.
Single bone fracture has a rare chance of FES (1–3%). The incidence may increase with number of bones involved. Bilateral femoral fracture demonstrated an incidence of as high as 33 percent.[7] An overall mortality of 5–15% has been described.[8] Intramedullary fat embolises as a result of fracture or intramedullary procedure. This embolic phenomenon has been confirmed echocardiographically.[9]
The fat enters torn venules which are kept patent in the Haversian canals and makes way into circulation.[10] Fat globule ranging from 7–10 μm in diameter has been documented to traverse the pulmonary vasculature. Systemic embolisation has been documented due to a patent foramen ovale which may be present in 25% of individuals.[11]
Even larger size fat globule can traverse the foramen if severe pulmonary hypertension is precipitated by fat embolism. Pulmonary hypertension can cause a pressure difference between the right and left atria which leads to embolisation of larger fat globule.[10]
Systemic liberation of fat can cause FES and fulminant FES by different pathophysiological mechanisms. FES is caused by perivascular hemorrhage and edema following the accumulation of fat in the pulmonary, cerebral or dermal microvasculature and local liberation of free fatty acids (FFAs).[12] Obstructive shock caused by fat globules may result in fulminant FES.
The timing of symptomatic presentation of FES may be variable. Most typically it presents 24–72 h after the initial injury. In rare cases it may be delayed as much as 2 weeks after the insult. In our case, the patient developed respiratory distress and encephalopathy within 18 hours of surgical manipulation of the fracture.[13]
Classically, it presents with asymptomatic interval followed by pulmonary, neurologic, and skin manifestations. It has a biphasic clinical course. The initial symptoms are caused by mechanical occlusion of blood vessels with fat globules that are too large to pass through the capillaries. Unlike other embolic events, the vascular occlusion in fat embolism is often temporary or incomplete since fat globules do not completely obstruct capillary blood flow. The late presentation is due to hydrolysis of the fat to more irritating FFAs which then migrate to other organs via the systemic circulation.[14]
The most common system involved is respiratory (95%) followed by the central nervous system.
The most common presentation with fat embolism includes severe hypoxemia and respiratory distress. The incidence has been reported to be 50% to 96% of the affected patients and most of them require ventilation.[15,16]
Petechial rash in conjunctiva, oral mucosa, and skin folds in axilla and neck occurs in up to 60% of cases. The described mechanism for development of petechiae is embolization of small dermal capillaries leading to extravasation of erythrocytes.[16] We noted oblivious petechiae in conjunctiva in both eyes of the patient [Figure 3].
Figure 3.

Conjunctival hemorrhage
A number of minor features of fat embolism syndrome may be present and these appear to result from the release of toxic mediators secondary either to the initial injury or to dysfunctional lipid metabolism. These include tachycardia, myocardial depression, ECG changes indicative of right heart strain, soft fluffy retinal exudates with macular edema, scotomata (Purtscher′s retinopathy), coagulation abnormalities (which mimics disseminated intravascular coagulation).[17]
Fat embolism can lead to diffuse alveolar hemorrhage. The described pathogenesis is an inflammatory response caused by lipoprotein lipase which is activated by catecholamine surge caused by stress. It acts on the fat deposited in the pulmonary or systemic capillary network liberating high concentrations of toxic FFAs locally. It causes platelet aggregation, a mild disseminated intravascular coagulation, and disruption of the pulmonary and cerebral capillary walls.[18]
Pulmonary histology usually reveals intra-alveolar hemorrhage, fat within pulmonary capillaries and oedema. Cerebral histology reveals diffuse cerebral edema with multiple hemorrhagic petechiae.[19]
FES is a clinical diagnosis and none of the evaluated laboratory parameters found to be sensitive or specific enough to diagnose FES. Various definitive criteria (include clinical and laboratory parameters) have been described, which include Gurd′s (four major and three minor criteria), Lindeque′s criteria (four criteria) and Schonfeld′s criteria (14 out of 15 points) [Chart 1].[20–23]
Chart 1.
