Abstract
During 2010 and 2011, 933 recently deceased birds, submitted as part of the dead bird surveillance program, tested positive for West Nile virus RNA at necropsy. The relative amount of RNA measured by qRT-PCR cycles ranged from 8.2 to 37.0 cycle threshold (Ct) and formed a bimodal frequency distribution, with maxima at 20 and 36 Ct and minima at 28–30 Ct. On the basis of frequency distributions among different avian species with different responses to infection following experimental inoculation, field serological data indicating survival of infection, and the discovery of persistent RNA in experimentally infected birds, dead birds collected in nature were scored as “recent” or “chronic” infections on the basis of Ct scores. The percentage of birds scored as having chronic infections was highest during late winter/spring, when all birds were after hatching year, and lowest during late summer, when enzootic transmission was typically highest as indicated by mosquito infections. Our data indicated that intervention efforts should not be based on dead birds with chronic infections unless supported by additional surveillance metrics.
Key Words: Surveillance, West Nile virus, Dead birds, Chronic infections, Overwintering
Introduction
Dead birds, especially those in the family Corvidae of the order Passeriformes, have been the hallmark of the West Nile virus (Flaviviridae, Flavivirus, WNV) epidemic in North America (Komar 2003) and have been useful in surveillance programs to track enzootic transmission activity (Eidson et al. 2001a, Eidson et al. 2001b, Carney et al. 2005, Johnson et al. 2006, Carney et al. 2011). In California, the public and vector control agencies have been encouraged to submit recently deceased specimens of all avian species for necropsy. Oral swabs from American Crows (Corvus brachyrhynchos) and kidney snips from other species are tested for WNV using qRT-PCR, allowing a rapid assessment of the relative amount of RNA present. Over time, a pattern has emerged, with a bifurcation of positive samples into low cycle threshold (Ct) values indicative of a high virus concentration and high Ct values indicative of very few RNA copies and little virus. This article describes this bifurcation and differentiates patterns among bird species with known responses to WNV based on experimental infection.
Experimental inoculation of competent birds with WNV is followed rapidly by a viremia that increases in concentration to a crisis point, when the infection either resolves due to a rise in antibody and subsequent decline in viremia or persists at a high viremia titer and the infected bird succumbs, usually within 5–8 days postinfection (Komar et al. 2003, Reisen et al. 2005). At this time, WNV can be found in almost every tissue (Kramer et al. 2002, Komar et al. 2003), probably because of high viremia titers within the circulatory system. Viremia levels and disease outcome vary markedly among bird species (Wheeler et al. 2009). In preliminary studies, some experimentally infected birds surviving acute infection were held for 4–6 weeks until antibody titers peaked and then were necropsied (Reisen et al. 2005). Viral RNA was recovered from multiple tissues including sera, and 4 of 6 House Finches (Carpodacus mexicanus) retained infectious virus detected after passage in C6/36 Aedes albopictus cells (Reisen et al. 2006). Subsequently, the period of WNV RNA detection in house Sparrows (Passer domesticus), House Finches, and Western Scrub-Jays (Aphelocoma californica) was extended through winter in both experimentally and naturally infected birds (Nemeth et al. 2009a; Wheeler et al. 2012), indicating that persistently infected birds potentially may serve as a virus overwintering mechanism and that birds testing positive in the spring probably were infected during the previous transmission season. This notion was supported by a time course study where WNV RNA was repeatedly recovered from sera and multiple organs at necropsy and where infectious WNV was recovered at 7 and 12 weeks postinfection by co-cultivation and blind passage (Wheeler 2012), in agreement with studies on rodents (Tesh et al. 2005, Appler et al. 2010). These data tentatively provided an explanation for the bifurcation of data seen in field-collected dead birds. The current article describes patterns in the quantity of WNV RNA found in dead birds of several species at necropsy and makes recommendations relevant to intervention.
