Abstract
Background:
Fat embolism syndrome (FES) is a clinical problem arising mainly due to fractures particularly of long bones and pelvis. Not much literature is available about FES from the Indian subcontinent.
Materials and Methods:
Thirty-five patients referred/admitted prospectively over a 3-year period for suspected FES to a north Indian tertiary care center and satisfying the clinical criteria proposed by Gurd and Wilson, and Schonfeld were included in the study. Clinical features, risk factors, complications, response to treatment and any sequelae were recorded.
Results:
The patients (all male) presented with acute onset breathlessness, 36-120 hours following major bone trauma due to vehicular accidents. Associated features included features of cerebral dysfunction (n = 24, 69%), petechial rash (14%), tachycardia (94%) and fever (46%). Hypoxemia was demonstrable in 80% cases, thrombocytopenia in 91%, anemia in 94% and hypoalbuminemia in 59%. Bilateral alveolar infiltrates were seen on chest radiography in 28 patients and there was evidence of bilateral ground glass appearance in 5 patients on CT. Eleven patients required ventilatory assistance whereas others were treated with supportive management. Three patients expired due to associated sepsis and respiratory failure, whereas others recovered with a mean hospital stay of 9 days. No long term sequelae were observed.
Conclusion:
FES remains a clinical challenge and is a diagnosis of exclusion based only on clinical grounds because of the absence of any specific laboratory test. A high index of suspicion is required for diagnosis and initiating supportive management in patients with traumatic fractures, especially in those having undergone an invasive orthopedic procedure.
Keywords: Accidents, ARDS, fat embolism syndrome, trauma
INTRODUCTION
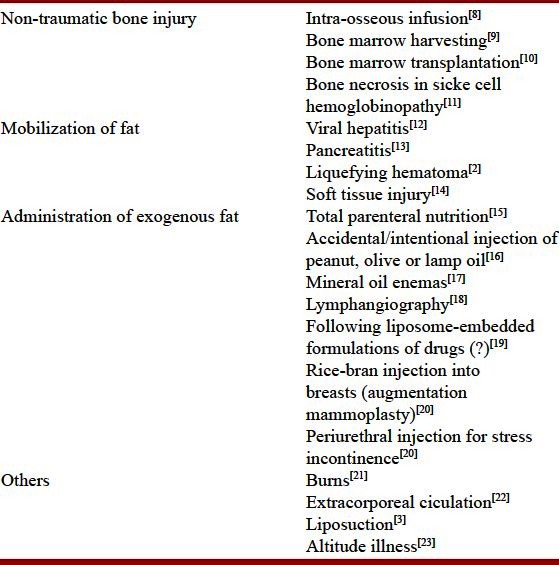
Fat embolism indicates the often asymptomatic presence of fat globules in the lung parenchyma and peripheral circulation,[1] generally following long bone or other major trauma, and cardiopulmonary resuscitation in medical patients.[2–5] Fat embolism syndrome (FES) is a serious consequence of this phenomenon producing a distinct pattern of clinical symptoms and signs, generally involving the skin, the lungs and the brain.[1–5] Fat released from disruption of the sinusoids and adipose tissue in the marrow allows the fat emboli and bone fragments to gain access to the venous circulation and embolization occurs.[6] Embolization of fat is almost universal in patients who sustain a pelvic or a long bone fracture, undergo endomedullary nailing of the fractures or placement of knee or hip prosthesis.[6] More commonly associated with fractures of long bones and the pelvis (marrow containing bones) and more frequent in closed rather than open fractures, the incidence increases with the number of fractures involved.[1,7] Thus, patients with a single long bone fracture have a 1–3% chance of developing the syndrome, but it has been reported in up to 33% of patients with bilateral femoral fractures.[4,5] While the majority (>95%) of fat embolism cases follow major trauma, non-trauma-related causes [Table 1] have also been reported to result in FES, albeit less likely than with trauma.[8–25] The mechanisms underlying the non-traumatic causes include mobilization of fat in conditions like viral hepatitis and pancreatitis; bone marrow necrosis after occlusion and activation of the clotting system in sickle cell disease or administration of exogenous fat in procedures like lymphangiography [Table 1].
Table 1.
