Abstract
Purpose
To evaluate in vivo ocular safety of an intravitreal hydrosilylated porous silicon (pSi) drug delivery system along with the payload of daunorubicin (DNR).
Methods
pSi microparticles were prepared from the electrochemical etching of highly doped, p-type Si wafers and an organic linker was attached to the Si-H terminated inner surface of the particles by thermal hydrosilylation of undecylenic acid. DNR was bound to the carboxy terminus of the linker as a drug-loading strategy. DNR release from hydrosilylated pSi particles was confirmed in the excised rabbit vitreous using liquid chromatography–electrospray ionization–multistage mass spectrometry. Both empty and DNR-loaded hydrosilylated pSi particles were injected into the rabbit vitreous and the degradation and safety were studied for 6 months.
Results
The mean pSi particle size was 30×46×15 μm with an average pore size of 15 nm. Drug loading was determined as 22 μg per 1 mg of pSi particles. An ex vivo drug release study showed that intact DNR was detected in the rabbit vitreous. An in vivo ocular toxicity study did not reveal clinical or pathological evidence of any toxicity during a 6-month observation. Hydrosilylated pSi particles, either empty or loaded with DNR, demonstrated a slow elimination kinetics from the rabbit vitreous without ocular toxicity.
Conclusions
Hydrosilylated pSi particles can host a large quantity of DNR by a covalent loading strategy and DNR can be slowly released into the vitreous without ocular toxicity, which would appear if an equivalent quantity of free drug was injected.
Introduction
Drug delivery to the posterior segment of the eye remains a challenging task due to the difficulty of crossing the blood–retinal barrier. Vitreoretinal diseases require sustained treatment regimens and frequent intravitreal injections over time. For example, in exudative age-related macular degeneration, a monthly intraocular injection of the antivascular endothelial growth factor (VEGF) has become a standard therapy.1 Reduction of the intravitreal injection frequency can adversely affect the treatment efficacy.2 Other diseases such as uveitis, diabetic retinopathy, or retinal vein occlusion also benefit from intravitreal injections of anti-VEGF agents and other therapeutics such as triamcinolone. Despite the success of the treatment, the need of frequent injections poses the risk of complications or infection, which can lead to severe visual morbidity. Therefore, various drug delivery systems have been investigated to lower the frequency of the injections and injection-related side effects.3,4
An optimal ocular drug delivery system should provide a sustained and long-lasting transport of medication to the vitreous and retina. Many ocular drug delivery systems involve surgical procedures,3,5,6 which have related complications when placing and replacing implants. To avoid surgery, intravitreal injection with a colloidal drug delivery system has been investigated.7 However, systems, such as polymer nanoparticles, microspheres, or liposomes, have a limited vitreous residence, impair vitreous clarity, and show localized vitreous foreign body reaction.8
Porous silicon (pSi) has been extensively studied as a drug carrier.9–11 The nanostructure of pSi provides reservoirs that can be loaded with the desired therapeutic and provide sustained release after a single intravitreal injection. We have demonstrated that native pSi particles degrade in rabbit eyes within 10 days without any toxicity; and surface chemistry modified pSi particles, either through silicon matrix oxidization or hydrosilylation, can safely reside in the rabbit eyes for months before complete degradation, thus demonstrating the capability to extend the drug action.12 In addition, we also demonstrated that drugs can be loaded into functionalized pSi particles through covalent bonding, which extended the half-life of daunorubicin (DNR) from 5 h to 15 days in a dynamic infusion chamber, which mimics the fluid dynamics of the human eye.13 Therefore, pSi has shown the potential to be an excellent candidate as a minimally invasive and long-acting drug delivery system for the treatment of intraocular diseases.12,14,15
Proliferative vitreoretinopathy (PVR) is the most common complication of retinal reattachment surgery.16 The current standard PVR treatment is vitrectomy which can restore the anatomical retina position for 60% to 80% of the cases; however, functional success (ambulatory vision 5/200 or better) is only 40% to 80% of those anatomically successful cases.17 To improve the functional success rate, various pharmacologic adjuncts targeting the different phases of the process of PVR has being explored, but success was limited without a sustainable drug delivery system.18–21 A drug appropriate for the control of PVR must inhibit cell proliferation effectively and must maintain the therapeutic level at vitreous for 4 months, which is the time window for high risk of PVR development.22 Previous studies have shown that the anthracycline drug DNR is effective in inhibiting PVR formation.23,24 Unfortunately, DNR has a short half-life in the eye vitreous coupled with a narrow therapeutic concentration range, thus requiring frequent injections over time.25 Therefore, a sustained delivery system is needed to control the drug release within its therapeutic window. In our previous in vitro experiments, we have shown that DNR can be loaded into hydrosilylated pSi particles through covalent bonding and released DNR was within the therapeutic level and below the toxic concentration, thus extending the safety as well as the efficacy of this delivery system.13 In that study, an enhanced in vitro potency of the elute from pSi microparticles, as compared to free drug, was observed. This effect was proposed to be caused by accelerated DNR reduction by Si-H species on pSi microparticles.26 The increased potency may improve the therapeutic index if the highest nontoxic dose remains the same. Since the drug will be delivered along with the pSi particles, an in vivo safety study using DNR-loaded pSi particles would be the immediate further effort to evaluate this drug and the delivery system. If DNR and the delivery system were not toxic in an in vivo setting, the increased potency can be harnessed to treat or prevent development of PVR. The aim of the current study was to assess the in vivo ocular behavior of this unique pSi microparticle drug delivery system using DNR as a model drug in the living rabbit eye.
