Abstract
Orientia tsutsugamushi, the etiologic agent of potentially fatal scrub typhus, is characterized by a high antigenic diversity, which complicates the development of a broadly protective vaccine. Efficacy studies in murine and nonhuman primate models demonstrated the DNA vaccine candidate pKarp47, based upon the O. tsutsugamushi Karp 47-kD HtrA protein gene, to be a successful immunoprophylactic against scrub typhus. To characterize 47-kD HtrA protein diversity among human isolates of Orientia, we sequenced the full open reading frame (ORF) of the 47-kD HtrA gene and analyzed the translated amino acid sequences of 17 patient isolates from Thailand (n=13), Laos (n=2), Australia (n=1), and the United Arab Emirates (UAE) (n=1) and 9 reference strains: Karp (New Guinea), Kato (Japan), Ikeda (Japan), Gilliam (Burma), Boryong (Korea), TA763, TH1811 and TH1817 (Thailand), and MAK243 (China). The percentage identity (similarity) of translated amino acid sequences between 16 new isolates and 9 reference strains of O. tsutsugamushi ranged from 96.4% to 100% (97.4% to 100%). However, inclusion of the recently identified Orientia chuto sp. nov. reduced identity (similarity) values to 82.2% to 83.3% (90.4% to 91.4%). These results demonstrate the diversity of Orientia 47-kD HtrA among isolates encountered by humans and therefore provide support for the necessity of developing a broadly protective scrub typhus vaccine that takes this diversity into account.
Key Words: Orientia tsutsugamushi, Scrub typhus, 47-kD HtrA protein gene, Antigenic diversity, vaccine development
Introduction
Scrub typhus is an acute, febrile, and potentially fatal disease caused by infection with the obligate intracellular bacteria of the genus Orientia. It can be characterized by fever, rash, eschar, pneumonitis, meningitis, and coagulopathies, in some cases leading to circulatory collapse (Kawamura et al. 1995). This disease is commonly seen in the Asia-Pacific region, with high population density and an estimated annual 1 million cases of scrub typhus (Rosenberg 1997, Kelly et al. 2002). Furthermore, it has become an identified risk to the increasing number of people traveling to this region (Jensenius et al. 2004, Jensenius et al. 2009). Scrub typhus can account for up to 23% of all febrile episodes and 27% of blood culture–negative fever patients in endemic areas (Brown et al. 1976, Phongmany et al. 2006). Mortality rates for scrub typhus range from <1% to 50%, depending on proper and timely antibiotic treatment, status of the individual infected, and the strain of Orientia encountered (Kelly et al. 2002). Recent reports of scrub typhus outbreaks (Kelly et al. 2002) and the recent detection of cases of scrub typhus outside previously described endemic regions (Izzard et al. 2010, Balcells et al. 2011) indicate that the disease is emerging and reemerging, and emphasize the need for characterizing antigen targets toward the development of a broadly protective scrub typhus vaccine.
The genus Orientia, is a member of the order Rickettsiales, which contains many other arthropod-vectored pathogens (e.g., Rickettsia, Anaplasma, Ehrlichia). Until very recently, it consisted of a single species, Orienta tsutsugamushi, with multiple antigenically disparate isolates characterized over the past 60 plus years. Initially, 3 antigenically distinct strains of O. tsutsugamushi were described: Karp (New Guinea), Kato (Japan), and Gilliam (Burma) (Kelly et al. 2009). In addition to these prototype strains, more than 20 antigenically distinct serotypes have been recognized. In addition to the various antigenic types, at least 9 phylogenetic groups include 135 isolates of O. tsutsugamushi based upon the extremely variable 56-kD type-specific antigen (TSA) gene have been described (Kelly et al. 2009).
Subunit vaccines based on the 47-kD high-temperature requirement A (HtrA) and 56-kD TSA proteins have been developed and evaluated (Chattopadhyay et al. 2007). Recombinant protein (truncated) and plasmid DNA 56-kD TSA vaccine candidates have shown the ability to provide mice with homologous protection (Ni et al. 2005, Chattopadhyay et al. 2007). Due to the type-specific nature of this protein, the more conserved antigen 47-kD HtrA protein (Oaks et al. 1989, Moree et al. 1992) was evaluated with the purpose of developing a vaccine with broad protection. This protein is a member of the HtrA protein family, which is characterized by serine protease and chaperone activities. The 47-kD HtrA gene and gene product of the Karp strain have been used to develop DNA (Niu et al. 2003, Xu et al. 2005) and recombinant protein (Niu et al. 2003, Yu et al. 2005) vaccine candidates displaying immunogenicity and efficacy in mouse scrub typhus models. Recent evaluations in our laboratory have shown not only that the pKarp47 DNA vaccine candidate is highly effective in protecting CD-1 outbred mice from homologous challenge, but also in protecting them from heterologous challenge from 4 of 17 disparate isolates using a single immunization (A.L.R., unpublished data). Most recently, the pKarp47 vaccine candidate was successful in completely protecting 2 and partially protecting 3 of 5 cynomolgus monkeys from intradermal injection with homologous challenge (D.H. Paris et al., manuscript in preparation).