Depicting reported causes of fat embolism syndrome unrelated to skeletal trauma
As mild cases go unnoticed, the exact incidence is usually underestimated and has ranged from as low as 1% to 29% by different investigators.[26,27] In a report on 50 autopsies, Eriksson et al. reported a virtual epidemic of pulmonary fat emboli being detected in 82% of trauma patients and 63% of non-trauma patients, 86% and 88% having received cardiopulmonary resuscitation respectively.[3] Comparing the clinical criteria with postmortem examination to diagnose FES, Georgopoulos et al.,[5] found that despite the evidence of FES in 20% of patients at autopsy, only 0.9% could be diagnosed on clinical grounds. Thus the diagnosis of FES remains a clinical challenge and a number of scoring systems have been devised to help arrive at a clinical diagnosis.[28–30] An overall mortality of 5–15% has been described.[2]
There is a paucity of literature regarding the FES from the Indian subcontinent. We herewith present our experience with FES in a tertiary care hospital setting in north India where road traffic accidents are common.
MATERIALS AND METHODS
All patients prospectively referred to the Sheri Kashmir Institute of Medical Sciences (a 750 bedded tertiary care hospital) as suspected FES (n = 102) and fulfilling the clinical criteria[28,29] for FES over a 2-year period were included in the study. All the patients were admitted in the ortho division of the hospital or other sister hospitals with fractures of long bones or pelvis requiring admission for traction or any surgical procedure and were suspected to have FES because of the development of symptoms like cough, breathlessness or skin rash. The patients were screened by fulfilling the criteria by Gurd as well as those by Schonfeld [Tables 2 and 3] by performing the screening clinical examination and relevant lab investigations like blood gas analysis, hemogram, etc., [Tables 2 and 3]. Fat Embolism Syndrome was diagnosed applying both Gurd′s as well as Schonfeld′s criteria, the diagnosis FES requiring at least two major criteria or one major and four minor criteria to be present as per the Gurd′s criteria [Table 1] and a Schonfeld score [Table 2] of greater or equal to 5. Patients with obvious cause of hypoxemia or mental obtundation like head injury, overt sepsis, rib fractures, spinal cord trauma, abdominal trauma, etc., were excluded from the study. Patients referred with a suspicion of FES and not satisfying the criteria were also excluded from the study. The criteria were applied at admission and also within first 48 hours of admission and only those fulfilling the criteria were included. Data collected included demographic profile, clinical presentation with time of onset of symptoms of FES. Various investigations included hemogram, serum biochemistry including urea, creatinine, glucose, lipids, albumin, proteins, bilirubin, AST, ALT, sodium, potassium and alkaline phosphatase. Arterial blood gases were performed at admission and repeated as required. Radiograph of the chest and an electrocardiogram was obtained and a CT of the chest was obtained in 6 cases and that of the head in 24 cases. All the patients were managed conservatively and the final and long term outcome of the patients was recorded. The study was approved by the Institutional review board and informed consent was obtained from all participants.
Table 2.
Showing diagnostic criteria for FES based on Gurd′s criteria
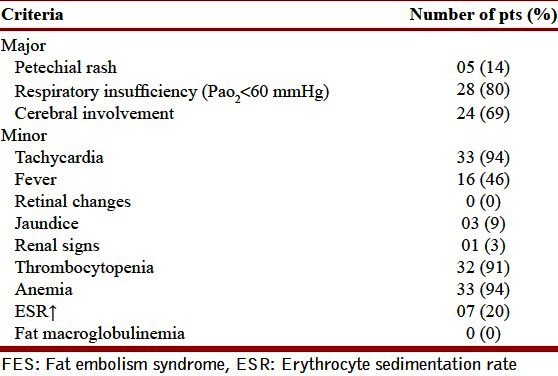
Table 3.
Showing the Schonfeld crieria for diagnosing FES, a cumulative score of >5 is required for diagnosis

RESULTS
Out of 102 suspected patients (with various fractures either of long bones of extremities or multiple trauma), a total of 35 patients fulfilled the criteria of FES, both on Gurd as well as on Schonfeld score scale and were thus included in the study. The 35 cases were all male with age ranging from 14 to 60 years (median 28 years). The clinical features and laboratory parameters are depicted in the Tables 2 and 4. The patients presented with acute onset breathlessness accompanied by altered sensorium (n = 24), petechial rash (n = 5, Figure 1) and fever, 36-120 hours (median 48 hours) following road traffic accidents. Thrombocytopenia, anemia and hypoxia were the commonest laboratory abnormalities. Out of three major criteria two patients fulfilled all major criteria of Gurd′s, whereas 26 had two major criteria. All cases fulfilled the Schonfeld criteria (score > 5) with max score of 15, minimum of 6 and mean of 10.