Methods
Synthesis of pSi microparticles
pSi microparticles were prepared from the electrochemical etching of highly doped, (100)-oriented, boron-doped p-type Si wafers with 0.99 mΩ·cm resistivity (Siltronix, Inc., Archamps, France) in a 3:1 solution of 48% aqueous hydrofluoric acid (HF):ethanol (Thermo Fisher Scientific, Pittsburg, PA).13 The pSi particles were chemically modified by microwave-assisted hydrosilylation with undecylenic acid (95%; Sigma-Aldrich, St. Louis, MO), as described by us.13 The particle size and the pore size were measured from the scanning electron microscopic (SEM) images using Adobe Photoshop software. The SEM microscopic images were randomly taken on several areas and an average value for the particle size as well as for the pore size was obtained.
Drug loading
About 5 mg of hydrosilylated pSi microparticles was suspended in 650 μL of 10% dimethyl sulfoxide (Sigma-Aldrich) in phosphate-buffered saline (PBS; Thermo Fisher Scientific) containing 50 mM N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC; Sigma-Aldrich) and 5 mM N-hydroxysulfosuccinimide (Sulfo-NHS; Pierce Biotechnology, Inc., Rockford, IL). Afterward, 200 μL of a 1 mg/mL aqueous solution of DNR hydrochloride (Tocris Biosciences, Minneapolis, MN) was added. The particles were then vortexed for 2 h at room temperature and rinsed with ethanol 5 times. Drug loading by covalent attachment was confirmed by Fourier-Transform-Infrared (FTIR) spectroscopy in an attenuated total reflectance mode in a Nicolet 6700 with Smart-iTR spectrometer (Thermo-Scientific); and by thermogravimetric analysis (TGA) in a TA Q600 apparatus (TA Instruments, Newcastle, DL) by constant heating from room temperature up to 800°C at 10°/min under a 10 mL/min nitrogen flow. To determine the amount of DNR loaded, pSi microparticles were completely dissolved in a solution of 1 M sodium hydroxide (Sigma-Aldrich) and the solution was neutralized with 1 M hydrochloric acid (Sigma-Aldrich) to recover the fluorescence spectrum of the free drug as previously described.26 The calculated quantity of DNR loaded was 22 μg per 1 mg of pSi particles.13
Ex vivo DNR confirmation in rabbit vitreous
It has been reported that DNR degrades in biological fluids.27,28 To confirm the presence of DNR in excised rabbit vitreous following incubation with DNR-loaded pSi particles at 37°C, an 8-day ex vivo study was performed, considering that beyond that time excised vitreous would alter substantially even decomposing. For this study, 3 mg of drug-loaded pSi microparticles in 100 μL of a balanced salt solution (BSS; Gibco, Carslbad, CA) was injected into a 1.5 mL excised rabbit vitreous in a vial using a 25-gauge needle. The vial was incubated at 37°C, thus mimicking the volume and temperature of the rabbit vitreous. At time points 1, 3, 5, 24, 120, and 192 h, the vial was centrifuged at 5,000 rpm for 20 min and 300 μL upper vitreous fluid was taken, protected from light, and stored at −20°C until analysis. About 300 μL of fresh BSS solution was added back to the system to maintain a constant volume. This ex vivo study was designed to mimic an in vivo intravitreal injection. In a living eye, the vitreous fluid is constantly turning over, which takes the released DNR away from the vitreous body. We tried looking at the dynamics of the vitreous-free DNR, which is the one to be able to exert a therapeutic effect. The DNR either trapped in pSi particles or bound with the vitreous collagen will not be available for biological process such as PVR formation. Therefore, instead of sampling the whole vitreous, the vitreous fluid was sampled and analyzed.
The drug released in the excised vitreous was analyzed by using a 1260 HPLC system (Agilent, Palo Alto, CA) coupled with a Thermo LCQdeca-MS spectrometer (Thermo Fisher Scientific). Positive ion mode electrospray ionization (ESI) was used as the ion source. The LC separation was performed on a Shiseido Capcell Pak MG III C-18 column (2.0 mm ID×50 mm length, 3 μm) (Phenomenex, Torrance, CA) with a guard column. Mobile phase A was 2.5% methanol (HPLC-grade; Sigma-Aldrich) in water with 0.1% formic acid (Sigma-Aldrich). Pure methanol with 0.1% formic acid was used as mobile phase B. The mobile phase was delivered at a flow rate of 200 μL/min under gradient conditions as follows: increased from 30% mobile phase B to 95% mobile phase B in 10 min, followed by 6 min at 95% mobile phase B, and then returned to 30% mobile phase B in 1 min followed by an additional 8 min at 30% mobile phase B to equilibrate the column. The liquid chromatography–electrospray ionization–multistage mass spectrometry (LC-ESI-MS/MS) was operated under a selected reaction monitoring (SRM) mode to detect DNR and the spiked internal standard doxorubicin (DOX; Fisher Bioreagents). A LC-ESI-MS/MS run of pure vitreous (DNR and DOX free) was used as a blank control. Processing of the vitreous with an equal volume of 2-propanol (HPLC-grade; Sigma-Aldrich) was used to extract DNR, precipitate proteins, and decrease the viscosity of the system before LC-MS/MS analysis.29 DNR was quantitated in the vitreous by spiking the sample with a known amount of internal standard (DOX) to compensate the variations in drug extraction efficiency as well as the instrument drift. In a typical experiment, 100 μL of a vitreous tap sample was spiked with 1 ng DOX (10 μL of a 100 ng/mL solution of DOX in milli-Q water). About 100 μL of 2-propanol was added to precipitate the proteins. The mixture was centrifuged at 12,500 rpm for 15 min and the supernatant was transferred to a clean tube and dried-down. The dried sample was resuspended in mobile phase and 20% of the sample was injected into the chromatographic system.