Materials and Methods
Dead birds were reported throughout the year by the public to the Dead Bird Hotline at the California Department of Public Health, collected by participating mosquito control and public health agencies (McCaughey et al. 2003, Carney et al. 2005), and shipped to the California Animal Health and Food Safety Laboratory (CAHFS) at the University of California at Davis for necropsy under BSL3 conditions. Oral swabs previously were found to be suitable for testing American Crows but not reliable for other species (Padgett et al. 2006), so kidney snips were collected from all other bird species as well as tree squirrels. During 2010 and 2011, swabs and snips were placed into ABI Lysis Buffer (Applied Biosystems, Life Technologies, Carlsbad, CA), and transferred to the Center for Vectorborne Diseases (CVEC) laboratory for testing. Here, specimens were triturated with copper BBs on a SPEX TissueLyser Mill (SPEX CertiPrep, Metuchen, NJ), RNA extracted using an ABI MagMAX system, and then tested for WNV RNA using quantitative RT-PCR with an ABI 7900 TaqMan® platform and primers/probe from the envelope gene (Lanciotti et al. 2000). Confirmations initially were attempted using tissue culture from second samples in virus diluent, re-extraction, and retesting by RT-PCR, and/or by testing with primers/probe from the NS1 region of the genome (Shi et al. 2001). Almost 100% of samples with <30 Ct with high amounts of viral RNA were confirmed, whereas virus from high Ct scored samples rarely could be isolated and were confirmed infrequently by qRT-PCR, leading to the discontinuation of confirmational testing during the 2010–2011 seasons.
To ascertain the relationship between virus titer and Ct score, the NY99 strain of WNV grown in Vero cell tissue culture was diluted 10-fold from 6 to 1 log10 plaque forming units (PFU)/mL and then tested by qRT-PCR using the envelope primers/probe. Ct scores for dead birds testing positive during 2010 and 2011 were combined over time and space into frequency distributions to ascertain virus load at the time of necropsy for each species. Data from virus loads as measured by qRT-PCR Ct values were used to identify chronic infections and then plotted over time to show seasonal trends.
Results
During 2010 and 2011, 20,582 dead birds were reported by the public to the Dead Bird Hotline, of which 3499 in 163 taxa were tested for WNV infection. Of these, 1092 (31%) birds in 77 taxa tested positive, with Ct scores for WNV RNA <40. Submission of dead birds for testing and WNV RNA prevalence varied seasonally, with 84 (2% positive) submitted during December to February, 915 (26% positive) submitted during March to May, 1790 (51% positive) submitted during June to August, and 710 (20% positive) submitted during September to November. Spatially, submissions varied among counties due to differences in avian species distributions, criteria used to submit birds (e.g., corvids only), human density and interest in finding and reporting dead birds, and the intensity of WNV activity. During 2010–2011 dead birds were widely submitted from 47 of 58 California counties, but most submissions came from Sacramento (19% of total) and Los Angeles (18%) counties; the remainder comprised <6% of the total tested. Among the most frequently tested birds (n >70), the proportion WNV RNA positive was highest among corvids (American Crow n=911 tested, 52% positive; Western Scrub-Jay 480, 44%; Yellow-Billed Magpie [Pica nuttalli], 74, 66%), followed by several peridomestic passerines [House Finch 228, 25%; House Sparrow 272, 24%; American Robin (Turdus migratorius), 165, 19%; Northern Mockingbird (Mimus polyglottos), 100, 25%; European Starling (Sturnus vulgaris), 114, 9%].