Table 4.
Various laboratory parameters
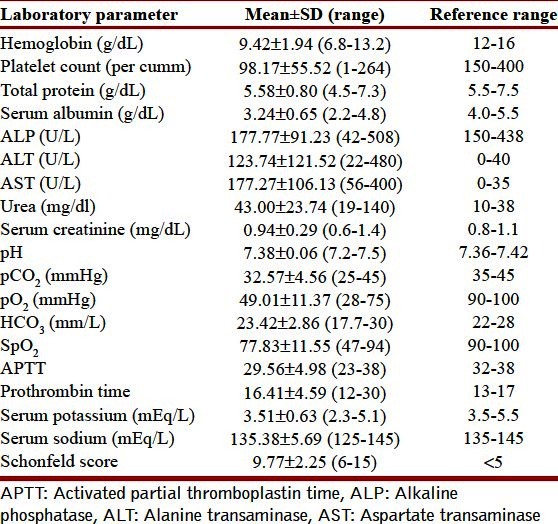
Figure 1.
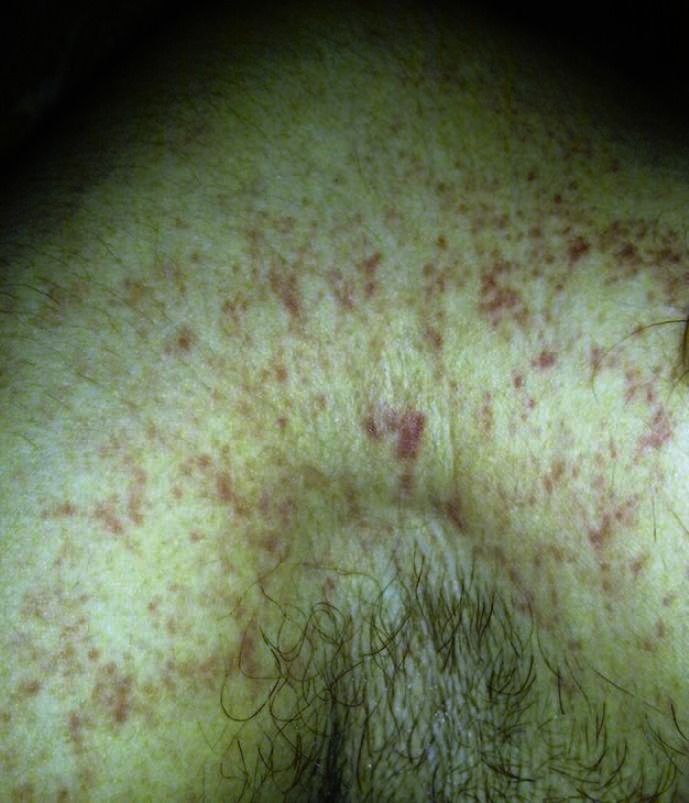
Evidence of petechial rash over the upper trunk
The various laboratory parameters at admission are depicted in Table 4. Radiographs of the chest showed bilateral alveolar infiltrates in 28 cases, 5 of these revealing evidence of bilateral ground glass appearance with septal thickening on high resolution tomography [Figure 2]. Serum albumin levels ranged from 2.2 to 4.8 g/l (median 3.15 g/dl) with 59% having hypoalbuminemia. Hyperbilirubinemia was present in 10 patients with clinically discernible jaundice in 3 whereas renal dysfunction occurred in one patient. Table 4 shows the laboratory values of various parameters collected.
Figure 2.
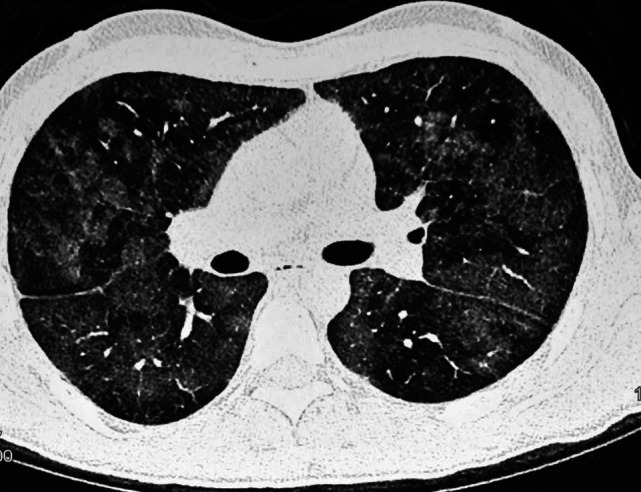
CT scan showing bilateral ground glass appearance and septal thickening
Eleven patients progressed to develop features of ARDS that required mechanical ventilation. All the patients received routine supportive care which included orthopedic care, oxygen therapy, albumin (n = 14) and antibiotics (n = 12). Three patients developed multi-organ dysfunction and succumbed whereas all the others had a full recovery over a period of 3–22 days (median 9d).