In vivo ocular toxicity studies
Fourteen rabbits were used for the in vivo safety studies. All animal experiments were carried out in the adherence of the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Under general anesthesia (intramuscular ketamine hydrochloride 25 mg/kg and xylazine 5.25 mg/kg) and topical 0.5% proparacaine, 1 eye of each rabbit was injected with the study agent; the fellow eye received the same amount of BSS to serve as a control. pSi particles, either empty or drug loaded, had gone through a final rinse with ethanol for sterilization purpose before quantitating the drug loading and the intravitreal injections.
Plain particle toxicity study: Four rabbit eyes were injected with empty hydrosilylated pSi particles to evaluate toxicity of surface chemistry modified pSi. Two rabbits were sacrificed at 1 month for short-term toxicity and the other 2 were sacrificed at 6 months for a long-term study.
DNR-loaded particle toxicity study: Ten rabbit eyes received DNR-loaded hydrosilylated pSi particles. Three rabbits were sacrificed at 1 month, the other 3 were sacrificed at 3 months, and the other 4 were observed for 6 months as a long-term toxicity study. For sacrifice, the rabbits were deeply anesthetized with intramuscular ketamine hydrochloride 35 mg/kg and xylazine 6 mg/kg before intracardiac injection of pentobarbital sodium (120 mg/kg).
For the intravitreal injection, 8 mg of the particles was suspended into 250 μL of BSS and 100 μL of the suspension was injected through the pars plana at 1.5 mm from the limbus into the rabbit midvitreous with a 25-gauge needle under direct view of a surgical microscope. A paracentesis was performed to normalize the intraocular pressure (IOP) immediately after the injection. After the injection procedure, topical tobradex ophthalmic ointment was applied once daily for 3 days. The anterior segment congestion, lens clarity, vitreous clarity, and fundus evaluation were performed as described previously.30 The anterior chamber migrating cell count was graded into 5 scales as we previously reported.31 For the eyes receiving DNR-loaded pSi particles, the calculated DNR in 3.2 mg of the particles was 70.4 μg. Following injection, both eyes of each rabbit were monitored by indirect ophthalmoscopy, tonometry, biomicroscopy, and fundus photography at day 3, 7, 14, and 25, and then month 2, 3, 4, 5, and 6. IOP was acquired using a hand-held tonometer Tonopen (Medtronic, Jacksonville, FL) after topical anesthesia. Three trials with 95% accuracy were averaged for each eye. Electroretinograms (ERG) were recorded from all eyes at day 14, day 28, month 3, and before rabbit sacrifice at month 6 using a standardized protocol described previously by us.32 Dark adapted ERG was recorded with the stimulus light strength of 0.34 candela-sec per meter squared (0.34 cd·s/m2) at the surface of the Ganzfeld bowl. After dark adapted ERG recording, the animals were light adapted for 5 min, then light adapted ERG and flicker (30 Hz/s) ERG were recorded using stimulus light of 0.79 cd·s/m2 at the surface of the Ganzfeld bowl against a background light of 72.6 cd/m2. After sacrifice, the globes were processed for light microscopy.
Statistical methods
In this study, IOP and ERG were recorded multiple times from the same eyes. In addition, each rabbit had its right eye as the study group and received empty pSi particles or DNR-loaded particles, while the left eye was used as control and received BSS injection. To take the fellow eye association and repeated measurements from the same animal into consideration, pooled IOP and ERG parameters were analyzed using a generalized estimating equation (GEE) to compare mean IOPs and ERG parameters among the groups. All analyses were performed using SAS version 9.2 and a P value smaller than 0.05 was considered to be significant.
Results
Characteristics of the pSi microparticles
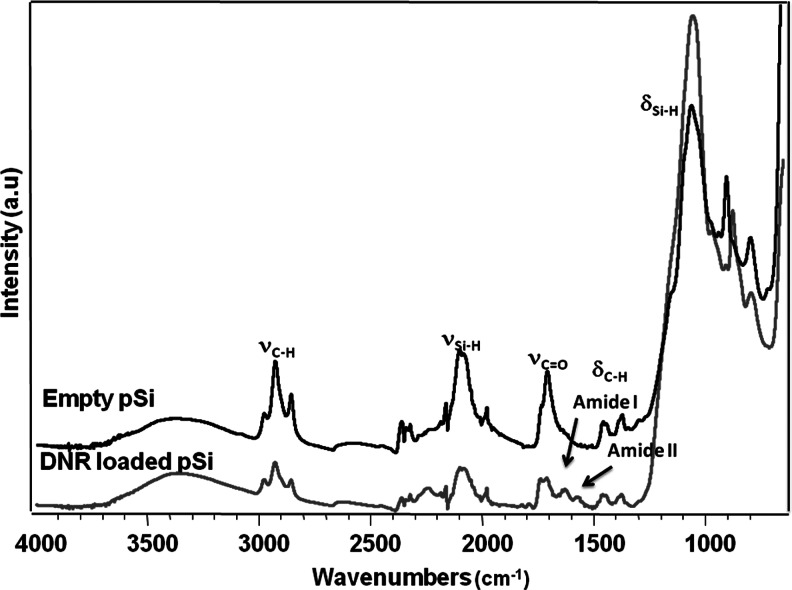
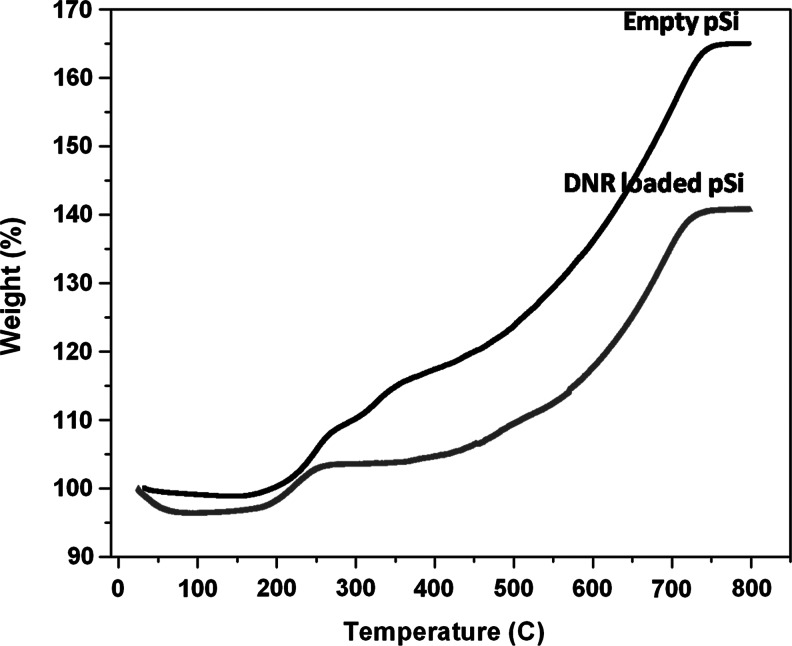
The pSi microparticles obtained showed an average particle size of 30×46×15 μm and a pore size of 15 nm, respectively, as measured from the SEM images (Fig. 1). The surface hydrosilylation and subsequent DNR loading by covalent attachment was confirmed by FTIR spectroscopy. Figure 2 shows successful hydrosilylation with undecylenic acid by observation of the ν (C-H) bands at 2,973 and 2857 cm−1 as well as the ν (C=O) band at 1,710 cm−1. Successful conjugation of the drug generates bands characteristics of amide I at 1,630 cm−1, and amide II at 1,573 cm−1. TGA further confirmed successful DNR loading in the particles, as seen by a weight difference between empty and drug-loaded pSi microparticles (Fig. 3).33
FIG. 1.