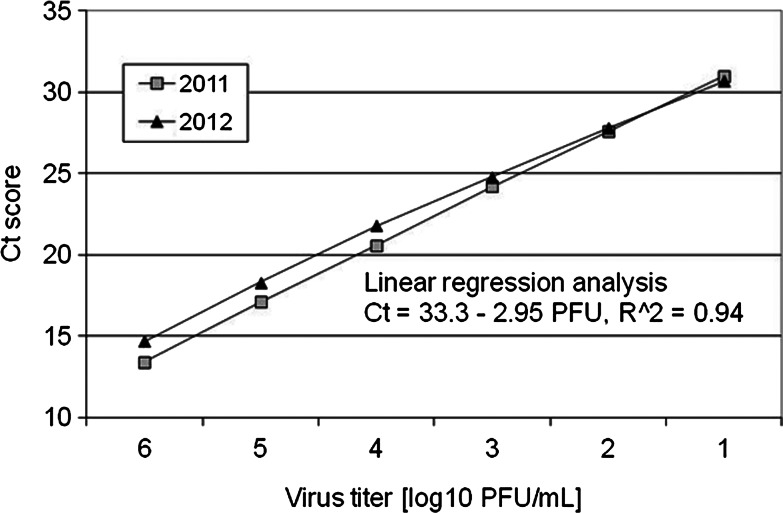
TaqMan qRT-PCR Ct scores measuring viral RNA copies were linearly related to WNV titers in Vero cell tissue culture between 6 to 1 log10 PFU/mL (Fig. 1). These assays were done as part of proficiency panel testing during 2011 and 2012 and yielded comparable results.
FIG. 1.
qRT-PCR Ct score plotted as a function of virus titer in log10 PFU/mL in duplicate assays done during 2011 and 2012. A regression analysis returned a linear equation that provided a significant fit for the data (p<0.01).
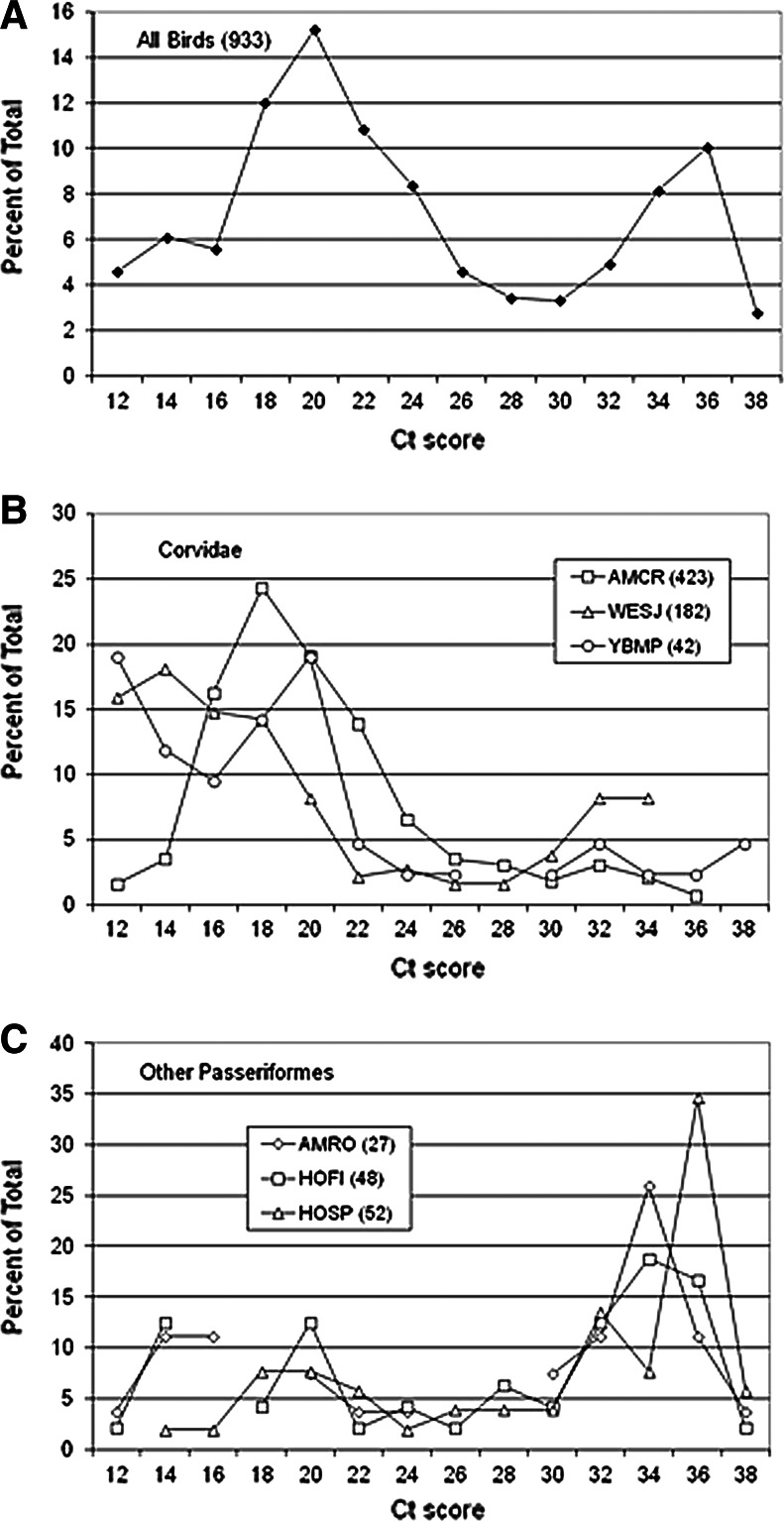
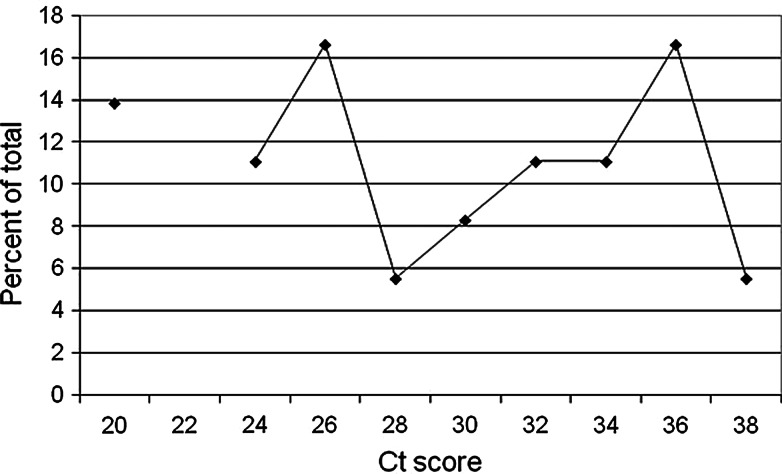
During 2010–2011, Ct scores were available for 933 bird tissue samples (85% of positives) and ranged from 8.2 to 37.0 Ct, indicating a >6 order of magnitude range from >9 to <1 log10 PFU/mL based on the curves in Figure 1. When Ct scores from all bird species from both years were grouped into a frequency distribution at 2 Ct increments, a bimodal distribution was resolved, with maxima at 20 and 36 Ct and minima at 28 to 30 Ct (Fig. 2A). These patterns varied among taxa, with Ct scores from 3 species of corvids peaking from 12 to 18 Ct (Fig. 2B) and 3 species of peridomestic passerines peaking from 34 to 36 Ct (Fig. 2C). Values for the American Crows were somewhat upwardly biased (lower RNA estimates) because these Ct scores were measured for oral swabs and not for kidney snips. In an ongoing comparison during 2012, Ct scores for 21 of 60 American Crows testing positive for both kidney snips and oral swabs averaged 19.2 Ct (standard error [SE]=3.6) for kidneys and 24.5 Ct (SE=5.6) for swabs. Kidney tissue samples from 34 of 220 tree squirrels (species not always determined) submitted for necropsy tested positive for WNV RNA and had Ct scores recorded. Ct scores ranged from 18.9 to 36.7 (Fig. 3), which agreed with laboratory infections and field samples of tree squirrels, including fox squirrels (Sciurus niger), that showed acute serum titers sufficient to infect mosquitoes (Padgett et al. 2007, Platt et al. 2008) and with long-term chronic WNV kidney infections found rodents such as hamsters (Tesh et al. 2005). These squirrel data have been used to augment the dead bird surveillance program and indicate that squirrel test reports perhaps also should be divided into “recent” and “chronic” at ∼30 Ct.