DISCUSSION
All our patients satisfied the criteria for FES proposed by Gurd as well as by Schonfeld [Tables 2 and 3]. Ever since its first description by Zenker in 1861, the diagnosis of FES is still based on clinical criteria and continues to be a clinical challenge. The clinical criteria[28–30] proposed by various investigators are helpful in arriving at a presumptive diagnosis, the one proposed by Gurd and Wilson being the most commonly employed one [Table 2].
As in our patients, FES mostly follows long bone or a pelvic fracture and is more frequent in closed fractures than open fractures or in those having undergone an orthopaedic procedure.[1] All of our patients followed trauma and intramedullary nailing had been undertaken in 10 of our patients. The affected patients classically suffer from a triad of hypoxemia, neurological abnormalities and a characteristic petechial rash in the upper body. Hypoxemia is the earliest finding which can progress to acute lung injury and half of these may go on to develop adult respiratory distress syndrome needing mechanical ventilation.[31] Majority of our patients (80%) also presented with hypoxemia with 31% progressing to ARDS like syndrome requiring mechanical ventilation.
Altered mental status was common in our patients, seen in about 60%. The altered mental status was persistent despite correction of accompanying hypoxemia. Most of the patients who develop FES develop neurological abnormalities ranging from an acute confusional state to altered level of consciousness and rarely seizures and focal neurological deficits.[32] All of our patients who recovered had a full recovery of their neurological abnormalities that was tested by mini-mental scale scoring. Neurological abnormalities are generally fully reversible without any long term sequalae.[32] However, patients have been reported to progress to brain death following cerebral fat embolism.[33] Residual neurological deficits may range from subtle personality changes to memory loss, cognitive dysfunction and long term focal deficits.[34] FES alone has not yet been reported to cause global anoxic injury, but it may play a contributory role, acting along with other cerebral insults.[34]
The characteristic petechial skin rash was seen in 14% our patients [Figure 1]. Considered pathognomonic of FES, it occurs in up to 60% of patients.[2,35] All of our patients had the rash in the axillae and the upper trunk. The rash is found most often on the head, neck, anterior thorax, axillae, and sub-conjunctiva. When present, the rash is the last component of the triad to develop and usually resolves in 5 to 7 days.[35] Occlusion of dermal capillaries by fat emboli with resultant extravasation of erythrocytes leads to the development of this rash.[2,35] Even as the rash could have been present in a higher number of patients, the darker skin of the Asian population might have led to the difficulty in its identification. Additionally the rash is short lived and hence may not be present at the time of diagnosis. The characteristic distribution of the rash has been attributed to the fat droplets accumulating in the aortic arch prior to embolization to nondependent skin via the subclavian and carotid vessels.[36] Direct systemic embolization of fat causing scotomata (Purtscher′s retinopathy) and lipiduria or the release of toxic mediators causing fever, disseminated intravascular coagulation like syndrome and myocardial depression have also been reported.[37]
Amongst the laboratory parameters, an unexplained drop in hematocrit and thrombocytopenia could be important pointers towards the diagnosis.[26] These were the commonest laboratory abnormalities in our study too. The mechanisms underlying thrombocytopenia are unclear but platelet activation by bone marrow emboli with thrombus formation as well as disseminated intravascular coagulation have been proposed as possible pathogenetic processes.[38] Hypoalbuminemia was seen in 59% in our patients. Hypoalbuminemia has also been suggested due to plasma free fatty acids (FFA) binding to albumin.[38] Besides these an elevated blood lipase levels, elevated FFA levels and hypocalcemia (due to binding of free fatty acids to calcium) have been described in these patients.[1,7]
Radiological features which have been described in patients with FES include chest X ray findings of bilateral fluffy shadows and the classical multiple flocculent shadows (“snow storm appearance”). These findings can persist up to 3 weeks.[39] Malagari et al., reported the high-resolution CT (HRCT) findings of the lungs in patients with mild FES and observed that there was evidence of bilateral ground-glass opacities and thickening of the interlobular septa, whereas in some cases centrilobular nodular opacities were present.[40] We also found bilateral ground glass opacity, confluent infiltrates and septal thickening on HRCT chest in 5 patients who underwent this imaging modality [Figure 2]. MRI of the brain may reveal characteristic high-intensity signal abnormalities located in the watershed areas perfused by perforating arteries and diffuse anatomic distribution of the lesions on T2WI.[41] None of our patients underwent an MRI of the brain.