Scanning electron microscope image of porous silicon (pSi) microparticles (A), close-up scanning electron microscopic view of one of the microparticles, revealing the mesoporous structure (B).
FIG. 2.
Fourier-Transform-Infrared (FTIR) spectra of empty and daunorubicin (DNR)-loaded porous silicon (pSi) microparticles. Conjugation of the drug molecule generates bands in the FTIR spectrum indicative of the formation of a linkage through amide bond (arrows).
FIG. 3.
Thermogravimetric analysis curves of pSi particles hydrosilylated with undecylenic acid (empty pSi) and pSi particles containing DNR covalently attached to the pore walls as described in the text (DNR-loaded pSi). Weight percent is reported relative to the initial weight of sample, before heating. A weight increase was observed due to the oxidation of pSi to porous silica (pSiO2). The weight difference between empty pSi and DNR-loaded pSi accounts for the organic matter corresponding to the drug loaded into the particles.
Ex vivo confirmation of DNR release in rabbit vitreous
DNR hydrochloride was dissolved into water, diluted by using 50% methanol and 50% water with 0.1% formic acid, and then infused to the ESI source to tune the instrument and obtain the fingerprint of DNR under LC-MS and LC-MS/MS analysis. The protonated molecular ion peak of DNR at m/z 528 and the typical MS/MS spectrum of this molecular ion peak were observed. The most intense daughter ion peak at m/z 381 was selected for SRM in subsequent DNR detection using LC-MS/MS analysis.
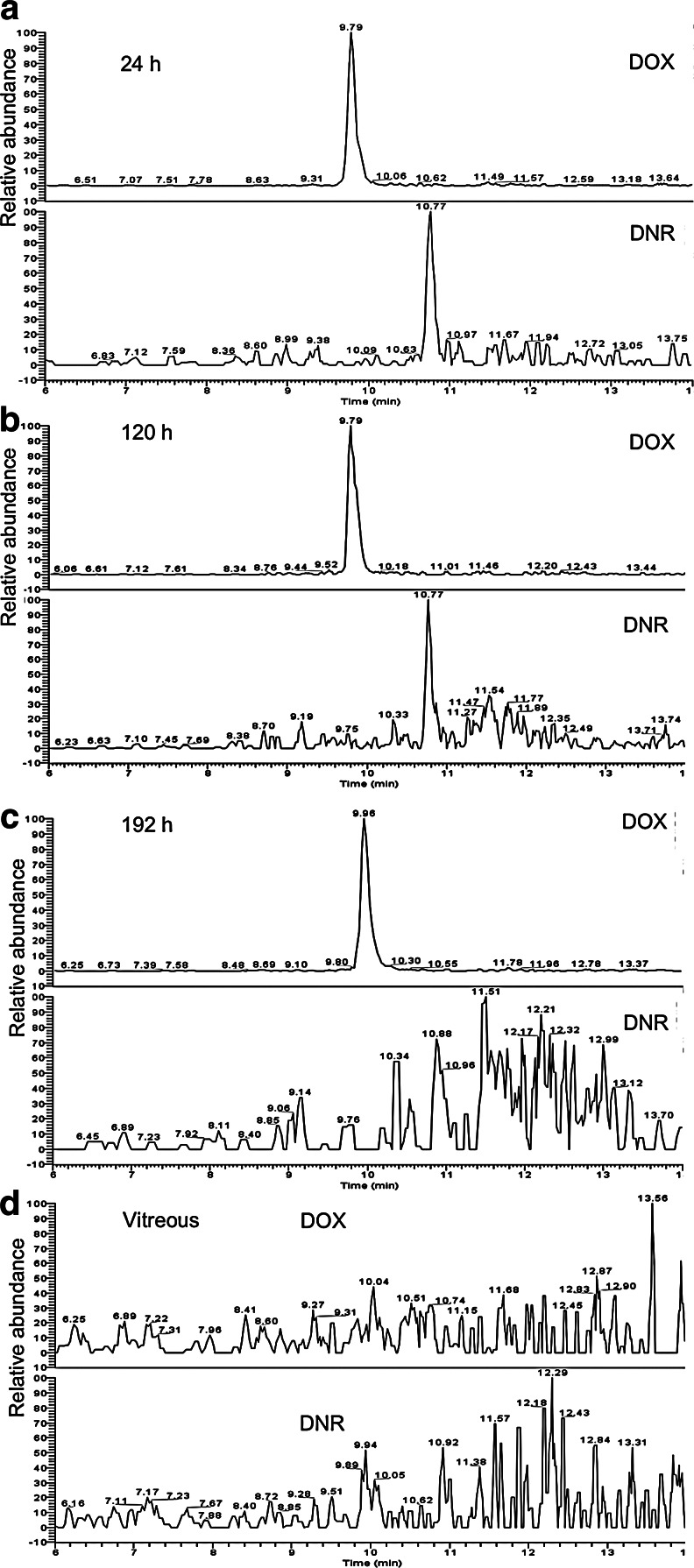
We performed LC-ESI-MS/MS on m/z 528 peak followed by SRM detection at m/z 381 on samples released from drug-loaded hydrosilylated particles in excised vitreous at time points 1, 2, 5, 24, 120, and 192 h. As shown in Fig. 4a and 4b, the LC-ESI-MS/MS measurements revealed a peak at a retention time of ∼10.77 min, confirming the detection of intact DNR at time points 24 h and 120 h, respectively. Only very trace amount of intact DNR was detected at time point 192 h (Fig. 4c), and as expected, no intact DNR was detected from the pure vitreous blank control (Fig. 4d). LC-ESI-MS/MS on m/z 544 (protonated molecular ion peak of internal standard DOX), followed by SRM at its most intense fragmental peak (m/z 397) showed the presence of the internal standard DOX, as expected. For a better understanding, the SRM plots of both DNR and DOX are showed in each chromatogram in Fig. 4.
FIG. 4.
Selected reaction monitoring liquid chromatography–electrospray ionization–multistage mass spectrometry of DNR released from DNR-loaded pSi microparticles in upper vitreous fluid taken at 24 h (a), 120 h (b), and 192 h (c). Pure vitreous showed as a control (d). Doxorubicin (DOX) was used as an internal standard.