FIG. 2.
Frequency distribution plotting percent of total numbers testing positive as a function of Ct scores at 2 Ct intervals from <12 to 38 Ct. (A) All positive birds tested during 2010 and 2011. (B) Three corivd species: American Crow (AMCR, Corvus brachyrhynchos), Western Scrub-Jay (WESJ, Aphelocoma californica), and Yellow-Billed Magpie (YBMP, Pica nuttalli). (C) Three passerine species: American Robin (AMRO, Turdus migratorius), House Finch (HOFI, Carpodacus mexicanus), and House Sparrow (HOSP, Passer domesticus). Numbers in parentheses are the number of positive birds included within the frequency distribution.
FIG. 3.
Frequency distribution plotting percent of total numbers as a function of Ct score at 2 Ct intervals from 20 to 38 Ct for 34 positive tree squirrels tested during 2010 and 2011.
Discussion
The distribution of Ct scores among frequently tested bird species agreed well with results from laboratory infection studies (Komar et al. 2003, Reisen et al. 2005, 2006) and field seroprevalence surveys (Wheeler et al. 2009) that indicated corvids frequently succumb during acute infection after WNV replicates to very high serum titers, whereas the 3 other passerine species reach a crisis point during acute infection, at which time some individuals succumb whereas others clear their viremia and survive. Extremely high viremia and mortality among American crows presumably was related to the presence of the NS3 T249P substitution in the nonstructural portion of the genome (Brault et al. 2007) that is known to be present in California strains of WNV (Deardorff et al. 2006). However, repeated “sweeps” through bird populations during the 7–8 years following the WNV invasion of California seems to have selected for genetic resistance to infection in corvid populations, because Western Scrub-Jays (Reisen et al. 2009), Yellow-Billed Magpies (Crosbie et al. 2008), and American Crows (Cummings 2012) have been found to he repeatedly seropositive in nature. Similarly, initial experimental infections in House Sparrows and House Finches documented very high viremias and mortality (Komar et al. 2003, Langevin et al. 2005, Reisen et al. 2005, Fang and Reisen 2006); however, recent studies have reported lower viremias in more individuals and lower mortality rates (Wheeler 2012). Interestingly, House Finches, House Sparrows, and Western Scrub-Jays surviving both natural and experimental infection frequently were found to have low, but repeatedly detectable, amounts of WNV RNA in tissues (i.e., 28–37 Ct) at necropsy (Reisen et al. 2006, Nemeth et al. 2009a, Wheeler et al. 2012), and these persistent infections appeared to contribute to long-lasting immunity (Nemeth et al. 2009b).