Recovery of fat globules in blood (upon pulmonary artery catheterization) and fat droplets within cells recovered by bronchoalveolar lavage (BAL) has been suggested as a rapid and specific method for establishing the diagnosis of the FES, although of uncertain significance.[42,43] None of the 14 patients in whom sputum was tested in our patients had fat globules in the sputum. Bronchoalveolar lavage was not performed in any of our patients. Although both fat from blood as well as BAL fluid lack sensitivity and specificity but the absence of staining of macrophages for fat on BAL might prompt a search for alternative cause of hypoxemia.[2]
Mortality in the current study was 8%. Mortality in FES has ranged from 5 to 15%,[2] the major cause of death being progressive respiratory failure as a result of evolution to ARDS. On occasion FES can present as acute cor pulmonale, respiratory failure, and/or embolic phenomena, leading to death within a few hours of injury.[44] Patients with increased age, multiple underlying medical problems, and/or decreased physiologic reserves have worse outcomes.[44]
The pathogenesis of FES remains controversial, and a mechanical as well as a biochemical theory has been proposed. According to the mechanical theory the organ dysfunction in FES is the result of the direct entry of depot fat globules from disrupted tissue into the bloodstream and travelling to the pulmonary vasculature.[45] According to the biochemical theory, inflammatory reactants, including lipoprotein lipase, cause the release of FFA with a resultant alteration of fat transport mechanisms of the plasma. The changed homeostasis results in fat droplet aggregation with systemic sequestration in the microvasculature.[46] The time delay in the occurrence of symptoms can be explained by this biochemical theory.[47]
Since there is no specific therapy for fat embolism syndrome; prevention, early diagnosis, and adequate supportive or alternate treatment are of paramount importance. The preventive therapies include early immobilization of fractures and early fixation of fractures together with external fixation or fixation with plate and screw.[48–50] Besides using smaller diameter unreamed nails have also been shown to produce lesser lung injury.[50] Additionally Pitto, et al., have demonstrated that limiting elevation of the intraosseous pressure during orthopedic procedures reduces the intravasation of intramedullary fat and other debris, which may in turn reduce the incidence of FES.[51] Multiple treatments have been evaluated without any significant changes on clinical outcome and these include clofibrate, dextran-40, ethyl alcohol, heparin, aspirin, and steroids.[2,52] Prophylactic corticosteroids have been shown to reduce the risk of FES in high risk patients. A recent meta-analysis of six randomized trials (389 patients with long-bone fractures) comparing systemic corticosteroids plus supportive care to supportive care alone has demonstrated reduced incidence of FES without any positive influence on mortality[53] However, further studies have been advised to conclusively ascertain the role of steroids.[54] None of our patients received steroids. The duration of FES is difficult to predict because FES is often subclinical or overshadowed by other illnesses or injuries. Increased alveolar-to-arterial oxygen gradient and neurology deficits, including altered consciousness, may last days or weeks. As in ARDS, the pulmonary sequelae usually resolve almost completely within a year. Residual subclinical diffusion capacity deficits may persist.[34]
Our study is limited by the fact that patients whose rash might have disappeared prior to the screening would clearly be missed as they might not have satisfied the scoring criteria. In addition, it can be argued altered mental status at admission could be partly contributed by other factors like hypoxemia and attendant sepsis that would affect the criteria for enrollment into the study. However all the patients fulfilled both Gurd as well as Schonfeld criteria for enrollment and the correction of hypoxemia led to the improvement of the mental status in all who recovered except in the 3 cases who had an inexorably downhill course that resulted in their demise. Another limitation was the inability to demonstrate lipiduria or lipids in sputum. Since BAL was not performed and cranial MRI not done, some patients with fat globules in BAL fluid or cerebral fat embolism could have been missed too.
