Abstract
Objectives:
To evaluate the prolongation of ventricular repolarization and proarrhythmic activity of antimalarial drug chloroquine in two rabbit proarrhythmia models viz., in vivo α1 adrenoceptor-stimulated anesthetized rabbit and ex vivo isolated Langendorff rabbit heart using clofilium as standard proarrhythmic agent.
Materials and Methods:
In the in vivo model, three groups of rabbits, anesthetized by pentobarbitone sodium and α-chloralose, sensitized with α1 agonist methoxamine followed by either continuous infusion of saline (control) or clofilium (3 mg/kg) or chloroquine (21 mg/kg) for 30 min. In ex vivo model, rabbit hearts were perfused with clofilium (10 μM) or chloroquine (300 μM) continuously after priming along with methoxamine, acetylcholine chloride and propranolol hydrochloride.
Results:
In these models, prolongation of repolarization during α1-adrenoceptor stimulation produced early after depolarization (EAD) and Torsade de pointes (TdP). Saline infusion did not induce any abnormality in the animals. Clofilium caused expected changes in the electrocardiogram in both the models including TdP (50.0% in in vivo and 66.67% in ex vivo). Chloroquine caused decrease in heart rate and increase in the corrected QT (QTc) interval in both the models. Further, apart from different stages of arrhythmia, TdP was evident in 33.33% in ex vivo model, whereas no TdP was observed in in vivo model.
Conclusions:
The results indicated that proarrhythmic potential of chloroquine and clofilium was well evaluated in both the models; moreover, both the models can be used to assess the proarrhythmic potential of the new drug candidates.
Keywords: α1 -Adrenoceptor stimulation, chloroquine, clofilium, torsade de pointes, in vivo rabbit model, ex vivo rabbit model
INTRODUCTION
Several non-cardiac drugs have the liability of unanticipated morbidity and mortality associated with adverse cardiac events.[1] This risk is often associated with the development of prolongation of QT, early after depolarization (EAD), and subsequently development of Torsade de pointes (TdP). Increase in QT interval usually occurs with the drugs, which have the potential to block the potassium channels and inhibit the delayed rectifier potassium current (IKr) leading to early after depolarization.[2] Therefore, it has become mandatory to screen the new chemical entity for its potential to block the IKr channels before first time administration in human.[3] However, there are clinical evidences that TdP may also develop in the absence of QT interval prolongation.[4] Therefore, the battery of in vitro, ex vivo and in vivo preclinical assays is required to assess the proarrhythmic potential of new chemical entities (NCEs). Over recent years numbers of in vivo and ex vivo models have been developed to predict TdP in humans.[5]
Reported dataset showed that not only the class III antiarrhythmic drugs but non-cardiac drugs like antihistamine (terfenadine), antimalarials (halofantrine) and antibiotics (sparfloxacin, moxifloxacin, erythromycin and telithromycin) are also associated with proarrhythmic liabilities.[6–9] Antimalarial drug, chloroquine, is known for its cardiovascular effects as it blocks Ik1, Ikr, INa and ICa-L. These findings provide the cellular mechanism for the prolonged action potentials and reduction in Vmax of cardiac action potentials.[10] However, there is a lack of information available about possibility of chloroquine to cause TdP. The present study was conducted to evaluate the proarrhythmic potential of chloroquine in in vivo rabbit model of arrhythmia and ex vivo model using clofilium as a standard proarrhythmic drug; a class III antiarrhythmic agent for the validation of both the models.
MATERIALS AND METHODS
Drugs
The following drugs were used: Clofilium tosylate (Alexis Biochemicals, Switzerland), chloroquine phosphate (Ranbaxy Research Laboratories, Gurgaon), hydroxy-β-cyclodextrine (Roquette, France), methoxamine HCl, propranolol hydrochloride, acetylcholine chloride (ACh chloride) and α-chloralose (Sigma Chemicals, St. Louis, MO, USA), and pentobarbitone sodium (LOBA Chemie, Mumbai, India). Pentobarbitone sodium, clofilium tosylate, methoxamine hydrochloride and chloroquine phosphate were dissolved in normal saline. α-chloralose solution was prepared in 3% hydroxy-β-cyclodextrine solution; 10 μM clofilium tosylate, 10 μM propranolol hydrochloride, 0.6 μM of methoxamine hydrochloride, 0.6 μM ACh chloride and 300 μM chloroquine phosphate solutions were prepared in filtered 1 mM modified Krebs-Henseleit solution.
Animals
New Zealand white rabbits weighing 1.5 to 2.5 kg (n = 30) were used in this study. The animals were handled according to protocol approved by Institutional Animal Ethics Committee (IAEC) and Standard Operating Procedures (SOPs), Ranbaxy Research Laboratories, Gurgaon, India. The method and number of animals employed to the study comply with ethical care and use of animal and was duly approved by IAEC.