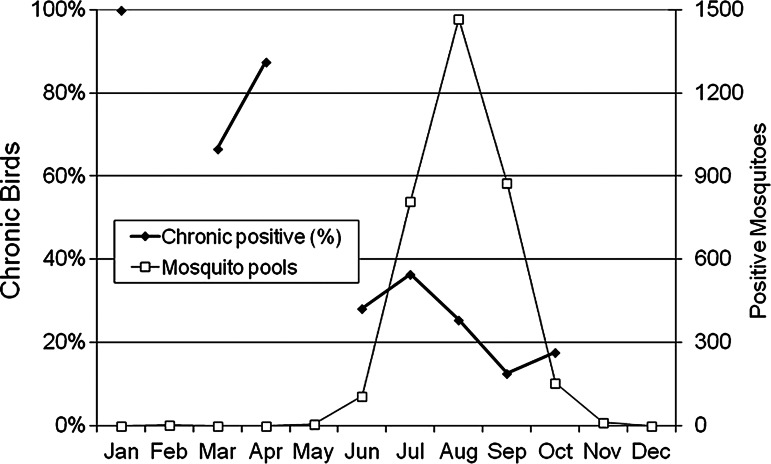
Collectively, these field and laboratory observations have led us to separate the dead bird laboratory test results into “recent” and “chronic” positive infection categories by Ct score. We have selected Ct=30 as the break point, with “recent” infections considered to be birds with a Ct value <30 that most likely were infected and died within the previous few weeks, whereas “chronic” or persistent infections had Ct values ≥30 and presumably had been infected sometime in the past. In the current data set, 242 (26%) of the 933 positive birds were considered to have “chronic” infections. The 691 birds with “recent” infections most likely succumbed during acute infection and therefore represented a measure of active enzootic transmission, whereas the 242 birds with “chronic” infections most likely were infected previously, died of other causes, and retained viral RNA in their kidneys that was detected at necropsy. We recognize that some of the higher Ct score values could be related to birds being dead longer than 24 h when collected, virus being lost under field conditions, or samples being inadequately collected, processed, or tested. However, when used in a decision support system, we recommend a conservative approach where mosquito control agencies should not respond to these reports and increase intervention until confirmation by additional dead bird results or other enzootic transmission measures such as mosquito infection or sentinel seroconversions. This was especially true during late winter and spring when all birds were after hatching year, most likely were infected during the previous season, and there was little virus transmission activity as indicated by the number of mosquito pools testing positive (Fig. 4). At this time although relatively few birds were positive, >60% were considered to be chronically infected. Furthermore, human cases were not detected in regions where only chronic positive birds and few other surveillance elements were positive (Anderson et al., 2012).
FIG. 4.
Seasonal variation in the percent of total WNV RNA positive birds tested per month that were scored as “chronic” infections with a Ct ≥30 and the total number of mosquito pools collected throughout California per month testing positive for WNV RNA combined over 2010–2011.
Frequent detection of chronically infected birds during spring may have implications for WNV overwintering. During a time course study, infectious WNV was rescued from kidney tissue taken from experimentally infected house sparrows by co-cultivation and blind passage as long as 12 weeks postinfection (Wheeler 2012), so some chronically infected birds could be harboring infectious virus. A previous time course study in Rock Doves (Columba livia) showed intermittent, but repeated, virus recovery (Semenov et al. 1973), supporting variable antibody results reported for some naturally infected birds, where low-grade viral recrudescence may intermittently stimulate the immune system to increase antibody titer (Reisen et al. 1992, Gruwell et al. 2000, Kwan et al. 2012). Further study is needed to determine if immunosuppression may allow WNV recrudescence and mosquito infection.
Acknowledgments
The authors especially thank CVEC personnel Bborie Park for data management; Helen Lu, Maureen Dannen, Sandra Garcia, and Nadira Chouicha for laboratory assays; and Jackie Parker and Amy Mikula from CAHFS for necropsies and specimen collection. This research was funded by Extended Laboratory Capacity funds from the Centers for Disease Control and Prevention and research grant AI55607 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health (NIH). W.K.R. acknowledges support from the Research and Policy in Infectious Disease Dynamics (RAPIDD) Program, Fogarty Center, NIH, and Department of Homeland Security.