In conclusion, FES is a clinical syndrome and laboratory tests are nonspecific for diagnosing this condition. A low threshold for suspicion is needed to diagnose patients with FES following vehicular trauma so that appropriate supportive measures are instituted. Further preventive strategies should be routine during management of traumatic fractures.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Gupta A, Reilly CS. Fat Embolism. Continuing education in Anasthesia. Crit care pain. 2007;7:148–51. [Google Scholar]
- 2.Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56:145–54. doi: 10.1046/j.1365-2044.2001.01724.x. [DOI] [PubMed] [Google Scholar]
- 3.Eriksson EA, Pellegrini DC, Vanderkolk WE, Minshall CT, Fakhry SM, Cohle SD. Incidence of pulmonary fat embolism at autopsy: An undiagnosed epidemic. J Trauma. 2011;71:312–5. doi: 10.1097/TA.0b013e3182208280. [DOI] [PubMed] [Google Scholar]
- 4.Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19:41–8. doi: 10.3928/0147-7447-19960101-09. [DOI] [PubMed] [Google Scholar]
- 5.Georgopoulos D, Bouros D. Fat embolism syndrome clinical examination is still the preferable diagnostic method. Chest. 2003;123:982–3. doi: 10.1378/chest.123.4.982. [DOI] [PubMed] [Google Scholar]
- 6.Koessler MJ, Fabiani R, Hamer H, Pitto RP. The clinical relevance of embolic events detected by transesophageal echocardiography during cemented total hip arthroplasty: A randomized clinical trial. Anesth Analg. 2001;92:49–55. doi: 10.1097/00000539-200101000-00010. [DOI] [PubMed] [Google Scholar]
- 7.Jorens PG, Van Marck E, Snoeckx A, Parizel PM. Nonthrombotic pulmonary embolism. Eur Respir J. 2009;34:452–74. doi: 10.1183/09031936.00141708. [DOI] [PubMed] [Google Scholar]
- 8.Hasan MY, Kissoon N, Khan TM, Saldajeno V, Goldstein J, Murphy SP. Intraosseous infusion and pulmonary fat embolism. Pediatr Crit Care Med. 2001;2:133–8. doi: 10.1097/00130478-200104000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Baselga J, Reich L, Doherty M, Gulati S. Fat embolism syndrome following bone marrow harvesting. Bone Marrow Transplant. 1991;7:485–6. [PubMed] [Google Scholar]
- 10.Lipton JH, Russell JA, Burgess KR, Hwang WS. Fat embolization and pulmonary infiltrates after bone marrow transplantation. Med Pediatr Oncol. 1987;15:24–7. doi: 10.1002/mpo.2950150106. [DOI] [PubMed] [Google Scholar]
- 11.Vichinsky EP, Neumayr LD, Earles AN, Williams R, Lennette ET, et al. Causes and outcomes of the acute chest syndrome in sickle cell disease. National acute chest syndrome study group. N Engl J Med. 2000;342:1855–65. doi: 10.1056/NEJM200006223422502. [DOI] [PubMed] [Google Scholar]
- 12.Schulz F, Trübner K, Hildebrand E. Fatal fat embolism in acute hepatic necrosis with associated fatty liver. Am J Forensic Med Pathol. 1996;17:264–8. doi: 10.1097/00000433-199609000-00016. [DOI] [PubMed] [Google Scholar]
- 13.Guardia SN, Bilbao JM, Murray D, Warren RE, Sweet J. Fat embolism in acute pancreatitis. Arch Pathol Lab Med. 1989;113:503–6. [PubMed] [Google Scholar]
- 14.Hiss J, Kahana T, Kugel C. Beaten to death: Why do they die? J Trauma. 1996;40:27–30. doi: 10.1097/00005373-199601000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Schulz PE, Weiner SP, Haber LM, Armstrong DD, Fishman MA. Neurological complications from fat emulsion therapy. Ann Neurol. 1994;35:628–30. doi: 10.1002/ana.410350521. [DOI] [PubMed] [Google Scholar]
- 16.Seifert SA, Dart RC, Kaplan EH. Accidental, intravenous infusion of a peanut oil-based medication. J Toxicol Clin Toxicol. 1998;36:733–6. doi: 10.3109/15563659809162624. [DOI] [PubMed] [Google Scholar]
- 17.Rabah R, Evans RW, Yunis EJ. Mineral oil embolization and lipid pneumonia in an infant treated for hirschsprung′s disease. Pediatr Pathol. 1987;7:447–55. doi: 10.3109/15513818709161406. [DOI] [PubMed] [Google Scholar]