Experimental protocol
In vivo model – α1 adrenoceptor methoxamine stimulated anesthetized rabbit
After sedation with pentobarbitone sodium (45 mg/kg i.v.), rabbits were anesthetized with α-chloralose at the dose of 100 mg/kg with the continuous infusion rate of 1 ml/min/kg into the left marginal ear vein. Tracheotomy was performed to control the respiration with ventilator (Columbus instruments, model CIV 101, USA). Respiratory rate and tidal volume were set at 40 strokes/min and 7 ml/kg, respectively. The animal body temperature was maintained at 36 to 38oC with DC Temperature Control Module (Stoelting model 40-90-8C, USA). The right femoral artery was cannulated for monitoring the blood pressure using Biopac MP100 Data Acquisition Unit (Biopac Systems, Inc., Santa Barbara, CA, USA). Both the ear veins were cannulated with 25 G × 3/4” infusion set. All catheters inserted in blood vessels were filled with heparinized saline (100 IU/ml). After stabilization for approximately 30 min, baseline measurement for heart rate (HR), mean arterial blood pressure (MBP) and electrocardiogram (ECG; lead II) parameters were recorded. Thereafter, methoxamine (15 μg/kg/min, 2 ml/kg/hour) was administered intravenously through right marginal ear vein up to 40 min through infusion pump. After 10 min of methoxamine administration, rabbits (6/group) were intravenously infused with normal saline (Group I) or clofilium 3 mg/kg (Group II) or chloroquine 21 mg/kg (Group III) for 30 min at the rate of 0.1 ml/kg/min. Methoxamine was continued simultaneously with the vehicle or clofilium or chloroquine throughout the experiment and animal was observed up to 1 hour.[11–13]
Langendorff isolated rabbit heart ex vivo model
The animals were divided into two groups of six animals each: Group I were administered methoxamine, ACh chloride, propranolol and clofilium (10 μM/0.9 ml/min); group II were administered methoxamine, ACh chloride, propranolol and chloroquine (300 μM/0.9 ml/min). Rabbits were injected sodium heparin (1000 IU/kg i.v.) and stunned by a blow to the neck. Hearts were excised via midsternal thoracotomy and immediately immersed in modified Krebs-Henseleit (KH) solution containing (in mM) NaCl 112.0, KCl 2.0, D-Glucose 11.5, NaHCO3 25.0, MgSO4 0.2, CaCl2 2.4 and KH2 PO4 1.0. Heart was mounted on the Langendorff isolated heart system (Model LF/01 Experimetria Ltd, Budapest, Hungary) and was retrogradely perfused with peristaltic pump (Harvard) at constant pressure (80 mmHg) with the modified Krebs-Henseleit buffer solution warmed to 37°C. A mixture of 95% O2 and 5% CO2 was bubbled through the buffer, which was equilibrated to pH 7.4. Heart was allowed to equilibrate for 20 min before baseline recording of HR and ECG (Lead II). After baseline recording, ACh chloride (0.6 μM/0.5 ml/min), methoxamine (0.6 μM/0.5 ml/min) and propranolol (10 μM/0.5 ml/min) were continuously infused throughout the experiment. After 10 min of methoxamine, ACh chloride and propranolol infusion, clofilium (10 μM/0.9 ml/min) or chloroquine (300 μM/0.9 ml/min) was infused for 30 min or until TdP occurred.[14]
Observation of different stages of arrhythmia and analysis
AcqKnowledge 3.9.0 software (BIOPAC Inc, Goleta, CA) and SPEL Advanced Haemosys software 2.45 (Experimetria Ltd. and Logirex Software Laboratory, Budapest, Hungary) were used to analyze the ECG waveforms of in vivo and ex vivo models, respectively.
ECG parameters were measured at different time points before and after methoxamine, clofilium or chloroquine administration. HR, RR and QT interval were measured for both the models by manual positioning on screen markers. Further QT interval was corrected by using Carlson formula (in vivo model) and Bazette, Fredericia and Van de water (ex vivo model). Additional parameters viz. MBP and PR interval were measured only for in vivo model. The QT interval was measured from the onset of Q wave to the end of T wave. Where the T or U wave overlapped the following P wave or the QRS complex of the subsequent sinus beat, interval was measured up to the end of U wave.[15] Premature ventricular contractions (PVC), ventricular tachycardia (VT), TdP, ventricular fibrillation (VF) and atrioventricular (AV) blocks were recorded as ECG changes. TdP was considered to occur when four or more closely coupled repetitive ventricular premature contractions with twisting of QRS complex were observed.[16]
Statistics
The effect of clofilium and chloroquine was evaluated separately in both the models. The percentage incidences of the various arrhythmias of each group were calculated. QT intervals were corrected for HR changes using the following formula: QTc Carlson (QTcC) = (QT-0.175)*RR-300 for in vivo model, QTcBazett (QTcB) = QT/square root RR interval, QTcFredericia (QTcF) = QT/cube root of RR interval and QTc Van de water (QTcV) = QT-0.087 (RR-1) for ex vivo model. MBP was calculated as 2/3 [systolic BP-diastolic BP] + diastolic BP. Data were expressed as mean ± SEM after subjecting to D’Agostino and Pearson omnibus normality test. After passing the normality test, Student's t-tests for paired and unpaired data was used for comparison with the baseline and vehicle, respectively at 5% and 1% level of significance using PC SAS 9.1.3 (SAS Institute Inc., Cary, NC).
RESULTS
In vivo anesthetized rabbit model
Effect on hemodynamics and electrocardiogram parameters
Effect of methoxamine administration on HR, MBP and QTc interval before administration of saline or clofilium or chloroquine are shown in [Table 1]. After 10 min of methoxamine administration, HR decreased significantly by 6.7% to 15.2% among the treated groups and MBP was found to increase significantly by 29% to 68%. Clofilium had no effect on MBP up to 30 min while chloroquine showed significant elevation in MBP at 5 min by 22.5% with the subsequent decrease up to 15 min. Progressive decrease in HR was found in saline (17.5%), clofilium (61%) and chloroquine (63%) group up to 30 min as compared to 10 min methoxamine infusion [Table 2].