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson J. Foss L. Fang Y. Woods L, et al. West Nile virus chronic positive infections in dead birds in California 2010–2011. Proc Mosq Cont Assoc Calif. 2012 (in press). [Google Scholar]
- Appler KK. Brown AN. Stewart BS. Behr MJ, et al. Persistence of West Nile virus in the central nervous system and periphery of mice. PLoS One. 2010;5:e10649. doi: 10.1371/journal.pone.0010649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brault AC. Huang CY. Langevin SA. Kinney RM, et al. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nat Genet. 2007;39:1162–1166. doi: 10.1038/ng2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney R. Padgett K. Cahoon-Young B. Woods L, et al. The California West Nile virus dead bird surveillance program—Challenges and solutions during 2004. Proc Calif Mosq Vector Control Assoc. 2005;73:105–106. [Google Scholar]
- Carney RM. Ahearn SC. McConchie A. Glasner C, et al. Early warning system for West Nile virus risk areas, California, USA. Emerg Infect Dis. 2011;17:1445–1454. doi: 10.3201/eid1708.100411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosbie SP. Koenig WD. Reisen WK. Kramer VL, et al. Early impact of West Nile virus on the Yellow-Billed Magpie (Pica nuttalli) Auk. 2008;125:542–550. [Google Scholar]
- Cummings RF. West Nile virus activity in Oragne County: Are patterns beginning to emerge? Proc Mosq Vector Control Assoc Calif. 2012 (in press). [Google Scholar]
- Deardorff E. Estrada-Franco J. Brault AC. Navarro-Lopez R, et al. Introductions of West Nile virus strains to Mexico. Emerg Infect Dis. 2006;12:314–318. doi: 10.3201/eid1202.050871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M. Komar N. Sorhage F. Nelson R, et al. Crow deaths as a sentinel surveillance system for West Nile virus in the northeastern United States, 1999. Emerg Infect Dis. 2001a;7:615–620. doi: 10.3201/eid0704.010402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eidson M. Kramer L. Stone W. Hagiwara Y, et al. Dead bird surveillance as an early warning system for West Nile Virus. Emerg Infect Dis. 2001b;7:631–635. doi: 10.3201/eid0704.010405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y. Reisen WK. Previous infection with West Nile or St. Louis encephalitis viruses provides cross protection during reinfection in house finches. Am J Trop Med Hyg. 2006;75:480–485. [PubMed] [Google Scholar]
- Gruwell JA. Fogarty CL. Bennett SG. Challet GL, et al. Role of peridomestic birds in the transmission of St. Louis encephalitis virus in southern California. J Wildl Dis. 2000;36:13–34. doi: 10.7589/0090-3558-36.1.13. [DOI] [PubMed] [Google Scholar]
- Johnson GD. Eidson M. Schmit K. Ellis A, et al. Geographic prediction of human onset of West Nile virus using dead crow clusters: An evaluation of year 2002 data in New York State. Am J Epidemiol. 2006;163:171–180. doi: 10.1093/aje/kwj023. [DOI] [PubMed] [Google Scholar]
- Komar N. West Nile virus: Epidemiology and ecology in North America. Adv Virus Res. 2003;61:185–234. doi: 10.1016/s0065-3527(03)61005-5. [DOI] [PubMed] [Google Scholar]
- Komar N. Langevin S. Hinten S. Nemeth N, et al. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg Infect Dis. 2003;9:311–322. doi: 10.3201/eid0903.020628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer LD. Wolfe TM. Green EN. Chiles RE, et al. Detection of encephalitis viruses in mosquitoes (Diptera: Culicidae) and avian tissues. J Med Entomol. 2002;39:312–323. doi: 10.1603/0022-2585-39.2.312. [DOI] [PubMed] [Google Scholar]
- Kwan JL. Kluh S. Reisen WK. Antecedent avian immunity limits tangential transmission of West Nile virus to humans. PLoS One. 2012;7:e34127. doi: 10.1371/journal.pone.0034127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanciotti RS. Kerst AJ. Nasci RS. Godsey MS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol. 2000;38:4066–4071. doi: 10.1128/jcm.38.11.4066-4071.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langevin SA. Brault AC. Panella NA. Bowen RA, et al. Variation in virulence of West Nile virus strains for house sparrows (Passer domesticus) Am J Trop Med Hyg. 2005;72:99–102. [PubMed] [Google Scholar]