With the intention of developing a broadly protective scrub typhus vaccine incorporating the 47-kD HtrA target, we investigated the diversity of this gene from recently acquired human clinical isolates of Orientia. We report here our success in determining the nucleic acid and translated amino acid sequence diversity based on the full open reading frame (ORF) of the 47-kD HtrA gene of 17 newly obtained Orientia isolates from Thailand, Laos, Australia, and the United Arab Emirates (UAE), and 9 reference strains from New Guinea, Japan, Burma, Korea, Thailand, and China.
Materials and Methods
Orientia isolates and nucleic acid preparations
Blood samples from patients with scrub typhus (clinically diagnosed and subsequently confirmed by serology) were collected at various study sites by the Mahidol–Oxford Tropical Medicine Research Unit, Mahidol University, Bangkok, Thailand (MORU), the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit, Mahosot Hospital, Vientiane, Laos (LOMWRU), and the Australian Rickettsial Reference Laboratory/Barwon Biomedical Research, the Geelong Hospital, Geelong, Victoria, Australia during 2003–2009. The site of blood collection and therefore georeferencing may not have been the site where the patient was bitten. The blood samples were inoculated into Vero cells and cultured at 37°C to obtain Orientia isolates (Luksameetanasan et al. 2007, Izzard et al. 2010). Nucleic acid was extracted from the cultures using the Wizard SV Genomic DNA purification system (Promega, USA) or patient blood samples using Qiagen Mini Blood kit (Qiagen, Valencia, CA). Seventeen dried nucleic acid preparations of Orientia isolates, 7 from cultures and 10 from patient blood samples (culture negative), were sent to Naval Medical Research Center (NMRC), Silver Spring, MD, where the nucleic acid preparations were reconstituted in TE buffer. Of the 17 DNA samples, 6 were from Udorn Thani province in northeastern Thailand, 2 from Tak province in northwestern Thailand (Luksameetanasan et al. 2007, Blacksell et al. 2008), 5 from Chiang Rai province in northern Thailand (this report), 2 from Laos (Phongmany et al. 2006), 1 from Australia (Unsworth et al. 2007), and 1 from the UAE (Izzard et al. 2010). The O. tsutsugamushi TA763, TH1811, and TH1817 (Thailand) and MAK243 (China) isolates' nucleic acids (NMRC preparations) were analyzed for comparison with the new isolates.
Quantitative real-time PCR (qPCR) assay
The Otsu47 qPCR assay was used to confirm the identity and presence of Orientia in the nucleic acid preparations of the new isolates. One microliter of the nucleic acid preparations was added to a final volume of a 25-μL reaction that included Platinum Quantitative PCR SuperMix-UDG (Invitrogen, Carlsbad, CA). The concentration of primers and probe used and reaction parameters were the same as previously described (Jiang et al. 2004).
PCR, nested PCR, and sequencing
Standard PCR and nested PCR (nPCR) were performed to amplify the entire ORF of the 47-kD HtrA genes. Primers used for PCR (Ot-145F, Ot-1780R) and nPCR (Ot-263F, Ot-1133R, and Otr47-1404R) amplification were selected from conserved regions of the 47-kD HtrA gene after alignment of the sequences from Karp, Kato, Ikeda, Gilliam, and Boryong strains of O. tsutsugamushi (GenBank accession numbers L31934, L11697, AP008981, L31933, and AM494475, respectively) using Vector NTI advance 11 software (Invitrogen). Due to difficulties in amplifying the 47-kD HtrA gene from the O. chuto isolate nucleic acid, specific primers (Chur 627F and Chur 669F) were identified and selected after a partial sequence was obtained. The sequences of the primers used for PCR, nPCR, and sequencing are listed in Table 1.
Table 1.
Primers Used for Standard PCR, Nested PCR, and Sequencing of the 47-kD HtrA Protein Gene of Orientia Isolates
| Name | Sequence (5′-3′) |
|---|---|
| Ot-145Fa,.b | ACAGGCCAAGATATTGGAAG |
| Ot-1780Ra,b | AATCGCCTTTAAACTAGATTTACTTATTA |
| OtsuFP630b | AACTGATTTTATTCAAACTAATGCTGCT |
| OtsuRP747b | TATGCCTGAGTAAGATACRTGAATRGAATT |
| Ot-263Fb,c | GTGCTAAGAAARGATGATACTTC |
| Ot-1133Rb,c | ACATTTAACATACCACGACGAAT |
| Chur 627Fa | GCGGGATATAGGTAGTTCAA |
| Chur 669Fb,c | TATTCAAACTAATGCTGTTGTGC |
| Otr47-1404Rb,c | GATTTACTTATTAATRTTAGGTAAAGCAATGT |
PCR primer.