Table 1.
Effect of methoxamine on mean blood pressure, heart rate, QT and corrected QT in anesthetized methoxamine sensitized rabbits
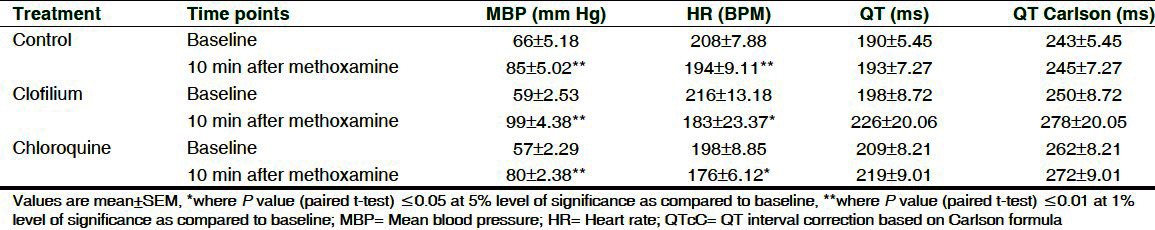
Table 2.
Effect of chloroquine and clofilium on PR interval, mean blood pressure and HR in anesthetized methoxamine sensitized rabbits
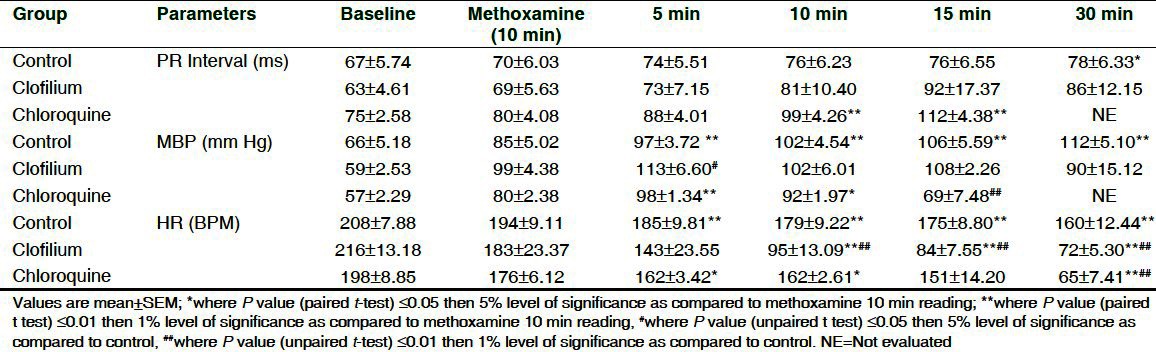
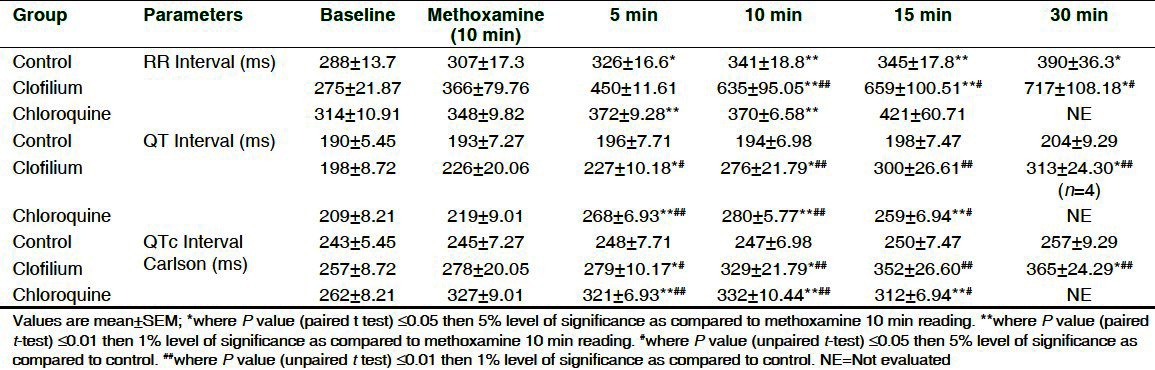
The effect of clofilium and chloroquine on ECG parameters as compared to 10 min methoxamine infusion and vehicle are shown in Table 2 and Table 3. Clofilium did not induce significant effect on PR interval during its infusion up to 30 min whereas RR interval, QT and QTc by Carlson were found to be significantly increased up to 30 min. Maximum increase in QT and QTcC was 53% and 42%, respectively as compared to vehicle. Chloroquine significantly prolonged QT and QTc up to 15 min. Maximum increase in QT and QTcC was 44% and 35%, respectively as compared to vehicle. Conduction disturbances observed after 30 min of clofilium and chloroquine administration prevented measurement of ECG parameters in 3 out of 6 rabbits. Administration of saline did not produce any changes in ECG of the control group.
Table 3.
Effect of chloroquine and clofilium on RR, QT and QTc interval in anesthetized methoxamine sensitized rabbits
Arrhythmia incidences and onset times
A marked increase in arrhythmic activity with greater incidences of PVC, VT and TdP was observed in 4 out of 6 animals in clofilium-treated group. Infusion of chloroquine produced I and II degree AV block and VF but failed to cause VT or TdP in any of the 6 animals tested. Most arrhythmias occurred within 20 min of infusion of the compounds. Incidences and types of arrhythmias after administration of clofilium or chloroquine are showed in Figure 1 and Table 4.
