Abstract
Tick-borne encephalitis virus (TBEV) is a zoonotic agent causing severe encephalitis in humans. A recent epizootiological survey indicated that endemic foci of TBEV have been maintained in the southern part of Hokkaido until recently. In this study, we sought to isolate TBEV from wild rodents in the area. One virus, designated Oshima 08-As, was isolated from an Apodemus speciosus captured in Hokuto in 2008. Oshima 08-As was classified as the Far Eastern subtype of TBEV and formed a cluster with the other strains isolated in Hokkaido from 1995 to 1996. Thirty-six nucleotide differences resulted in 12 amino acid changes between Oshima 08-As and Oshima 5–10 isolated in 1995. Oshima 08-As caused high mortality and morbidity in a mouse model compared with Oshima 5–10. Although similar transient viral multiplication in the spleen was observed in the mice infected with Oshima 08-As and Oshima 5–10, greater viral multiplication with an inflammatory response was noted in the brains of mice infected with Oshima 08-As than those infected with Oshima 5–10. These data indicate that a few naturally occurring mutations affect the pathogenicity of the Oshima strains endemic in the southern part of Hokkaido.
Key Words: Tick-borne encephalitis virus, Oshima 08-As, Oshima 5–10, Hokkaido
Introduction
Tick-borne encephalitis virus (TBEV), a member of the genus Flavivirus within the family Flaviviridae, causes tick-borne encephalitis (TBE) in humans. Although most of the cases are asymptomatic, TBEV produces a variety of clinical symptoms. TBEV is prevalent over a wide area of Eurasia, including Europe, Russia, Far Eastern Asia, and Japan (Blaskovic et al. 1967, Korenberg and Kovalevskii 1999, Lindgren and Gustafson 2001) and has a significant impact on public health in these endemic regions. On the basis of phylogenetic analysis, TBEV can be divided into three subtypes—the Far Eastern subtype, known as Russian Spring summer encephalitis (RSSE) virus; the European subtype; and the Siberian subtype (Gritsun et al. 1993, Wallner et al. 1995, Gritsun et al. 1997, Ecker et al. 1999, Heinz et al. 2000). In a recent study, other possible genotypes were also identified in Siberia (Tkachev et al. 2011). TBEV is transmitted by tick bites and is maintained in the zoonotic transmission cycle between Ixodes ticks and wild vertebrate hosts. Humans are accidental hosts. The most important vertebrate hosts for TBEV are rodents, which have the highest population densities within an endemic area (generally Apodemus, Myodes, and Microtus species).
In 1993, the first confirmed case of serologically diagnosed TBE was reported in Hokuto, Hokkaido Prefecture, Japan (Takashima et al. 1997). TBEV was isolated from dogs, ticks, and rodents in the area where the patient with TBE lived (Takeda et al. 1998, Takeda et al. 1999), and the virus was identified as the Far Eastern subtype of TBEV after nucleotide sequence analysis. Although no TBE case has been reported on Hokkaido since the first case, our epizootiological survey indicated that endemic foci of TBEV were maintained in Hokuto until recently (Yoshii et al. 2011). Therefore, isolating and characterizing TBEV endemic in the area is necessary to evaluate the epidemiological risk of TBE.
In this study, we sought to isolate TBEV from wild rodents captured in southern Hokkaido, and investigated the genetic and biological characteristics of the isolated virus.