Sequencing primer.
Nested PCR primer.
The 25-μL reaction mixtures, which contained 2 μL of the nucleic acid preparations, 0.3 μM of the forward and reverse primers, and Platinum PCR SuperMix High Fidelity (Invitrogen), were incubated on a T-Gradient Thermocycler (Biometra, Goettingen, Germany) at 95°C for 2 min followed by 40 cycles of 3-step amplification at 94°C for 30 sec, 54°C for 30 sec, and 68°C for 2 min, followed by a final extension hold at 72°C for 7 min. PCR amplicons were visualized and compared to molecular weight standards (1-kbp Ladder, Invitrogen) on 1.5% agarose gels with ethidium bromide (Gibco BRL Life Technologies, Inc. Gaithersburg, MD) staining following electrophoresis. The PCR products were purified by QIAquick PCR Purification Kit (Qiagen, Valencia, CA). The sequencing reactions were performed using BigDye Terminator v 3.1 Ready Reaction Cycle Sequencing Kits (Applied Biosystems, Foster City, CA), after cleanup with Gel Filtration Cartridges (EdgeBio, Gaithersburg, MD). The reaction products were run on an automated ABI Prism 3130xl genetic analyzer (Applied Biosystems). The sequences, from both directions of the DNA strands, were assembled by Vector NTI advance 11 software (Invitrogen), and compared to each new human isolate and with the reference strains Karp (New Guinea), Kato (Japan), Ikeda (Japan), Gilliam (Burma), and Boryong (Korea) from GenBank and Kato (RDD10-001), TA763 (Thailand), TH1811 (Thailand) TH1817 (Thailand), and MAK243 (China) sequenced in this study.
GenBank accession numbers
The 47-kD HtrA gene sequences of the new Orientia isolates and 5 reference strains reported herein have been deposited in GenBank with accession numbers listed in Table 2.
Table 2.
Reference Strains and New Isolates of Orientia Used in the Study
| No. | Isolate | GenBank no. | Geographic location | Region/Province | District/Village | Latitude | Longitude | Ct of Otsu47 qPCR | ORF Length |
|---|---|---|---|---|---|---|---|---|---|
| 1 | CRF27 | HM156047 | Thailand N | Chiang Rai | Thoeng | 19.683333 | 100.2 | 38.28a | 1401 |
| 2 | CRF79 | HM156048 | Thailand N | Chiang Rai | Muang | 19.95234 | 99.88293 | 44.27a | 1401 |
| 3 | CRF93 | HM156049 | Thailand N | Chiang Rai | Fang | 19.91667 | 99.21667 | 41.56a | 1401 |
| 4 | CRF116 | HM156050 | Thailand N | Chiang Rai | Muang | 19.95234 | 99.88293 | 40.66a | 1401 |
| 5 | CRF136 | HM156051 | Thailand N | Chiang Rai | Muang | 19.95234 | 99.88293 | 34.99a | 1401 |
| 6 | FPW1038 | HM156052 | Thailand NW | Tak | Mae Ramat | 16.96667 | 98.51667 | 23.61b | 1404 |
| 7 | FPW2016 | HM156053 | Thailand NW | Tak | Phop Phra | 16.38611 | 98.69028 | 21.87b | 1401 |
| 8 | UT76 | HM156054 | Thailand NE | Udorn Thani | Muang | 17.38644 | 102.7883 | 20.86b | 1401 |
| 9 | UT169 | HM156055 | Thailand NE | Udorn Thani | Muang | 17.38644 | 102.7883 | 21.25b | 1401 |
| 10 | UT176 | HM156056 | Thailand NE | Udorn Thani | Ban Phu | 17.13417 | 102.9183 | 20.29b | 1401 |
| 11 | UT221 | HM156057 | Thailand NE | Udorn Thani | Muang | 17.38644 | 102.7883 | 21.09b | 1401 |
| 12 | UT418 | HM156058 | Thailand NE | Udorn Thani | Muang | 17.38644 | 102.7883 | 21.18b | 1401 |
| 13 | UT661 | HM156059 | Thailand NE | Udorn Thani | Muang | 17.38644 | 102.7883 | 36.77a | 1401 |
| 14 | TM1320 | HM156060 | Laos | Vientiane capital | Sangthong/Samphanna | 18.05579 | 102.31616 | 38.33a | 1401 |
| 15 | TM1324 | HM156061 | Laos | Vientiane capital | Xaysettha/Kham ngoi | 17.98086 | 102.68386 | 38.95a | 1401 |
| 16 | SIDO | HM156062 | Australia | NA | NA | NA | NA | 26.94a | 1401 |
| 17 | Chuto | HM156063 | UAE | NA | NA | NA | NA | 42.82a | 1404 |
| r-1 | TH1811 | HM595489 | Thailand | NA | NA | NA | NA | -ND | 1401 |
| r-2 | TH1817 | HM156064 | Thailand | Nakorn Ratchasima | Pak Tong Chai/Pak Tong Chai | 14.7167 | 102.0167 | 19.73b | 1401 |
| r-3 | TA763 | HM595490 | Thailand | Ubon Ratchathani | Sirinthorn/Chong Mek | 15.1333 | 105.4667 | 19.2b | 1401 |
| r-4 | MAK243 | HM595491 | Taiwan | Pescadore Islands | NA | NA | NA | 18.98b | 1401 |
| r-5-1 | Kato RDD10-001 | HM595493 | Japan | Nigata | NA | 37.9112 | 139.0052 | 21.8b | 1401 |
| r-5-2 | Kato | L11697 | Japan | Nigata | NA | 37.9112 | 139.0052 | 21.44b | 1401 |
| r-6 | Karp | L31934 | New Guinea | NA | NA | NA | NA | 17.56b | 1401 |
| r-7 | Gilliam | L31933 | Burma | NA | NA | NA | NA | 14.9b | 1401 |
| r-8 | Ikeda | AP008981 | Japan | Nigata | NA | 37.9112 | 139.0052 | -ND | 1401 |
| r-9 | Boryong | AM494475 | South Korea | South Chungcheong | / Boryong | NA | NA | -ND | 1401 |
Ct values for the10 DNA preparations obtained from blood samples of scrub typhus patients.