Figure 1.
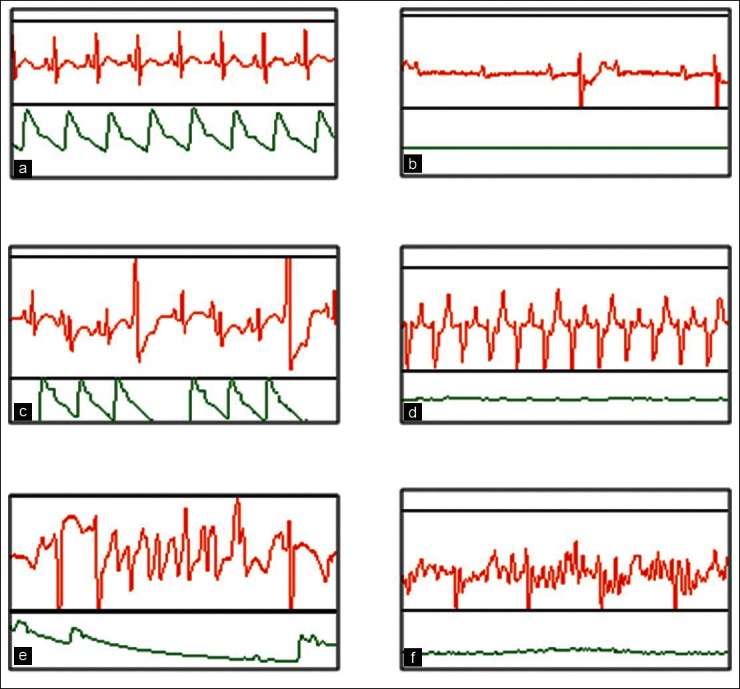
Incidences of arrhythmia in rabbit in-vivo model where clofilium treated animals showed PVC, II AV Block, VT and TdP while chloroquine treated animals showed PVC, I AV block, II AV Block and VF. (a) Normal sinus rhythm. (b) Atrioventricular (AV) block.(c) Premature ventricular contractions. (d) Ventricular tachycardia (e) Torsade De Pointes (TdP). (f) Ventricular Fibrillation (VF)
Table 4.
Percentage of occurrence of arrhythmias in different groups in anesthetized, methoxamine sensitized rabbits
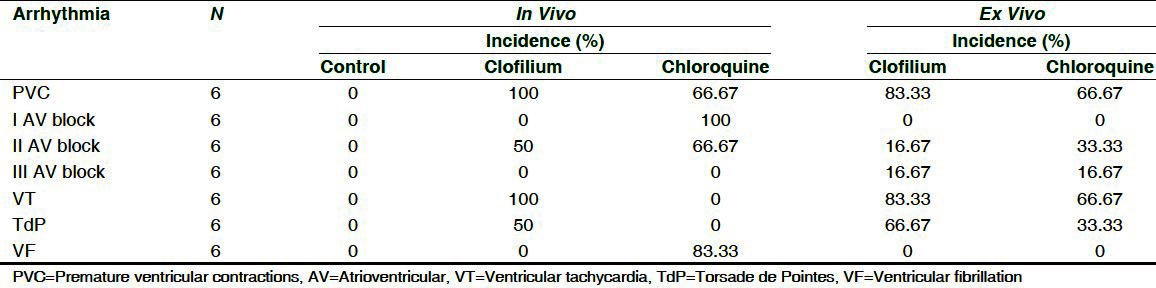
On an average the onset time for PVC in clofilium group was 5.50 ± 2.08 min after its administration at dose of 0.55 ± 0.21 mg/kg and in chloroquine group it was 18.0 ± 1.12 min at the dose of 12.6 ± 0.78 mg/kg. The average onset time for occurrence of VT in clofilium group was 10.00 ± 2.13 min at the dose of 1.00 ± 0.21 mg/kg and TdP was exhibited in 50% of the animals with the average onset time of 14 to 25 min at the dose of 1.4 mg/kg to 2.5 mg/kg. There were no incidences of VT and TdP found in chloroquine group while occurrence of VF was 83.33% with an average onset time of 31.67 ± 2.93 min at the dose of 22.52 ± 2.31 mg/kg.
Langendorff isolated rabbit heart ex vivo model effect on ECG and heart rate
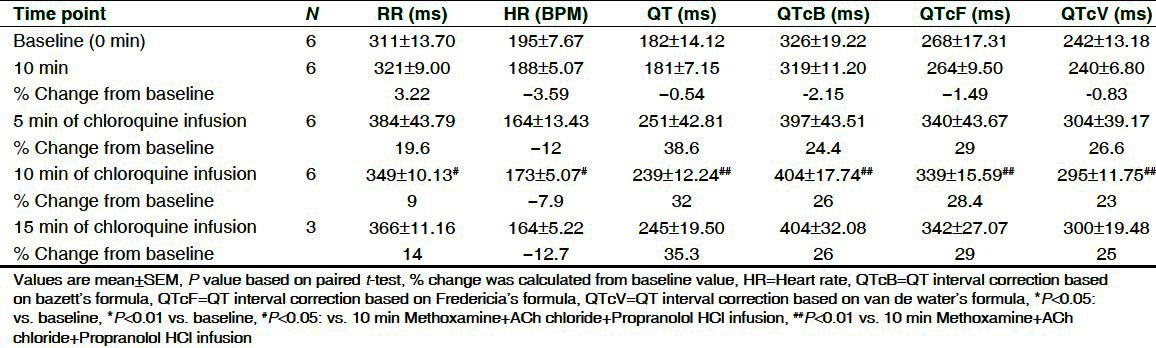
Effect on ECG parameters and HR is shown in Tables 5 and 6. No arrhythmic incidences were observed after combined infusion of methoxamine, ACh chloride and propranolol; only a slight reduction in HR (3-5%) and non-significant increase in QTc were seen in both the groups. Clofilium and chloroquine induced progressive time-dependent decrease in mean HR by 19.5% and 12.7%, while increase in RR was by 16.9% and 14%, respectively. Both the drugs were associated with significant increase in QT and QTc at 10 min of drug infusion as compared to 10 min methoxamine, ACh chloride and propranolol infusion.