- 18.Francis RA, Barnes PA, Libshitz HI. Pulmonary oil embolism after lymphangiography. J Comput Assist Tomogr. 1983;7:170–1. doi: 10.1097/00004728-198302000-00040. [DOI] [PubMed] [Google Scholar]
- 19.Tolentino LF, Tsai SF, Witt MD, French SW. Fatal fat embolism following amphotericin B lipid complex injection. Exp Mol Pathol. 2004;77:246–8. doi: 10.1016/j.yexmp.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Kiyokawa H, Utsumi K, Minemura K, Kasuga I, Torii Y, Yonemaru M, et al. Fat embolism syndrome caused by vegetable oil injection. Intern Med. 1995;34:380–3. doi: 10.2169/internalmedicine.34.380. [DOI] [PubMed] [Google Scholar]
- 21.Currie I, Drutz HP, Deck J, Oxorn D. Adipose tissue and lipid droplet embolism following periurethral injection of autologous fat: Case report and review of the literature. Int Urogynecol J Pelvic Floor Dysfunct. 1997;8:377–80. doi: 10.1007/BF02765599. [DOI] [PubMed] [Google Scholar]
- 22.Levy D. The fat embolism syndrome. A review. Clin Orthop Relat Res. 1990;35:628–30. [PubMed] [Google Scholar]
- 23.Hodge AJ, Dymock RB, Sutherland HD. A case of fatal fat embolism syndrome following cardiopulmonary bypass. J Thorac Cardiovasc Surg. 1976;72:202–5. [PubMed] [Google Scholar]
- 24.Laub DR, Jr, Laub DR. Fat embolism syndrome after liposuction: A case report and review of the literature. Ann Plast Surg. 1990;25:48–52. doi: 10.1097/00000637-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Haymaker W, Davidson C. Fatalities resulting from exposure to simulated high altitudes in decompression chambers; a clinicopathologic study of five cases. J Neuropathol Exp Neurol. 1950;9:29–59. doi: 10.1097/00005072-195001000-00003. [DOI] [PubMed] [Google Scholar]
- 26.Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome: A 10 years review. Arch Surg. 1997;132:435–9. doi: 10.1001/archsurg.1997.01430280109019. [DOI] [PubMed] [Google Scholar]
- 27.Fabian TC, Hoots AV, Stanford DS, Patterson CR, Mangiante EC. Fat embolism syndrome, prospective evaluation in 92 fractured patients. Crit Care Med. 1990;18:42–6. [PubMed] [Google Scholar]
- 28.Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56:408–16. [PubMed] [Google Scholar]
- 29.Schonfeld SA, Ploysongsang Y, DiLisio R, Crissman JD, Miller E, Hammerschmidt DE, et al. Fat embolism prophylaxis with corticosteroids. Ann Intern Med. 1983;99:438–43. doi: 10.7326/0003-4819-99-4-438. [DOI] [PubMed] [Google Scholar]
- 30.Lindeque B, Schoeman H, Dommisse G, Boeyens MC, Vlok AL. Fat embolism and the fat embolism syndrome. J Bone Joint Surg. 1987;69B:128–31. doi: 10.1302/0301-620X.69B1.3818718. [DOI] [PubMed] [Google Scholar]
- 31.King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15:561. [PubMed] [Google Scholar]
- 32.Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36:847–51. doi: 10.1212/wnl.36.6.847. [DOI] [PubMed] [Google Scholar]
- 33.Eriksson EA, Schultz SE, Cohle SD, Post KW. Cerebral fat embolism without intracardiac shunt: A novel presentation. J Emerg Trauma Shock. 2011;4:309–12. doi: 10.4103/0974-2700.82233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shaikh N. Emergency management of fat embolism syndrome. J Emerg Trauma Shock. 2009;2:29–33. doi: 10.4103/0974-2700.44680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kaplan RP, Grant JN, Kaufman AJ. Dermatologic features of the fat embolism syndrome. Cutis. 1986;38:52–5. [PubMed] [Google Scholar]
- 36.Tachakara SS. Distribution of skin petechiae in fat embolism rash. Lancet. 1976;1:284–5. doi: 10.1016/s0140-6736(76)91408-2. [DOI] [PubMed] [Google Scholar]
- 37.Carlson DS, Pfadt E. Fat embolism syndrome. Nursing. 2011;41:72. doi: 10.1097/01.NURSE.0000395312.91409.7f. [DOI] [PubMed] [Google Scholar]