Ct values determined for DNA preparations from cultures of patient and reference samples.
Ct, cycle threshold values; ORF, open reading frame; NA, not available; ND, not determined.
Phylogenetic analyses
Phylogenetic analyses were performed using MEGA5 software using the multialignment of the 47-kD HtrA gene and 47-kD protein sequences from 17 new human isolates and 9 reference strains. The evolutionary history was inferred by using the maximum likelihood method based on the Tamura–Nei (for the 47-kD gene) and point accepted mutation (PAM) matrix-based (for 47-kD protein) models (Schwarz and Dayhoff 1979, Nei and Kumar 2000). The constructed trees were evaluated with bootstrapping from 1000 replications. The calculated translated amino acid sequences were determined by Vector NTI advance 11 software (Invitrogen). The heat maps depicting similarity/diversity of the nucleotide and amino acid sequences among 16 new human isolates (without O. chuto isolate) and 9 reference strains were generated using the web tool (www.hiv.lanl.gov/content/sequence/HEATMAP/heatmap.html/).
Ethics statement
All clinical samples in this study (TM [Typhus Mahosot], CRF [Chiang Rai Fever], UT [Udorn Thani], and FPW [Fever in Pregnant Women]) were collected as part of studies investigating the causes of fever at the corresponding study sites. Ethical clearance was obtained from the Faculty of Tropical Medicine, Mahidol University (CRF, UT, FPW), the Thai Ministry of Public Health (CRF, UT, FPW), the National Ethics Committee for Health Research, Ministry of Public Health, Lao PDR (TM), and the Oxford Tropical Research Ethics Committee (all studies). All patients provided informed written consent before sample collection.
Results
qPCR Assay with DNA from patients' blood samples
All 17 dried nucleic acid preparations of Orientia isolates (7 from cultures and 10 from patient blood samples) sent to NMRC were positive by the Otsu47 qPCR assay. The cycle threshold (Ct) values for the 10 DNA preparations from patient blood samples represented approximately 12 to 207,157 (median=111) genome equivalents/mL.
DNA sequence analysis
Of the 17 new isolates, CRF116 and CRF136 from northern Thailand had identical sequences, and UT76, UT169, and UT661 from northeastern Thailand and TM1324 from Laos had identical sequences within the gene studied. Thus, 13 (76.5%) new nucleotide sequences of the 47-kD HtrA gene were identified from the 17 available isolates; 10 from Thailand (4, 2, and 4 from northeastern, northwestern, and northern Thailand, respectively), and 1 each from Laos, Australia, and the UAE.
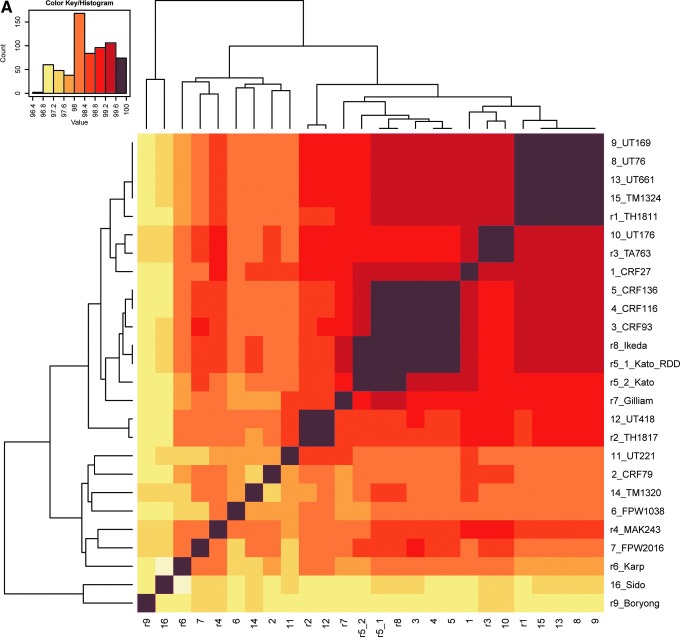
Nucleotide sequence identities of the 47-kD HtrA gene ranged from 96.7% (Karp vs. Sido) to 100% for 25 isolates without including O. chuto (Fig. 1a). However, the percent identities of the geographically remote O. chuto isolate to the other 25 isolates ranged between 84.1% and 84.6%.