Table 5.
Effect of methoxamine, ACh chloride, propranolol and clofilium on ECG parameters on isolated rabbit hearts
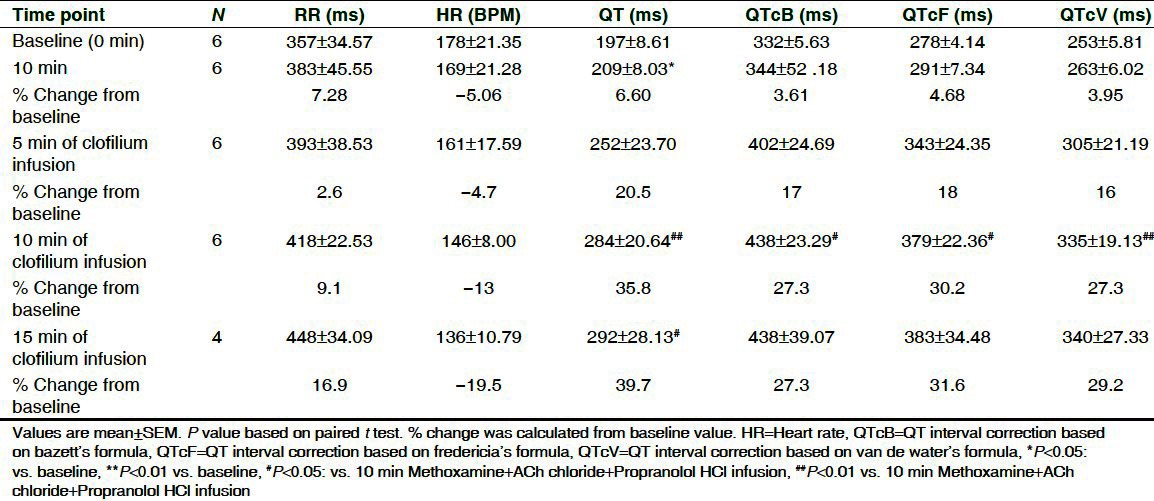
Table 6.
Effect of methoxamine, ACh chloride, propranolol HCl and chloroquine on ECG parameters on isolated rabbit hearts
Arrhythmia incidences
The ECG changes initiated with QT interval prolongation followed by T wave alteration. Ectopic beats and EAD were found before initiation of TdP. None of the heart showed any type of arrhythmic incidences during baseline recording [Figure 2a]. Incidence of premature ventricular contraction (PVC) was observed in 5 out of 6 clofilium-treated hearts with an average onset time of 24-28 min, whereas it was 4 out of 6 hearts in chloroquine-treated group with an average onset time of 15-20 min [Figure 2b, Table 4]. Infusion of clofilium and chloroquine produced VT, II and III degree AV block and incidence of TdP without the occurrence of VF as shown in Figures 2c-f. In the presence of methoxamine, ACh chloride and propranolol, clofilium elicited TdP in 66.67% and chloroquine in 33.33% treated hearts with an average onset time between 55-65 min and 30-40 min, respectively.
Figure 2.