By using standard controls (pure vitreous samples spiked with known amount of DNR and DOX), the concentration of intact DNR detected at 24, 120, and 192 h was determined to be around 1.0, 0.6, and 0.3 ng/mL, respectively. The decrease of the DNR concentration is likely due to drug degradation resulted from the reduction by the pSi matrix in a closed system, as observed in our previous in vitro study in PBS.13
In vivo rabbit eye toxicity study
Following intravitreal injection, the empty particles were observed to be suspended in the vitreous of the injection site, while the drug-loaded particles settled in the inferior vitreous cavity over 24 to 72 h. During the time course of the experiment, measurements of IOP (BSS vs. Empty pSi vs. DNR-loaded pSi=17.35±3.3 vs. 17.93±3.5 vs. 16.72±3.0, P=0.3, GEE) and ERG showed to be normal (Table 1). Table 1. Summary of ERG after intravitreal injection of hydrosilylated pSi microparticles (empty and DNR loaded).
Table 1.
Summary of Electroretinogram Parameters by Study Groups
| Group | Eye | Dark B ampa | Light B ampb | Flicker ampc |
|---|---|---|---|---|
| Empty pSi | OD | 82.8±11.4 | 37.6±16.0 | 17.6±8.4 |
| DNR-loaded pSi | OD | 101.5±18.7 | 50.4±11.1 | 24.7±6.2 |
| Control | OS | 96.5±15.9 | 49.3±8.5 | 25.4±5.9 |
| P valued | 0.31 | 0.14 | 0.16 |
Dark adapted b wave amplitude (μV).
Light adapted b wave amplitude (μV).
30 Hz flicker electroretinograms amplitude (μV).
Derived from generalized estimating equation analysis.
OD, right eye; OS, left eye; pSi, porous silicon; DNR, daunorubicin.
During the time course of the in vivo experiment, the observed degradation rate of the empty and DNR-loaded hydrosilylated pSi particles was similar. However, the appearance of 2 types of particles was very different. Due to the orange color of DNR itself, the drug-loaded particles were deep orange color (Fig. 5A, B), while the empty particles were light reflective with vivid color at early observation, but lost the vivid light reflex later due to erosion of the nanostructure from degradation (Fig. 5C, D). In addition, the empty particles floated in midvitreous (Fig. 5C, D), while drug-loaded particles settled down in the inferior vitreous by gravity (Fig. 5A, B). Figure 5 shows fundus photographs of empty and DNR-loaded hydrosilylated pSi particles at different time points. In the beginning, the hydrosilylated pSi showed very slow degradation. At postinjection month 1, the particles degraded up to 10%, and then some acceleration of the degradation process over time was noted. Most of the particles showed degradation up to 40% within the first 3 months as judged using indirect ophthalmoscopy by 2 independent observers (KIH and JK) at serial predetermined exam time points and fundus photography. At month 6, the particles were still visible in the vitreous cavity and showed a degradation of over 80% with a notable color change for the empty pSi particles (Fig. 5C vs. D) though degradation slightly varied among the different rabbit eyes.
FIG. 5.