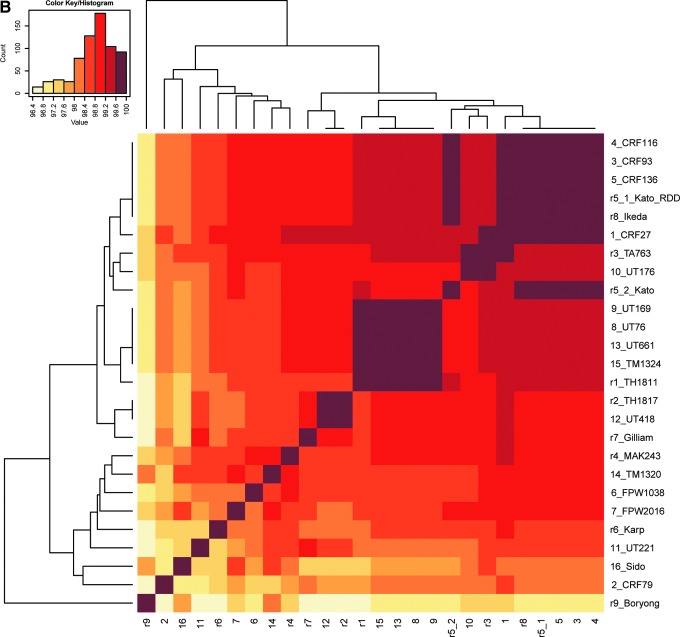
FIG. 1.
Heat maps of percent identities of the 47-kD HtrA DNA (A) and amino acid (B) sequences among 25 Orientia isolates (without O. chuto). Heat maps were generated using the pairwise identity matrix tables with hierarchical clustering method. The name of each isolate was labeled on the right side of the graphs with its corresponding number code, which was used at the bottom of the graphs. Dendrograms were placed to the left side and on top of the graphs.
The 47-kD HtrA gene sequences of 2 different passage preparations of reference strain Kato (plaque purified) archived in the NMRC, L5/90, and RDD10-001 were identical to each other and surprisingly identical to Ikeda, but they had a 2-bp difference compared to the Kato sequence listed in GenBank (accession number L11697). To confirm the identity of the NMRC Kato nucleic acid preparations, 56-kD TSA sequencing was performed. Two 1114-bp sequences from the NMRC Kato preparations demonstrated 100% identity to each other and to the Kato strain 56-kD TSA gene sequence in GenBank (accession number M63382), but only 82.4% identity to the Ikeda 56-kD TSA sequence (accession number AP008981). Thus, the 2 NMRC preparations were correctly identified as belonging to the Kato strain.
Amino acid sequence analysis
The nucleotide sequences of the full ORF 47-kD HtrA gene from the 17 new isolates led to 10 new amino acid sequences. Isolates CRF93, CRF116, and CRF136 had the same translated sequence and were identical to Ikeda. Sequence of sample UT418 from northeastern Thailand was identical to the sequence obtained from TH1817. Percent identities (similarities) of calculated translated amino acid sequences for the 47-kD HtrA between the 16 new isolates (not including the O. chuto isolate) and 9 reference strains were 96.4% to 100% (97.4% to 100%) (Fig. 1). The identities (similarities) of the translated amino acid sequences between O. chuto and the other 25 isolates ranged from 82.2% to 83.3% (90.4% to 91.4%).