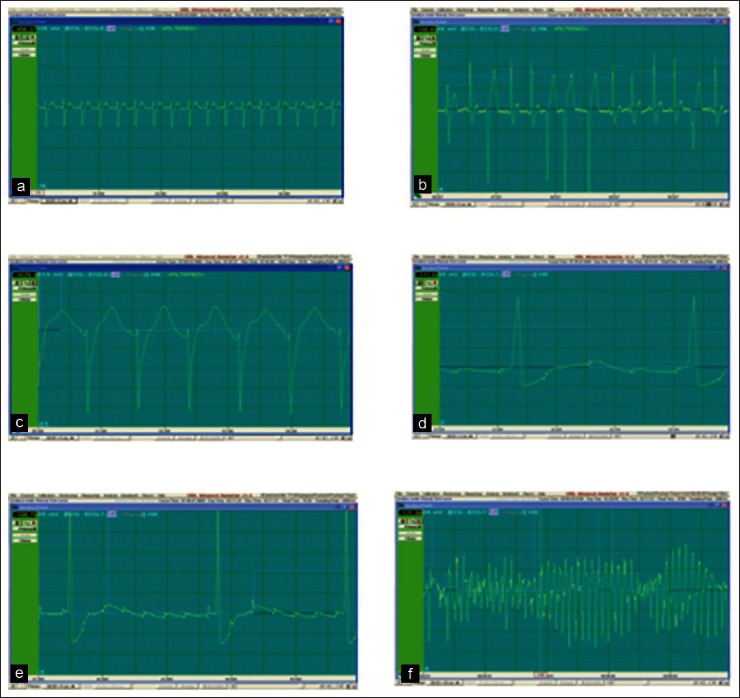
Incidences of arrhythmia in rabbit ex vivo model where clofilium and chloroquine treated animals showed PVC, II and III AV Block, VT and TdP (a) Normal sinus rhythm. (b) Premature ventricular contractions. (c) Ventricular tachycardia (VT). (d) 2rnd Degree Atrioventricular (AV) block. (e) 3rd Degree Atrioventricular (AV) block. (f) Torsade De Pointes (TdP)
DISCUSSION
In the present study, in vivo and ex vivo animal models of arrhythmia were used to assess the potential of chloroquine to cause arrhythmia and TdP. Animal models used in the present study are well accepted, inexpensive, easily accessible, reproducible and sensitive for mechanistic evaluation of proarrhythmic activity. Moreover, these models provided important insights into the etiology of TdP and systematic applications of these models contributed to the risk assessment of non-antiarrhythmic and antiarrhythmic agents. Rational for the use of rabbit in these models was the presence of high density of IKr channels, making this species more sensitive for proarrhythmic potential. Studies in sheep ventricular muscle and purkinje fibers, demonstrated that chloroquine prolongs the action potential duration and refractory period. This is usually attributed to block of K+ currents contributing to the excessive prolongation of QT interval and conduction disturbances.[17] The present study described that administration of chloroquine during α1 stimulation resulted in arrhythmia in both the models.
Effect of anesthesia
In the present study pentobarbitone sodium, a short- to intermediate-acting barbiturate used as induction anesthesia and α-chloralose was used to produce non-recoverable long-acting anesthesia throughout the experiment. The proarrhythmogenic potential of anesthetics has been reported by many researchers and therefore the type of anesthesia used may affect development of TdP in experimental animals. Vincze, et al.[18] reported that the model of TdP induction with dofetilide in rabbits succeeded cent percent when chloralose alone was used as anesthetic whereas success rate of this model with pentobarbitone sodium and propofol was only 40% and 70%, respectively. Contradictory to this, Carlsson et al.;[11] White et al.[19] and Orth, et al.[20] reported the incidence of clofilium-induced TdP in pentobarbitone anesthetized rabbits was close to 100% while in our experiment it was 50%. Reduction in TdP percentage in our experiment may be attributed to the action of pentobarbitone, which homogeneously prolongs the duration of the action potential of the canine endocardium, epicardium than the midmyocardium. This significantly reduces the ability of the repolarization prolonging drugs to increase transmural dispersion of repolarization.[21]
Effect of methoxamine
Methoxamine is a α1 adrenoreceptor agonist that causes vasoconstriction and reflex bradycardia by modulating the levels of intracellular inositol triphosphate and diacylglycerol resulting in an increased release of Ca++ in the sarcoplasmic reticulum.[22,23] Intracellular Ca++ rise elicit instability of Ca++ and membrane potential during the EADs may convert the heart to TdP.[24] Buchanan et al.[13] observed increase in blood pressure and decrease in HR after 15 min of methoxamine infusion. Our observations of increase in blood pressure and decrease in HR in in vivo model were in line with the reported findings.
In ex vivo model, methoxamine alone failing to produce bradycardia and/or TdP in our preliminary experiments when used with clofilium is in accordance with the previous reported studies.[14,25] D’Alonzo et al.[14] studied the combined effect of methoxamine, ACh and nadolol on the ability of dofetilide to elicit TdP. HR was reduced by 7% following ACh and methoxamine treatment. The addition of β-adrenoceptor antagonist (nadolol) caused a further reduction of 6% in HR. The combination of ACh, methoxamine and nadolol with dofetilide developed TdP in 100% of hearts. Acetylcholine induced slowing of HR by increasing conductance through acetylcholine-sensitive potassium channels and stimulation of muscaranic M2 receptors, which is associated with increases in inositol triphosphate responsible for increase in intracellular calcium concentration. The ability of β-adrenoceptor antagonist to enhance the proarrhythmic action of drugs in combination of ACh and methoxamine may be explained by further reduction of HR along with elevated calcium level and decreased current of slow component of the delayed rectifier activity, which leads to further generation of TdP more easily.[14] Therefore, combinations of ACh chloride, non-selective beta blocker propranolol along with methoxamine were used to produce bradycardia and/or TdP in our experiment, which further sensitized the hearts to develop TdP in clofilium and chloroquine treated heart.
Effect of chloroquine and clofilium in in vivo and ex vivo model
Chloroquine, at therapeutic concentration is known to produce different cardiovascular effects such as fall in blood pressure, slowing of ventricular conduction and ECG changes such as lengthening of QRS, QT interval and AV blocks.[10,26] It also decreased Vmax, prolonged action potential duration (APD) and decreased maximum diastolic potential in cat isolated purkinje fibers and ventricular myocytes.[11] Chloroquine increased cyclic GMP level in the smooth muscle cells by inducing nitric oxide (NO) synthesis.[27] Nitric oxide diffuses to nearby smooth muscle cells in which it stimulates the soluble guanylate cyclase resulting in enhanced synthesis of cyclic GMP. This increase in cyclic GMP in the smooth muscle cells leads to their relaxation and reduced the blood pressure.[28] The finding in our study is in accordance with reported data as chloroquine caused decrease in blood pressure and HR in both the models.