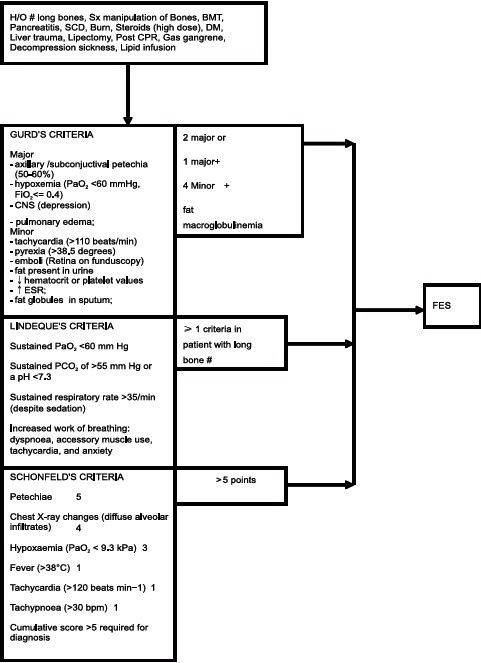
Diagnostic criteria for fat embolism syndrome
Investigations are usually performed to support the clinical diagnosis or to monitor therapy which include: Hematology and biochemistry: An unexplained anemia (70% of patients) and thrombocytopenia (platelet count <1,50,000/cumm in up to 50% of patients). Hypocalcaemia (due to binding of free fatty acids to calcium) and elevated serum lipase have also been reported.[6,24] Hypofibrinogenemia, raised erythrocyte sedimentation rate (ESR), and prolongation of prothrombin time may be present.[15,25] Isolated case report suggests microemboli detected by Trans Cranial Doppler (TCD) and Magnetic Resonance Imaging (MRI) can add in diagnosing FES.[6,26–28]
Corticosteroids act as anti-inflammatory agents and reduce the perivascular hemorrhage and edema. They have been used extensively and recommended by some for the management of FES. There is insufficient scientific evidence to support initiation of steroid therapy in established FES. An experimental study showed no beneficial effect.[24]
Use of aspirin has been advocated. Heparin is thought to have potential for activating lipoprotein lipase thereby increases the clearance of lipemic serum. On the other hand, there is a potential risk of increase in free fatty acid level there-by possible enhancement of inflammation.[24]
Bloody bronchoalveolar lavage specimens (with numerous erythrocytes and siderophages) establish the diagnosis of diffuse alveolar hemorrhage.
Treatment of alveolar hemorrhage include steroid. Majority of cases of pulmonary hemorrhge have an autoimmune cause and mainstay of the treatment include steroids and immunosuppression.[29]
As in our case the cause is nonimmune, we used high dose steroids (methyl prednisolone started at 60 mg twice daily and tapered over 2 weeks with improvement of clinical state).
CONCLUSION
FES is a rare but devastating complication in patients presenting with traumatic fracture of long bones or procedure related and involving manipulation of the same. The diagnosis of FES is clinical, though use of diagnostic criteria may be helpful. Alveolar hemorrhage is a rare presentation of FES and the management is essentially supportive. Though none of the available studies have clearly supported the role of steroids, we found good result with early use of steroid in our case.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Weisz GM. Fat embolism. Curr Probl Surg. 1977;14:1–54. doi: 10.1016/s0011-3840(74)80001-8. [DOI] [PubMed] [Google Scholar]
- 2.Dudney TM, Elliott CG. Pulmonary embolism from amniotic fluid, fat, and air. Prog Cardiovasc Dis. 1994;36:447–74. doi: 10.1016/s0033-0620(94)80053-7. [DOI] [PubMed] [Google Scholar]
- 3.Levy DL. The fat embolism syndrome: A review. Clin Orthop Relat Res. 1990;2612:281–6. [PubMed] [Google Scholar]
- 4.Latif A, Bashir A, Aurangzeb, Ghani U. Fat embolism and fat embolism syndrome. Prof Med J. 2008;15:407–13. [Google Scholar]
- 5.Akhtar S. Fat embolism. Anesthesiol Clin. 2009;27:533–50. doi: 10.1016/j.anclin.2009.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Gupta B, D′Souza N, Sawhney C, Kamran Farooque K, Kumar A, Agrawal P, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS apex trauma center, New Delhi, India. J Emerg Trauma Shock. 2011;4:337–41. doi: 10.4103/0974-2700.83859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19:41. doi: 10.3928/0147-7447-19960101-09. [DOI] [PubMed] [Google Scholar]