Phylogenetic analyses
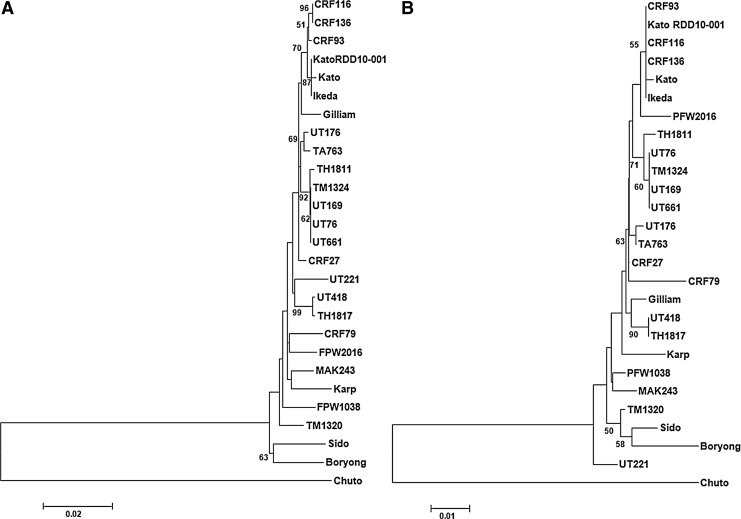
The phylogenetic analyses conducted using the 47-kD HtrA gene among the new Orientia isolates and the reference strains showed that CRF93, CRF116, and CRF136 clustered together with reference strains Ikeda and Kato; UT418 joined with TH1817; UT76, UT169, UT661, and TM1324 formed a cluster with reference strain TH1811; and UT176 placed closely with TA763. The clusters formed were supported by high bootstrap values. The rest of the new isolates did not form clusters with each other or with the reference isolates (Fig. 2A). The dendrogram from the heat map by hierarchical clustering showed similar evolutionary relationships (Fig. 1A). The similar phylogenetic patterns were observed in 47-kD protein among those 26 O. tsutsugamushi isolates/strains (Fig. 2B).
FIG. 2.
Evolutionary relationships among 17 new Orientia isolates and 9 reference strains. Phylogenetic trees were constructed based on multiple sequence alignment of 47-kD HtrA gene open reading frame (ORF) (A) and calculated amino acid sequence of the 47-kD HtrA (B) using the maximum likelihood method. Bootstrap values above 50 are labeled at the nodes.
Discussion
The aim of this study was to assess the isolate-associated divergence of the 47-kD HtrA DNA and amino acid sequences among the Orientia isolates studied (n=26). The importance of the divergence may help explain why the vaccine candidate pKarp47 derived from a single strain of O. tsutsugamushi, Karp, has been found to protect mice from challenge with just 4 of 17 disparate strains of O. tsutsugamushi (A.L.R., unpublished data).
In agreement with previous reports that the 47-kD HtrA gene is relatively conserved (Oaks et al. 1989, Moree et al. 1992), the variation in our nucleotide sequences was seen at only 3.3% divergence among 25 isolates (not including the O. chuto). This compares with other conserved protein genes such as the 22-kD antigen gene, where percentage sequence divergence between 7 strains was less than 5% (Ge et al. 2005), the 58-kD GroEL protein gene with divergence under 3.5% among Korean and Thai strains (Lee et al. 2003, Paris et al. 2009), and the 16S rRNA gene with a maximum divergence of 1.5% (Ohashi et al. 1995). The conserved nature of the 47-kD HtrA nucleotide sequence stands in stark contrast to that of the 56-kD TSA gene, where divergence among isolates of up to and greater than 80% were noted (Blacksell et al. 2008, Fournier et al. 2008, Kelly et al. 2009). Moreover, the 9 distinct clusters of genotypes described for the 56-kD TSA gene were not seen with the phylogenetic tree constructed for the 47-kD HtrA gene (Fig. 1A). However, even though the 47-kD HtrA gene is relatively conserved within the genus, Orientia isolates could be seen to cluster based upon differences in nucleotide sequences. The 4 clusters identified included: (1) UT661, TM1324, UT76, UT169, and TH1811; (2) Kato, Ikeda, CRF93, CRF116, and CRF136; (3) UT418 and TH1817; and (4) TA763 and UT176. In addition, certain Orientia isolates did not cluster similarly in both the 47-kD HtrA and 56-kD TSA phylogenetic trees (e.g., Kato and Ikeda).
Further characterization of the 47-kD HtrA protein among Orientia isolates included calculating the amino acid sequences; as with the nucleotide sequences, unique differences were seen between Orientia isolates (Fig. 1B). The greatest difference was seen between the amino acid sequence of the O. chuto isolate from the UAE and all other isolates, including reference strains, supporting the evidence that this isolate represents a new species of Orientia, as recently reported (Izzard et al. 2010). The large divergence was seen more impressively when unique differences were identified in the nucleotide sequences of the 56-kD TSA (n=31) and 16S rRNA (n=9) gene of the O. chuto when compared to O. tsutsugamushi (Izzard et al. 2010).
Additional differences in the amino acid sequences from the consensus sequence were seen in the Boryong, Sido, and Karp isolates. The observation made that the Karp strain was divergent from other isolates based upon the 47-kD HtrA sequence was unexpected because it has been considered the type strain of O. tsutsugamushi. This divergence is also notable in the 56-kD phylogenetic analyses, which showed Karp and similar isolates grouping away from other strains and isolates (Kelly et al. 2009). In addition, a phylogenetic analysis of 22-kD protein gene sequences of 7 O. tsutsugamushi isolates also suggested that the Karp strain is relatively distant from the other isolates (Ge et al. 2005). This implies that vaccine candidates based upon the Karp strain may need to be augmented by other gene/gene products from isolates more represented in the genus and especially from those isolates associated with human disease, i.e., obtained from scrub typhus patients (Ruang-Areerate et al. 2011).