Fundus photographs of DNR-loaded hydrosilylated pSi particles (A, B) and empty hydrosilylated pSi particles (C, D) in the rabbit eyes. Focus of the images was on the particles instead of on the retina. The photograph (A) shows DNR-loaded particles in the vitreous 1 week after intravitreal injection (arrow), (B) demonstrates a reduction in the amount of particles after 3 months in the same eye, but the color was barely changed (arrow), indicating simultaneous particle degradation and drug release. (C) shows the typical bright vivid color of empty pSi (arrow) taken at 2 months with a major change of amount and color after 6 months (D) and the degraded pSi looked whitish and dull (arrow). Color images available online at www.liebertpub.com/jop
Histology was performed at month 3 and month 6. The light microscopy demonstrated the normal structures of the retina, retinal pigment epithelium (RPE), and choroid (Fig. 6). The samples were selected at visual streak (dense ganglion cell population region) and paired from the 2 eyes of the same rabbit that had the right eye injected with DNR-loaded pSi particles and left eye with BSS. Compared to its own control, the injected eyes showed similar morphology of ganglions, cells in the inner nuclear layer, and photoreceptors, including outsegments and inner segments. There were no discernible changes in the choroid between the study and control eyes, either.
FIG. 6.
Retinal cross sections imaged by light microscopy of the eyes harvested from a rabbit 3 months (R83) and another rabbit 6 months (R32) after intravitreal injection of DNR-loaded hydrosilylated porous Si particles (taken at 62.5×magnification). Images show a normal retina and choroid in pairs of 2 eyes of the same rabbit. The left eyes (OS) were the control eyes and the right eyes (OD) were the study eyes injected with DNR-loaded pSi particles.
Discussion
pSi has been investigated as a drug delivery system due to its large free pore volume, ability to host various therapeutics, as well as its good biocompatibility, and resorbability.11,34 The hypothesis is that the drug chemically grafted to the inner pore walls of the pSi microparticles will be released in the body as the matrix dissolves.35 By contrast, drug loaded into freshly etched pSi microparticles by simple physical infiltration often led to fast leaching of the adsorbed drug, usually within a few hours.34,36 For many vitreoretinal diseases such as PVR, the pathologic evolution lasts for months during which inhibitory therapeutics are needed to suppress the unwanted excessive cell proliferation.22 It is well known that the dissolution rate of silicon matrices can be slowed if the surface is modified with Si-C bonded species, which increases stability toward oxidation and hydrolysis in aqueous media.37 Furthermore, the Si-C chemistry provides a convenient means to attach drugs through covalent bonding, intended for slow release. In this study, we demonstrated a slow degradation of pSi and drug release in the rabbit vitreous without notable toxicity. In the current in vivo study, after pSi intravitreal injection (3.2 mg pSi loaded with 70.4 μg DNR), no toxicity was seen by ERG and histology during a 6-month observation. In both ex vivo and in vivo experiments, DNR-loaded pSi particles appeared reddish due to the color of DNR, also confirming loading was successful. In rabbit eyes, the amount of the reddish colored pSi particles in the vitreous decreased over time, which indicated that DNR was released as the pSi matrix degraded. In addition, the lack of retinal toxicity was indicative of a slow release process; otherwise, the dose would have been significantly greater than 15 μg per rabbit eye, which would have caused retinal toxicity.23 The pSi particles were visible in the eyes through a fundus camera for at least 6 months, indicating a slow degradation rate of this hydrosilylated pSi formulation in vivo.
Due to the narrow therapeutic window of DNR, delivery of a large bolus of free DNR to extend the therapeutic concentration is not possible. By sequestering DNR into pSi microparticles, the present study demonstrated that longer action times without toxicity is possible in vivo. It is evident that pSi particles can provide a reservoir for DNR in the vitreous.
In our previous in vitro study, DNR was loaded into pSi particles and drug release was confirmed in PBS. Since in vitro results are not always translated into in vivo behavior, we tested the DNR release in the excised rabbit vitreous, which is much more complicated biological fluid than PBS. The ex vivo study also demonstrated that DNR-loaded pSi microparticles released detectable DNR into the excised rabbit vitreous, athough the amount of DNR detected was less in the longer incubated rabbit vitreous. We hypothesize that the released DNR experienced chemical reduction in the biological fluid27 (excised vitreous) and Si-Si or Si-H species in the pSi carrier may have accelerated this reduction process.26 In addition, accumulation of silicic acid in the ex vivo static dissolution study (excised vitreous) may have further aggravated drug degradation. It is not clear if this degree of degradation will be observed in a living eye, where the vitreous fluid is dynamic and silicic acid and other silicon-related byproducts may not accumulate to the extent that is reached in a quiescent test tube. On the other hand, DNR is a redox-sensitive compound that is unstable and degrades rapidly in biological fluids at body temperature. This is a major factor influencing the pharmacokinetics of these anthracycline drugs in vivo.27 Of the possible DNR degradation products, semiquinone-free radicals produced from reduction of the quinone group of DNR are cytotoxic, and this may be one of the chemical pathways by which DNR exerts its effect against unwanted cellular proliferation. In our previous in vitro study, reduced DNR was observed, and the degradation products from this hydrosilylated pSi delivery system showed increased potency toward RPE cells.26 In the present in vivo study, the same delivery system and the payload did not induce any signs of ocular toxicity or other deleterious effects during 6 months of the study. It is known that pSi degrades into silicic acid, which is presumably cleared with physiological vitreous fluid turnover. The routes of silicic acid clearance need to be further studied. In addition, drug-loaded pSi particles will settle down into inferior vitreous as shown in this study. This will help to maintain a clear optical visual axis, otherwise, pSi particles may interfere with the patient's vision.
It is surprising to note that this pSi system degraded faster in PBS in vitro dissolution chamber than in living rabbit eyes of the current study.13 This again highlighted the difference between the in vitro and in vivo study. The vitreous is a unique viscous medium, which contains various salts, proteins, and water, which is substantially different from PBS. In addition, for the in vitro study, the particles were continually subjected to a flow/shear stress. Although the system included a 5-μm-pore size mesh, it is possible that, as the particles degrade, some can go through the mesh and be removed from the chamber. The continuous pressure from the flow and the loss of some particles may contribute to the faster degradation and drug release observed in vitro.