Chloroquine is known for cardiac rhythm abnormalities,[29] which were reflected in in vivo experiment in the form of QT prolongation, PVC, VF and AV block, which further leads to mortality in all the animals. However except VF, TdP was observed along with other ECG abnormalities in ex vivo model. Inhibition of inward rectifying potassium current IK1 > (IKr)> (INa)> (ICa-L) mainly blockade of rapid component of the delayed rectifying outward current IKr but not the slow component IKs. It is clear that these events precede cardiac rhythm abnormalities of chloroquine.[10]
The positive control clofilium in the present study showed occurrence of conduction abnormality along with TdP in both the models. It is explained by blocking activity of clofilium toward IKr, IKs and Ito channels, which further leads to prolong QT interval, prolong APD, lower HR and cardiac repolarization.[30]
CONCLUSION
Our study has provided the evidence that chloroquine phosphate, a non-cardiac, antimalarial drug, evokes arrhythmia in the presence of α1 adrenoceptor stimulation in anesthetized rabbit in vivo model and Langendorff isolated heart rabbit ex vivo model. Results indicate that rabbits treated with clofilium and chloroquine displayed several forms of arrhythmia in both the models. This raises the need to evaluate the proarrhythmic potential of non-cardiac drugs also for the pharmaceuticals in the development of safer drugs. The results demonstrated that both the models were efficient in exhibiting the proarrhythmic changes; hence, this is value addition for better predictions and risk assessment of arrhythmias in human beings.
ACKNOWLEDGMENTS
The authors wish to extend their thanks to the Ranbaxy Research Laboratories for the provision of facility to carry out the work. They are also grateful to Dr. M. R. Srinivasan and Mr. K. N. Nanjappa for their helpful discussions. They wish to extend their thanks to Dr. Milind Deore and Mr. Pravin J. Patil for peer review of the article.
Footnotes
Source of Support: Ranbaxy Research Laboratories
Conflict of Interest: None declared.
REFERENCES
- 1.Woosley RL. Drugs that prolong the QT interval and/or induce Torsades de pointes (Arizon CERT web site) [Last accessed on 2012 March 02]. Available from: http://www.azcert.org/medical-pros/drug-lists/browse-drug-list.cfm .
- 2.Haverkamp W, Breithardt G, Camm AJ, Janse MJ, Rosen MR, Antzelevitch C, et al. The potential for QT prolongation and proarrhythmia by non-antiarrhythmic drug: Clinical and regulatory implications. Report on a conference of the European society of cardiology. Eur Heart J. 2000;21:1216–31. doi: 10.1053/euhj.2000.2249. [DOI] [PubMed] [Google Scholar]
- 3.The non-clinical evaluation of the potential for delayed ventricular repolarization (QT interval prolongation) by human pharmaceuticals ICH Topic S7B guideline. 2005 Nov; (CPMP/ICH/423/02) [Google Scholar]
- 4.Morganroth J. Comparative efficacy of oral mexiletine and quinidine in benign or potentially lethal ventricular arrhythmia. Am J Cardiol. 1987;60:1276–81. doi: 10.1016/0002-9149(87)90608-4. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence CL, Pollard CE, Hammond TG, Valentine JP. Non-clinical proarrhythmia models: Predicting torsades de pointes. J Pharmacol Toxicol Methods. 2005;52:46–59. doi: 10.1016/j.vascn.2005.04.011. [DOI] [PubMed] [Google Scholar]
- 6.Crumb WJ, Wible B, Arnold DJ, Payne PJ, Brown AM. Blockade of multiple human cardiac potassium currents by the antihistamine terfenadine: Possible mechanism for terfenadine-associated cardiotoxicity. Mol Pharmacol. 1995;47:181–90. [PubMed] [Google Scholar]
- 7.Castot A, Rapoport P, Coz PL, Monlun E, Pillet O, Cochard JF, et al. Prolonged QT-interval with halofantrine. Lancet. 1993;341:1541–2. [PubMed] [Google Scholar]
- 8.Nosten F, Terkuile FO, Luxemburger C, Woodrow C, Chongsuphajaisiddhi T, White NJ, et al. Cardiac effects of antimalarial treatment with halofantrine. Lancet. 1993;341:1054–6. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- 9.Lu HR, Vlaminckx E, Van de water A, Rohrbacher J, Herman A, Gallacher DJ. In vitro experimental models for the risk assessment of antibiotic- induced QT prolongation. Eur J Pharmacol. 2006;553:229–39. doi: 10.1016/j.ejphar.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Chapula JA, Salinas-Stefanon E, Torres-Jacome J, Banavides-Haro DE, Navarro-Polanco RA. Blockade of currents by antimalarial drug chloroquine in feline ventricular myocytes. J Pharmacol Exp Ther. 2001;297:437–45. [PubMed] [Google Scholar]
- 11.Carlsson L, Almgren O, Duker G. QTU-prolongation and Torsade de pointes induced by putative class III antiarrhythmic agents in the rabbit-etiology and interventions. J Cardiovasc Pharmacol. 1990;16:276–85. doi: 10.1097/00005344-199008000-00014. [DOI] [PubMed] [Google Scholar]