In contrast to the overall large extent of amino acid conservation displayed among 25 Orientia isolates, the bulk of the observed amino acid substitutions were associated with O. chuto. The observed amino acid sequence changes could suggest that the 47-kD HtrA protein may be able to withstand a large amount of variation, at least in the UAE, where the ecology maybe significantly different from previously described endemic regions of Asia, northern Australia, and the western Pacific. A less plausible alternative is that although the protein is expressed, it is nonfunctional. The ability of O. chuto to survive/thrive needs further investigation.
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the Department of the Navy, Department of Defense, nor the U.S. Government. Authors, as employees of the U.S. Government, conducted the work as part of their official duties. Title 17 U.S.C. § 105 provides that “Copyright protection under this title is not available for any work of the United States Government.” Title 17 U.S.C § 101 defines a US Government work as a work prepared by an employee of the US Government as part of the person's official duties.
This work was supported by the U.S. DoD MIDRP work unit # 6000.RAD1.J.A0310. Specimen collection in Laos was part of the Lao-Oxford-Mahosot Hospital-Wellcome Trust Research Unit funded by the Wellcome Trust of Great Britain. We thank the Directors of Mahosot Hospital, the staff of the Microbiology Laboratory and Infectious Disease Ward, Mahosot Hospital, and Sayaphet Rattanavong. D.H.P. was supported by a Wellcome Trust Clinical Research Training Fellowship (grant no. 0789900/Z/06/Z).
We are grateful to Francois Nosten and Rose McGready, Shoklo Malaria Research Unit, MaeSot, Thailand for providing the FPW samples.
Author Disclosure Statement
There is no commercial relationship between any of the authors and products used in the study, thus no competing financial interests exist.
References
- Balcells ME. Rabagliati R. Garcia P. Poggi H, et al. Endemic scrub typhus-like illness, Chile. Emerg Infect Dis. 2011;17:1659–1663. doi: 10.3201/eid1709.100960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacksell SD. Luksameetanasan R. Kalambaheti T. Aukkanit N, et al. Genetic typing of the 56-kDa type-specific antigen gene of contemporary Orientia tsutsugamushi isolates causing human scrub typhus at two sites in north-eastern and western Thailand. FEMS Immunol Med Microbiol. 2008;52:335–342. doi: 10.1111/j.1574-695X.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- Brown GW. Robinson DM. Huxsoll DL. Ng TS, et al. Scrub typhus: A common cause of illness in indigenous populations. Trans Royal Soc Trop Med Hyg. 1976;70:444–448. doi: 10.1016/0035-9203(76)90127-9. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay S. Richards AL. Scrub typhus vaccines: Past history and recent developments. Hum Vaccines. 2007;3:73–80. doi: 10.4161/hv.3.3.4009. [DOI] [PubMed] [Google Scholar]
- Ge H. Tong M. Li A. Mehta R. Ching WM. Cloning and sequence analysis of the 22-kDa antigen genes of Orientia tsutsugamushi strains Kato, TA763, AFSC 7, 18-032460, TH1814, and MAK 119. Ann NY Acad Sci. 2005;1063:231–238. doi: 10.1196/annals.1355.036. [DOI] [PubMed] [Google Scholar]
- Fournier PE. Siritantikorn S. Rolain JM. Suputtamongkol Y, et al. Detection of new genotypes of Orientia tsutsugamushi infecting humans in Thailand. Clin Microbiol Infect. 2008;14:168–173. doi: 10.1111/j.1469-0691.2007.01889.x. [DOI] [PubMed] [Google Scholar]
- Izzard L. Fuller A. Blacksell SD. Paris DH, et al. Isolation of a novel Orientia species (O. chuto sp. nov.) from a patient infected in Dubai. J Clin Microbiol. 2010;48:4404–4409. doi: 10.1128/JCM.01526-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensenius M. Fournier PE. Raoult D. Rickettsioses and the international traveler. Clin Infect Dis. 2004;39:1493–1499. doi: 10.1086/425365. [DOI] [PubMed] [Google Scholar]
- Jensenius M. Davis X. von Sonnenburg F. Schwartz E, et al. Multicenter GeoSentinel analysis of rickettsial diseases in international travelers, 1996–2008. Emerg Infect Dis. 2009;15:1791–1798. doi: 10.3201/eid1511.090677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J. Chan TC. Temenak JJ. Dasch GA, et al. Development of a quantitative real-time polymerase chain reaction assay specific for Orientia tsutsugamushi. Am J Trop Med Hyg. 2004;70:351–356. [PubMed] [Google Scholar]