In summary, DNR was successfully loaded into hydrosilylated pSi particles through covalent bonding. An estimated 3.2 mg dose of the particles containing 70.2 μg of DNR administered by intravitreal injection showed no ocular toxicity in a 6-month observation and slow degradation of the pSi particles with slow release of the loaded DNR otherwise 70.2 μg DNR in a rabbit eye would cause extreme toxicity. The DNR release was confirmed in an ex vivo vitreous incubation study using SRM LC-MS/MS. As shown in our previous in vitro studies, the Si-H species on hydrosilylated pSi surface may have accelerated drug degradation, which happens in biological fluid by DNR itself. This suggests that a nonredox compound will work better with this hydrosilylated pSi delivery system formulation although this DNR delivery system may still be useful in the prevention of postoperative or post-trauma PVR formation because reduction products are biologically active to inhibit cell proliferation.13 Future studies will focus on the validation of efficacy of this delivery system in a PVR animal model.
Acknowledgments
A.N. wants to acknowledge Fundacion Alfonso Martin Escudero for funding.
Financial support: This study was supported by NIH EY020617 and the NSF under Grant DMR-0806859. Commercial relationship policy: NS. Scientific Section Code: RE.
Author Disclosure Statement
M.J. Sallor, Spinnaker Biosciences (I); W.R. Freeman, Spinnaker Biosciences (C); L. Cheng, Spinnaker Biosciences (C).
References
- 1.Chappelow A.V. Kaiser P.K. Neovascular age-related macular degeneration: potential therapies. Drugs. 2008;68:1029–1036. doi: 10.2165/00003495-200868080-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin D.F. Maguire M.G. Fine S.L., et al. Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology. 2012;119:1388–1398. doi: 10.1016/j.ophtha.2012.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Taban M. Lowder C.Y. Kaiser P.K. Outcome of fluocinolone acetonide implant (retisert(tm)) reimplantation for chronic noninfectious posterior uveitis. Retina. 2008;28:1280–1288. doi: 10.1097/IAE.0b013e31817d8bf2. [DOI] [PubMed] [Google Scholar]
- 4.Lee S.S. Robinson M.R. Novel drug delivery systems for retinal diseases. Ophthalmic Res. 2009;41:124–135. doi: 10.1159/000209665. [DOI] [PubMed] [Google Scholar]
- 5.MacCumber M.W. Sadeghi S. Cohen J.A. Deutsch T.A. Suture loop to aid in ganciclovir implant removal. Arch. Ophthalmol. 1999;117:1250–1254. doi: 10.1001/archopht.117.9.1250. [DOI] [PubMed] [Google Scholar]
- 6.Sieving P.A. Caruso R.C. Tao W., et al. Ciliary neurotrophic factor (cntf) for human retinal degeneration: phase i trial of cntf delivered by encapsulated cell intraocular implants. Proc. Natl. Acad. Sci. U. S. A. 2006;103:3896–3901. doi: 10.1073/pnas.0600236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Short B.G. Safety evaluation of ocular drug delivery formulations: techniques and practical considerations. Toxicol. Pathol. 2008;36:49–62. doi: 10.1177/0192623307310955. [DOI] [PubMed] [Google Scholar]
- 8.Bourges J.L. Bloquel C. Thomas A., et al. Intraocular implants for extended drug delivery: therapeutic applications. Adv. Drug Deliv. Revi. 2006;58:1182–1202. doi: 10.1016/j.addr.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 9.Anglin E.J. Cheng L. Freeman W.R. Sailor M.J. Porous silicon in drug delivery devices and materials. Adv. Drug Deliv. Rev. 2008;60:1266–1277. doi: 10.1016/j.addr.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hettiarachchi K. Lee A.P. Polymer-lipid microbubbles for biosensing and the formation of porous structures. J. Colloid Interface Sci. 2010;344:521–527. doi: 10.1016/j.jcis.2010.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrew J.S. Anglin E.J. Wu E.C., et al. Sustained release of a monoclonal antibody from electrochemically prepared mesoporous silicon oxide. Adv. Funct. Mater. 2010;20:4168–4174. doi: 10.1002/adfm.201000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng L. Anglin E. Cunin F., et al. Intravitreal properties of porous silicon photonic crystals: a potential self-reporting intraocular drug-delivery vehicle. Br. J. Ophthalmol. 2008;92:705–711. doi: 10.1136/bjo.2007.133587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu E.C. Andrew J.S. Cheng L., et al. Real-time monitoring of sustained drug release using the optical properties of porous silicon photonic crystal particles. Biomaterials. 2011;32:1957–1966. doi: 10.1016/j.biomaterials.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Low S.P. Voelcker N.H. Canham L.T. Williams K.A. The biocompatibility of porous silicon in tissues of the eye. Biomaterials. 2009;30:2873–2880. doi: 10.1016/j.biomaterials.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Kashanian S. Harding F. Irani Y., et al. Evaluation of mesoporous silicon/polycaprolactone composites as ophthalmic implants. Acta Biomaterialia. 2010;6:3566–3572. doi: 10.1016/j.actbio.2010.03.031. [DOI] [PubMed] [Google Scholar]
- 16.Retina Society Terminology Committee. The classification of retinal detachment with proliferative vitreoretinopathy. Ophtlamology. 1983;90:121–125. doi: 10.1016/s0161-6420(83)34588-7. [DOI] [PubMed] [Google Scholar]