Materials and Methods
Rodent survey
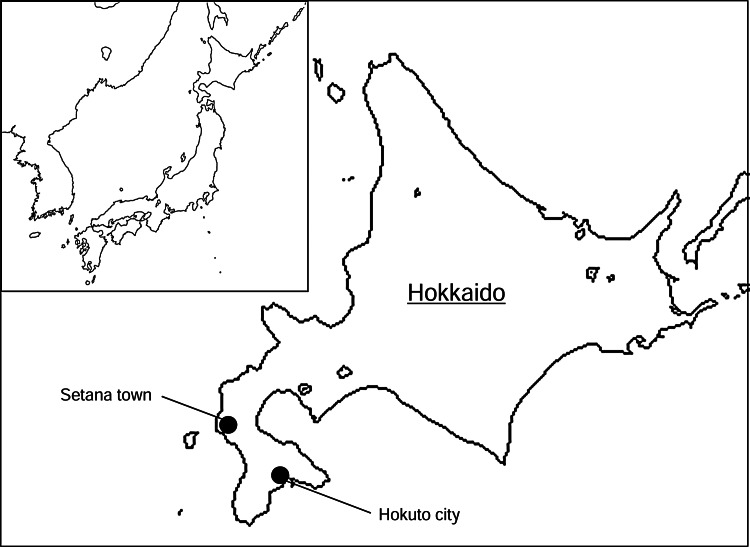
Fifty-nine wild rodents were captured using Sherman box traps in grass and shrub areas within forests bordering on pastures in Hokuto and Setana in 2008 (Fig. 1, Table 1) (Yoshii et al. 2011). The spleens were collected and stored at −80°C until virus isolation.
FIG. 1.
Geographical location of the study areas.
Table 1.
Isolation of TBEV from Wild Rodents Captured in Hokkaido in 2008
| |
Place of survey |
|
|
|---|---|---|---|
| Rodent species | Hokuto | Setana | Total |
| Apodemus speciosus | 13 (3)a | — | 13 (3) |
| Apodemus argenteus | 10 (3) | 7 (2) | 17 (5) |
| Myodes rufocanus | 11 (3) | 18 (4) | 29 (7) |
Number of rodents (number of spleen pools used for isolation).
Virus isolation and identification
The spleens of the wild rodents were homogenized using a cold mortar and pestle and suspended in phosphate-buffered saline (PBS) containing 10% fetal bovine serum (FBS). Homogenates from 3–5 rodents of the same rodent species were pooled and used as the inocula. Each of 10–13 1-day-old suckling mice was inoculated intracerebrally with 20 μL of the inoculum. The mice were observed daily for 14 days, and a moribund mouse was killed and stored at −80°C.
The virus isolate was identified by immunofluorescence assay (IFA) using anti-tick-borne flavivirus antibodies and RT-PCR. Briefly, a 10% suspension of suckling mouse brain was prepared and inoculated onto a monolayer of baby hamster kidney (BHK) cells. After 3 days of incubation, the cells were fixed with 3% paraformaldehyde and permeabilized with 0.2% Triton X-100. After blocking with 2% bovine serum albumin (BSA), the cells were incubated with polyclonal hyperimmune murine ascites fluid from Langat virus-infected mice that is cross-reactive to TBEV, followed by Alexa555-conjugated anti-mouse immunoglobulin G (IgG) antibodies. Viral RNA was extracted from the BHK cells using Isogen (Nippon Gene, Tokyo, Japan) and reverse-transcribed with random primers using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA). The TBEV-specific sequence was amplified using Platinum Taq polymerase (Invitrogen) using the forward primer, 5′-CGGAGACCTGTCCTTGTTAT-3′ and reverse primer 5′-GTATGCATAATTGTCATACC-3′.
The nucleic acid sequences of the viral genomes were determined by direct sequencing. The cycle sequencing reactions were performed using a BigDye™ Terminator Cycle Sequencing Kit (Life Technologies, Carlsbad, CA), and the sequences were determined with a 3130 Genetic Analyzer (Life Technologies).