- Kawamura A. Tanaka H. Tamura A. Tsutsugamushi Disease. Tokyo, Japan: University of Tokyo Press; 1995. [Google Scholar]
- Kelly DJ. Richards AL. Temenak J. Strickman D, et al. The past and present threat of rickettsial diseases to military medicine and international public health. Clin Infect Dis. 2002;34:S145–S169. doi: 10.1086/339908. [DOI] [PubMed] [Google Scholar]
- Kelly DJ. Fuerst PA. Ching WM. Richards AL. Scrub typhus: The geographic distribution of phenotypic and genotypic variants of Orientia tsutsugamushi. Clin Infect Dis. 2009;489(Suppl 30):S203–S230. doi: 10.1086/596576. [DOI] [PubMed] [Google Scholar]
- Lee JH. Park HS. Jang WJ. Koh SE, et al. Differentiation of rickettsiae by groEL gene analysis. J Clin Microbiol. 2003;41:2952–2960. doi: 10.1128/JCM.41.7.2952-2960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luksameetanasan R. Blacksell SD. Kalambaheti T. Wuthiekanun V, et al. Patient and sample-related factors that effect the success of in vitro isolation of Orientia tsutsugamushi. Southeast Asian J Trop Med Public Health. 2007;38:91–96. [PubMed] [Google Scholar]
- Moree MF. Hanson B. Growth characteristics and proteins of plaque-purified strains of Rickettsia tsutsugamushi. Infect Immun. 1992;60:3405–3415. doi: 10.1128/iai.60.8.3405-3415.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M. Kumar S. Molecular Evolution and Phylogenetics. New York: Oxford University Press; 2000. [Google Scholar]
- Ni YS. Chan TC. Chao CC. Richards AL, et al. Protection against scrub typhus by a plasmid vaccine encoding the 56-KD outer membrane protein antigen gene. The Am J Trop Med Hyg. 2005;73:936–941. [PubMed] [Google Scholar]
- Niu D. Chen W. Zhang X. Chen M, et al. Immunogenicity of a 40kDa fragment of the 47kDa recombinant protein and DNA vaccine from Karp strain of Orientia tsutsugamushi. Ann NY Acad Sci. 2003;990:527–534. doi: 10.1111/j.1749-6632.2003.tb07423.x. [DOI] [PubMed] [Google Scholar]
- Oaks EV. Rice RM. Kelly DJ. Stover CK. Antigenic and genetic relatedness of eight Rickettsia tsutsugamushi antigens. Infect Immun. 1989;57:3116–3122. doi: 10.1128/iai.57.10.3116-3122.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohashi N. Fukuhara M. Shimada M. Tamura A. Phylogenetic position of Rickettsia tsutsugamushi and the relationship among its antigenic variants by analyses of 16S rRNA gene sequences. FEMS Microbiol Lett. 1995;125:299–304. doi: 10.1111/j.1574-6968.1995.tb07372.x. [DOI] [PubMed] [Google Scholar]
- Paris DH. Aukkanit N. Jenjaroen K. Blacksell SD, et al. A highly sensitive quantitative real-time PCR assay based on the groEL gene of contemporary Thai strains of Orientia tsutsugamushi. Clin Microbiol Infect. 2009;15:488–495. doi: 10.1111/j.1469-0691.2008.02671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phongmany S. Rolain JM. Phetsouvanh R. Blacksell SD, et al. Rickettsial infections and fever, Vientiane, Laos. Emerg Infect Dis. 2006;12:256–262. doi: 10.3201/eid1202.050900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg R. Drug-resistant scrub typhus: Paradigm and paradox. Parasitol Today. 1997;13:131–132. doi: 10.1016/s0169-4758(97)01020-x. [DOI] [PubMed] [Google Scholar]
- Ruang-Areerate T. Jeamwattanalert P. Rodkvamtook W, et al. Genotype diversity and distribution of Orientia tsutsugamushi causing scrub typhus in Thailand. J Clin Microbiol. 2011;49:2584–2589. doi: 10.1128/JCM.00355-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R. Dayhoff M. Matrices for detecting distant relationships. In: Dayhoff M, editor. Atlas of Protein Sequences. National Biomedical Research Foundation; 1979. pp. 353–358. [Google Scholar]
- Unsworth NB. Stenos J. Faa AG. Graves SR. Three rickettsioses, Darnley Island, Australia. Emerg Infect Dis. 2007;13:1105–1107. doi: 10.3201/eid1307.050088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G. Chattopadhyay S. Jiang J. Chan TC, et al. Short- and long-term immune responses of CD-1 outbred mice to the scrub typhus DNA vaccine candidate: p47Kp. Ann NY Acad Sci. 2005;1063:266–269. doi: 10.1196/annals.1355.043. [DOI] [PubMed] [Google Scholar]
- Yu Y. Wen B. Wen B. Niu D, et al. Induction of protective immunity against scrub typhus with a 56-kilodalton recombinant antigen fused with a 47-kilodalton antigen of Orientia tsutsugamushi Karp. Am J Trop Med Hyg. 2005;72:458–464. [PubMed] [Google Scholar]