- 8.Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56:145–54. doi: 10.1046/j.1365-2044.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 9.Christie J, Robinson CM, Pell AC, McBirnie J, Burnett R. Transcardiac echocardiography during invasive intramedullary procedures. J Bone Joint Surg Br. 1995;77:450–5. [PubMed] [Google Scholar]
- 10.Fabian TC. Unraveling the fat embolism syndrome. N Engl J Med. 1993;329:961–3. doi: 10.1056/NEJM199309233291313. [DOI] [PubMed] [Google Scholar]
- 11.Pell AC, Hughes D, Keating J, Christie J, Busuttil A, Sutherland GR. Brief report: Fulminating fat embolism syndrome caused by paradoxical embolism through a patent foramen ovale. N Engl J Med. 1993;329:926–9. doi: 10.1056/NEJM199309233291305. [DOI] [PubMed] [Google Scholar]
- 12.Moylan JA, Birnbaum M, Katz A, Everson MA. Fat emboli syndrome. J Trauma. 1976;16:341. doi: 10.1097/00005373-197605000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Carr J, Hansen S. Fulminant fat embolism. Orthopedics. 1990;13:258–61. doi: 10.3928/0147-7447-19900201-20. [DOI] [PubMed] [Google Scholar]
- 14.Jawaid M, Naseem M. An update on fat embolism syndrome (FES) Pak J Med Sci. 2005;21:389–92. [Google Scholar]
- 15.King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15:561–80. [PubMed] [Google Scholar]
- 16.Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38:52–5. [PubMed] [Google Scholar]
- 17.Jones JP., Jr Fat embolism, intravascular coagulation, and osteonecrosis. Clin Orthop Relat Res. 1993;292:294–308. [PubMed] [Google Scholar]
- 18.Glover P, Worthley LI. Fat embolism. Critical Care Resusc. 1999;1:276–84. [PubMed] [Google Scholar]
- 19.Nijsten MW, Hamer JP, Ten Duis HJ, Posma JL. Fat embolism and patent foramen ovale. Lancet. 1989;1:1271. doi: 10.1016/s0140-6736(89)92370-2. [DOI] [PubMed] [Google Scholar]
- 20.Gurd AR. Fat embolism: An aid to diagnosis. J Bone Joint Surg Br. 1970;52:732–7. [PubMed] [Google Scholar]
- 21.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56:408–16. [PubMed] [Google Scholar]
- 22.Lindeque BG, Schoeman HS, Dommissen GF, Boeyens MC, Vlok AL. Fat embolism syndrome: A double blind therapeutic study. J Bone Joint Surg Br. 1987;69:128–31. doi: 10.1302/0301-620X.69B1.3818718. [DOI] [PubMed] [Google Scholar]
- 23.Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroids. Ann Intern Med. 1983;99:438–43. doi: 10.7326/0003-4819-99-4-438. [DOI] [PubMed] [Google Scholar]
- 24.Gupta A, Reilly CS. Fat embolism. Cont Edu Anaesth Crit Care Pain. 2007;7:148–51. [Google Scholar]
- 25.Blake DR, Fisher GC, White T, Bramble MG. Ionized calcium in fat embolism. Br Med J. 1979;13:902. doi: 10.1136/bmj.2.6195.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shobha N, Bermejo PG, Bhatia R, Choi Y, Smith EE, Demchuk AM. Multimodal imaging tools for diagnosis of fat embolism. J Emerg Trauma Shock. 2011;4:306–8. doi: 10.4103/0974-2700.82232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36:847–51. doi: 10.1212/wnl.36.6.847. [DOI] [PubMed] [Google Scholar]
- 28.Yoshida A, Okada Y, Nagata Y, Hanaguri K, Morio M. Assessment of cerebral fat embolism by magnetic resonance imaging in the acute stage. J Trauma. 1996;40:437–40. doi: 10.1097/00005373-199603000-00019. [DOI] [PubMed] [Google Scholar]
- 29.Ioachimescu OC, Stoller JK. Diffuse alveolar hemorrhage: Diagnosing it and finding the cause. Cleve Clin J Med. 2008;75:258, 260, 264–5. doi: 10.3949/ccjm.75.4.258. [DOI] [PubMed] [Google Scholar]