- McCaughey K. Miles SQ. Woods L. Chiles RE, et al. The California West Nile virus dead bird surveillance program. Proc Mosq Vector Control Assoc Calif. 2003;71:38–43. [Google Scholar]
- Nemeth N. Young G. Ndaluka C. Bielefeldt-Ohmann H, et al. Persistent West Nile virus infection in the house sparrow (Passer domesticus) Arch Virol. 2009a;154:783–789. doi: 10.1007/s00705-009-0369-x. [DOI] [PubMed] [Google Scholar]
- Nemeth NM. Oesterle PT. Bowen RA. Humoral immunity to West Nile virus is long-lasting and protective in the house sparrow (Passer domesticus) Am J Trop Med Hyg. 2009b;80:864–869. [PMC free article] [PubMed] [Google Scholar]
- Padgett KA. Cahoon-Young B. Carney R. Woods L, et al. Field and laboratory evaluation of diagnostic assays for detecting West Nile virus in oropharyngeal swabs from California wild birds. Vector Borne Zoonotic Dis. 2006;6:183–191. doi: 10.1089/vbz.2006.6.183. [DOI] [PubMed] [Google Scholar]
- Padgett KA. Reisen WK. Kahl-Purcell N. Fang Y, et al. West Nile virus infection in tree squirrels (Rodentia: Sciuridae) in California, 2004–2005. Am J Trop Med Hyg. 2007;76:810–813. [PMC free article] [PubMed] [Google Scholar]
- Platt KB. Tucker BJ. Halbur PG. Blitvich BJ, et al. Fox squirrels (Sciurus niger) develop West Nile virus viremias sufficient for infecting select mosquito species. Vector Borne Zoonotic Dis. 2008;8:225–233. doi: 10.1089/vbz.2007.0182. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Hardy JL. Presser SB. Evaluation of domestic pigeons as sentinels for detecting arbovirus activity in southern California. Am J Trop Med Hyg. 1992;46:69–79. doi: 10.4269/ajtmh.1992.46.69. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Martinez VM. Avian host and mosquito (Diptera: Culicidae) vector competence determine the efficiency of West Nile and St. Louis encephalitis virus transmission. J Med Entomol. 2005;42:367–375. doi: 10.1093/jmedent/42.3.367. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Fang Y. Lothrop HD. Martinez VM, et al. Overwintering of West Nile virus in Southern California. J Med Entomol. 2006;43:344–355. doi: 10.1603/0022-2585(2006)043[0344:oownvi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Reisen WK. Carroll BD. Takahashi R. Fang Y, et al. Repeated West Nile virus epidemic transmission in Kern County, California, 2004–2007. J Med Entomol. 2009;46:139–157. doi: 10.1603/033.046.0118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenov BF. Chunikhin SP. Karmysheva VI. Iakovleva NI. Study of chronic forms of arbovirus infections in birds. 1. Experiments with West Nile, Sindbis, Bhandja and Sicilian mosquito fever viruses. Vestn Akad Med Nauk SSSR. 1973;28:79–83. [PubMed] [Google Scholar]
- Shi PY. Kauffman EB. Ren P. Felton A, et al. High-throughput detection of West Nile virus RNA. J Clin Microbiol. 2001;39:1264–1271. doi: 10.1128/JCM.39.4.1264-1271.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesh RB. Siirin M. Guzman H. Travassos da Rosa AP, et al. Persistent West Nile virus infection in the golden hamster: studies on its mechanism and possible implications for other flavivirus infections. J Infect Dis. 2005;192:287–295. doi: 10.1086/431153. [DOI] [PubMed] [Google Scholar]
- Wheeler SS. University of California; Davis: 2012. Persistent West Nile virus in avian hosts: A potential overwintering mechanism. PhD Dissertation; pp. 1–120. [Google Scholar]
- Wheeler SS. Barker CM. Armijos MV. Carroll BD, et al. Differential impact of West Nile virus on California birds. Condor. 2009;111:1–20. doi: 10.1525/cond.2009.080013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler SS. Langevin SA. Brault AC. Woods L, et al. Detection of persistent West Nile virus RNA in experimentally and naturally-infected avian hosts. Am J Trop Med Hyg. 2012;87:559–564. doi: 10.4269/ajtmh.2012.11-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]