- 12.Farkas A, Lepran I, Papp JG. Comparison of antiarrhythmic and the proarrhythmic effect of almokalant in anesthetized rabbits. Eur J Pharmacol. 1998;346:245–53. doi: 10.1016/s0014-2999(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan LV, Kabell G, Brunden MN, Gibson JK. Comparative assessment of ibutilide, D-sotalol, clofilium, E-4031 and UK-68, 798 in a rabbit model of proarrhythmia. J Cardiovasc Pharmacol. 1993;220:540–9. [PubMed] [Google Scholar]
- 14.D’Alonzo AJ, Zhu JL, Darbenzio RB. Effects of class III antiarrhythmic agents in an in vitro rabbit model of spontaneous torsades de pointe. Eur J Pharmacol. 1999;369:57–64. doi: 10.1016/s0014-2999(99)00057-6. [DOI] [PubMed] [Google Scholar]
- 15.Farkas A, Batey AJ, Coker SJ. How to measure electrocardiograph QT interval in the anaesthetized rabbit. J Pharmacol Toxicol Methods. 2004;50:175–85. doi: 10.1016/j.vascn.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 16.Farkas A, Lepran I, Papp JG. Proarrhythmic effects of intravenous quinidine, amidarone, D-sotalol and almokalant in the anaesthetized rabbit model of Torsades de pointes. J Cardiovasc Pharmacol. 2002;39:287–97. doi: 10.1097/00005344-200202000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Harris L, Downar E, Shaikh NA, Chen T. Antiarrhythmic potential of chloroquine: New use for an old drug. Can J Cardiol. 1988;4:295–300. [PubMed] [Google Scholar]
- 18.Vincze D, Farkas AS, Rudas L, Makra P, Cs#x131;k N, Lepran I, et al. Relevance of anesthesia for dofetilide-induced torsades de pointes in α1-adrenoceptor stimulated rabbits. Br J Pharmacol. 2008;153:75–89. doi: 10.1038/sj.bjp.0707536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White CM, Xie J, Chow MS, Kluger J. Prophylactic magnesium to decrease the arrhythmogenic potential of class III antiarrhythmic agents in a rabbit model. Pharmacotherapy. 1999;19:635–40. doi: 10.1592/phco.19.8.635.31528. [DOI] [PubMed] [Google Scholar]
- 20.Orth PM, Hesketh JC, Mak CK, Yang Y, Lin S, Beatch GN, et al. RSD 1235 blocks late INa and suppresses early after depolarizations and torsade de pointes induced by class III agents. Cardiovasc Res. 2006;70:486–96. doi: 10.1016/j.cardiores.2006.01.026. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu W, McMahon B, Antzelevitch C. Sodium pentobarbital reduces transmural dispersion of repolarization and prevent. J Cardiovasc Electrophysiol. 1999;10:154–64. doi: 10.1111/j.1540-8167.1999.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 22.Westfall TC, Westfall David P. Adrenergic agonists and antagonists. In: Brunton LL, Chabner BA, Knollmann BC, editors. Goodman and Gilman's manual of pharmacology and therapeutics. 12th ed. USA: McGraw- Hill companies; 2008. pp. 277–334. [Google Scholar]
- 23.Braun AP, Fedida D, Clark RB, Giles WR. Intracellular mechanisms for α1-adrenergic regulation of the transient outward current in rabbit atrial myocytes. J Physiol. 1990;431:689–712. doi: 10.1113/jphysiol.1990.sp018355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi BR, Burton F, Salama G. Cystolic Ca2+ triggers early after depolarization and torsode de pointes in rabbit hearts with type 2 long QT syndrome. J Physiol. 2002;543:615–31. doi: 10.1113/jphysiol.2002.024570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farkas AS, Acsai K, Toth A, Dezsi L, Orosz O, Forster T, et al. Importance of extracardiac α1-adrenoceptor stimulation in assisting dofetilide to induce torsade de pointes in rabbit hearts. Eur J Pharmacol. 2006;537:118–25. doi: 10.1016/j.ejphar.2006.03.014. [DOI] [PubMed] [Google Scholar]
- 26.Bustos MD, Gay F, Diquet B, Thomare P, Warot D. The pharmacokinetics and electrocardiographic effects of chloroquine in healthy subjects. Trop Med Parasitol. 1994;45:83–6. [PubMed] [Google Scholar]
- 27.Anigbogu CN, Adigun SA, Inyang I, Adegunloye BJ. Chloroquine reduces blood pressure and forearm vascular resistance and increases forearm blood flow in healthy young adults. Clin Physiol. 1993;13:209–16. doi: 10.1111/j.1475-097x.1993.tb00381.x. [DOI] [PubMed] [Google Scholar]
- 28.Ghigo D, Aldieri E, Todde R, Costamagna C, Garbarino G, Pescarmona G, et al. Chloroquine stimulates Nitric Oxide synthesis in murine, porcine, and human endothelial Cells. J Clin Invest. 1998;102:595–605. doi: 10.1172/JCI1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guedira N, Hajjaj-Hassouni N, Srairi JE, el Hassani S, Fellat R, Benomar M. Third-degree atrioventricular block in a patient under chloroquine therapy. Rev Rhum Engl. 1998;65:58–62. [PubMed] [Google Scholar]
- 30.Li Q, Himmel HM, Ravens U. Selectivity of class-III antiarrhythmic action of clofilium in guinea pig ventricular myocytes. J Cardiovasc Pharmacol. 1996;27:401–10. doi: 10.1097/00005344-199603000-00013. [DOI] [PubMed] [Google Scholar]