- 38.Riseborough EJ, Herndon JH. Aletrations in pulmonary functions, coagulation and fat metabolism in patients with fractures of the lower limbs. Clin Orthop Relat Res. 1976;115:248–67. [PubMed] [Google Scholar]
- 39.Liljedahl SO, Westermark L. Aetiology and treatment of fat embolism. Reports of five cases. Acta Anaesthesiol Scand. 1967;11:177–94. doi: 10.1111/j.1399-6576.1967.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 40.Malagari K, Economopoulos N, Stoupis C, Daniil Z, Papiris S, Müller NL, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003;123:1196–201. doi: 10.1378/chest.123.4.1196. [DOI] [PubMed] [Google Scholar]
- 41.Takahashi M, Suzuki R, Osakabe Y, Asai J, Miyo T, Nagashima G, et al. Magnetic resonance imaging findings in cerebral fat embolism: Correlation with clinical manifestations. J Trauma. 1999;46:324–7. doi: 10.1097/00005373-199902000-00021. [DOI] [PubMed] [Google Scholar]
- 42.Chastre J, Fagon JY, Soler P, Fichelle A, Dombert MC, Huten D, et al. Bronchoalveolar Lavage for rapid diagnosis of the fat embolism syndrome in trauma patients. Ann Intern Med. 1990;113:583–8. doi: 10.7326/0003-4819-113-8-583. [DOI] [PubMed] [Google Scholar]
- 43.Godeau B, Schaeffer A, Bachir D, Fleury-Feith J, Galacteros F, Verra F, et al. Bronchoalveoalr lavage in adult sickle cell patients with acute chest syndrome value for diagnostic assessment of fat embolism. Am J Resp Crit Care Med. 1996;153:1691–6. doi: 10.1164/ajrccm.153.5.8630622. [DOI] [PubMed] [Google Scholar]
- 44.Nikoliæ S, Miciæ J, Saviæ S, Gajiæ M. Factors which could affect the severity of post-traumatic pulmonary fat embolism: A prospective histological study. Srp Arch Celok Lek. 2003;131:244–8. doi: 10.2298/sarh0306244n. [DOI] [PubMed] [Google Scholar]
- 45.Gossling HR, Pellegrini VD., Jr Fat embolism syndrome: A review of the pathophysiology and physiological basis of treatment. Clin Orthop Relat Res. 1982;165:68–82. [PubMed] [Google Scholar]
- 46.Fabian TC. Unravelling the fat embolism syndrome. N Engl J Med. 1993;329:961–3. doi: 10.1056/NEJM199309233291313. [DOI] [PubMed] [Google Scholar]
- 47.Müller C, Rahn BA, Pfister U, Meinig RP. The incidence, pathogenesis, diagnosis, and treatment of fat embolism. Orthop Rev. 1994;23:107–17. [PubMed] [Google Scholar]
- 48.Parisi DM, Koval K, Egol K. Fat embolism syndrome. Am J Orthop (Belle Mead NJ) 2002;31:507–12. [PubMed] [Google Scholar]
- 49.Brundage SI, McGhan R, Jurkovich GJ, Mack CD, Maier RV. Timing of femur fracture fixation: Effect on outcome in patients with thoracic and head injuries. J Trauma. 2002;52:299–307. doi: 10.1097/00005373-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 50.Behrman SW, Fabian TC, Kudsk KA, Taylor JC. Improved outcome with femur fracture: Early VS delayed fixation. J Trauma. 1990;30:792–7. doi: 10.1097/00005373-199007000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Pitto RP, Schramm M, Hohmann D, Kössler M. Relevance of the drainage along the linea aspera for the reduction of fat embolism during cemented total hip arthroplasty. A prospective, randomized clinical trial. Arch Orthop Trauma Surg. 1999;119:146–50. doi: 10.1007/s004020050378. [DOI] [PubMed] [Google Scholar]
- 52.Taviloglu K, Yanar H. Fat embolism syndrome. Surg Today. 2007;37:5–8. doi: 10.1007/s00595-006-3307-5. [DOI] [PubMed] [Google Scholar]
- 53.Kubota T, Ebina T, Tonosaki M, Ishihara H, Matsuki A. Rapid improvement of respiratory symptoms associated with fat embolism by high-dose methylpredonisolone: A case report. J Anesth. 2003;17:186–9. doi: 10.1007/s00540-003-0164-x. [DOI] [PubMed] [Google Scholar]
- 54.Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures. A meta-analysis? Can J Surg. 2009;52:386–93. [PMC free article] [PubMed] [Google Scholar]