- 17.Sadaka A. Giuliari G.P. Proliferative vitreoretinopathy: current and emerging treatments. Clin. Ophthalmol. 2012;6:1325–1333. doi: 10.2147/OPTH.S27896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wickham L. Bunce C. Wong D. McGurn D. Charteris D.G. Randomized controlled trial of combined 5-fluorouracil and low-molecular-weight heparin in the management of unselected rhegmatogenous retinal detachments undergoing primary vitrectomy. Ophthalmology. 2007;114:698–704. doi: 10.1016/j.ophtha.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 19.Cheng L.Y. Hostetler K. Valiaeva N., et al. Intravitreal crystalline drug delivery for intraocular proliferation diseases. Invest. Ophthalmol. Vis. Sci. 2010;51:474–481. doi: 10.1167/iovs.09-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiedemann P. Hilgers R.D. Bauer P. Heimann K. Adjunctive daunorubicin in the treatment of proliferative vitreoretinopathy: results of a multicenter clinical trial. Daunomycin study group. Am. J. Ophthalmol. 1998;126:550–559. doi: 10.1016/s0002-9394(98)00115-9. [DOI] [PubMed] [Google Scholar]
- 21.Shinohara K. Tanaka M. Sakuma T. Kobayashi Y. Efficacy of daunorubicin encapsulated in liposome for the treatment of proliferative vitreoretinopathy. Ophthalmic Surg. Lasers Imaging. 2003;34:299–305. [PubMed] [Google Scholar]
- 22.Mietz H. Heimann K. Onset and recurrence of proliferative vitreoretinopathy in various vitreoretinal disease. Br. J. Ophthalmol. 1995;79:874–877. doi: 10.1136/bjo.79.10.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiedemann P. Kirmani M. Santana M. Sorgente N. Ryan S.J. Control of experimental massive periretinal proliferation by daunomycin: dose-response relation. Graefes Arch. Clin. Exp. Ophthalmol. 1983;220:233–235. doi: 10.1007/BF02308080. [DOI] [PubMed] [Google Scholar]
- 24.Khawly J.A. Saloupis P. Hatchell D.L. Machemer R. Daunorubicin treatment in a refined experimental model of proliferative vitreoretinopathy. Graefes Arch. Clin. Exp. Ophthalmol. 1991;229:464–467. doi: 10.1007/BF00166311. [DOI] [PubMed] [Google Scholar]
- 25.Wiedemann P. Sorgente N. Bekhor C., et al. Daunomycin in the treatment of experimental proliferative vitreoretinopathy. Effective doses in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 1985;26:719–725. [PubMed] [Google Scholar]
- 26.Wu E.C. Andrew J.S. Buyanin A. Kinsella J.M. Sailor M.J. Suitability of porous silicon microparticles for the long-term delivery of redox-active therapeutics. Chem. Commun. 2011;47:5699–5701. doi: 10.1039/c1cc10993f. [DOI] [PubMed] [Google Scholar]
- 27.Maniez-Devos D.M. Baurain R. Lesne M. Trouet A. Degradation of doxorubicin and daunorubicin in human and rabbit biological fluids. J. Pharm. Biomed. Anal. 1986;4:353–365. doi: 10.1016/0731-7085(86)80057-7. [DOI] [PubMed] [Google Scholar]
- 28.Houee-Levin C. Benzineb K. Gardes-Albert M. Abedinzadeh Z. Ferradini C. Reduction of daunorubicin in the presence of sulfur-containing peptides. Free Radic. Biol. Med. 1991;11:573–580. doi: 10.1016/0891-5849(91)90138-s. [DOI] [PubMed] [Google Scholar]
- 29.Marney L.C. Laha T.J. Baird G.S. Rainey P.M. Hoofnagle A.N. Isopropanol protein precipitation for the analysis of plasma free metanephrines by liquid chromatography-tandem mass spectrometry. Clin. Chem. 2008;54:1729–1732. doi: 10.1373/clinchem.2008.104083. [DOI] [PubMed] [Google Scholar]
- 30.Cheng L. Hostetler K.Y. Chaidhawangul S., et al. Intravitreal toxicology and duration of efficacy of a novel antiviral lipid prodrug of ganciclovir in liposome formulation. Invest. Ophthalmol. Vis. Sci. 2000;41:1523–1532. [PubMed] [Google Scholar]
- 31.Brar M. Cheng L. Yuson R., et al. Ocular safety profile and intraocular pharmacokinetics of an antagonist of ephb4/ephrinb2 signalling. Br. J. Ophthalmol. 2010;94:1668–1673. doi: 10.1136/bjo.2010.182881. [DOI] [PubMed] [Google Scholar]
- 32.Cheng L. Hostetler K.Y. Chaidhawangul S., et al. Treatment or prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-o-hexadecylpropanediol-3-phospho-ganciclovir. Invest. Ophthalmol. Vis. Sci. 2002;43:515–521. [PubMed] [Google Scholar]
- 33.Lehto V.P. Vaha-Heikkila K. Paski J. Salonen J. Use of thermoanalytical methods in quantification of drug load in mesoporous silicon microparticles. J. Ther. Anal. Calorim. 2005;80:393–397. [Google Scholar]
- 34.Salonen J. Laitinen L. Kaukonen A.M., et al. Mesoporous silicon microparticles for oral drug delivery: Loading and release of five model drugs. J. Controlled Release. 2005;108:362–374. doi: 10.1016/j.jconrel.2005.08.017. [DOI] [PubMed] [Google Scholar]
- 35.Wu E.C. Park J-H. Park J., et al. Oxidation-triggered release of fluorescent molecules or drugs from mesoporous si microparticles. ACS Nano. 2008;2:2401–2409. doi: 10.1021/nn800592q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Anglin E.J. Schwartz M.P. Ng V.P. Perelman L.A. Sailor M.J. Engineering the chemistry and nanostructure of porous silicon fabry-pérot films for loading and release of a steroid. Langmuir. 2004;20:11264–11269. doi: 10.1021/la048105t. [DOI] [PubMed] [Google Scholar]
- 37.Canham L.T. Reeves C.L. Newey J.P., et al. Derivatized mesoporous silicon with dramatically improved stability in simulated human blood plasma. Adv. Mater. 1999;11:1505. [Google Scholar]