Phylogenetic analysis
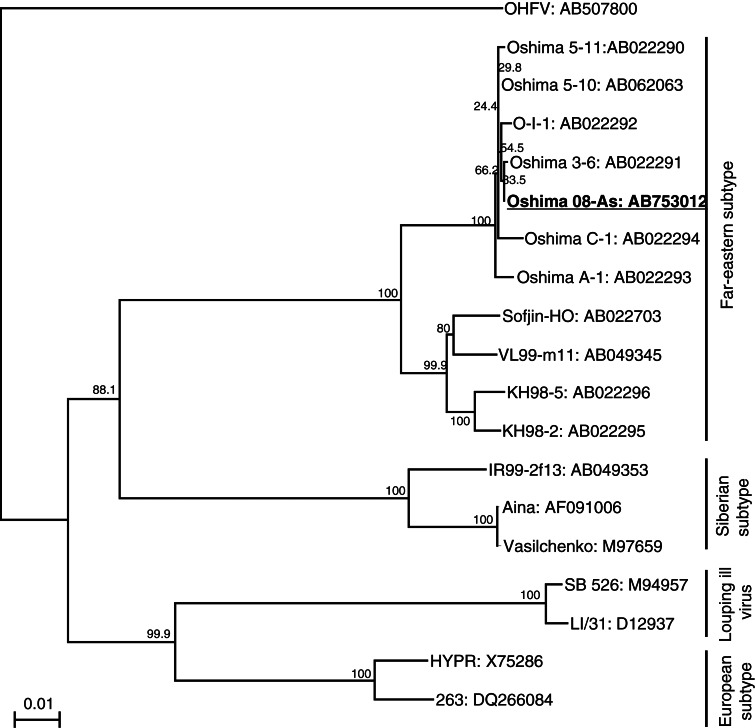
A phylogenetic analysis was performed using the complete E gene sequences of the TBEV strains, including strains isolated in Hokkaido (the accession numbers are shown in Fig. 3, below). Omsk hemorrhagic fever virus (OHFV) was used as the outgroup. ClustalX version 2.1 was used to generate the multiple alignments (Thompson et al. 1997), MEGA 4 (www.megasoftware.net/mega.html) was used to generate phylogenetic trees by the neighbor-joining method. The reliability of the dendrogram was evaluated using 1000 bootstrap replicates.
FIG. 3.
Phylogenetic tree of the tick-borne encephalitis virus (TBEV) strains The tree was constructed using 1488 nucleotides of the viral E gene and Omsk hemorrhagic fever virus (OHFV) as the outgroup. Horizontal distances are proportional to the minimum number of nucleotide differences. The numbers beside the branches are bootstrap values. Accession numbers are shown after the virus strains.
Growth curve in cell culture
Subconfluent BHK cells were grown in 24-well plates. The cells were inoculated with virus at a multiplicity of infection (MOI) of 0.01 plaque forming unit (pfu). Cells were incubated at 37°C in 5% CO2. The supernatant was harvested 12, 24, 36, and 48 h postinoculation and stored in aliquots at −80°C before titration.
For titration, cell monolayers prepared in 12-well plates were incubated with serial dilutions of virus for 1 h, and then overlaid with minimal essential medium (MEM) containing 2% FBS and 1.5% carboxymethyl cellulose (CMC; Sigma, St. Louis, MO) and incubated for 5 days. After incubation, the cells were fixed and stained with 0.25% Crystal Violet in 10% buffered formalin. Plaques were counted and expressed as pfu/mL.
Animal model
Viruses were inoculated subcutaneously into 5-week-old female C57BL/6J mice (Jackson ImmunoResearch, West Grove, PA). Morbidity was defined as a >10% weight loss. Surviving mice were monitored for 28 days postinfection to determine survival curves and mortality rates. To analyze the viral distribution in tissues, serum, brains, and spleens were collected from mice 3, 6, 9, and 12 days postinfection. The organs were weighted individually and homogenized, and prepared as 10% suspensions (wt/vol) in PBS that contained 10% FBS. The suspensions were clarified by centrifugation (4000 rpm for 5 min, 4°C), and the supernatants were titrated by plaque assay on BHK cells.
Histopathological examination
Mice infected with 1000 pfu of TBEV were killed 10–11 days postinfection, and fixed brain tissues were embedded in paraffin, sectioned, and stained with Hematoxylin & Eosin, as described previously (Nagata et al. 2007). Immunohistochemical detection of TBEV antigens was performed using rabbit polyclonal antibodies against E protein (Yoshii et al. 2004).
Results
Isolation and identification of TBEV in Hokuto
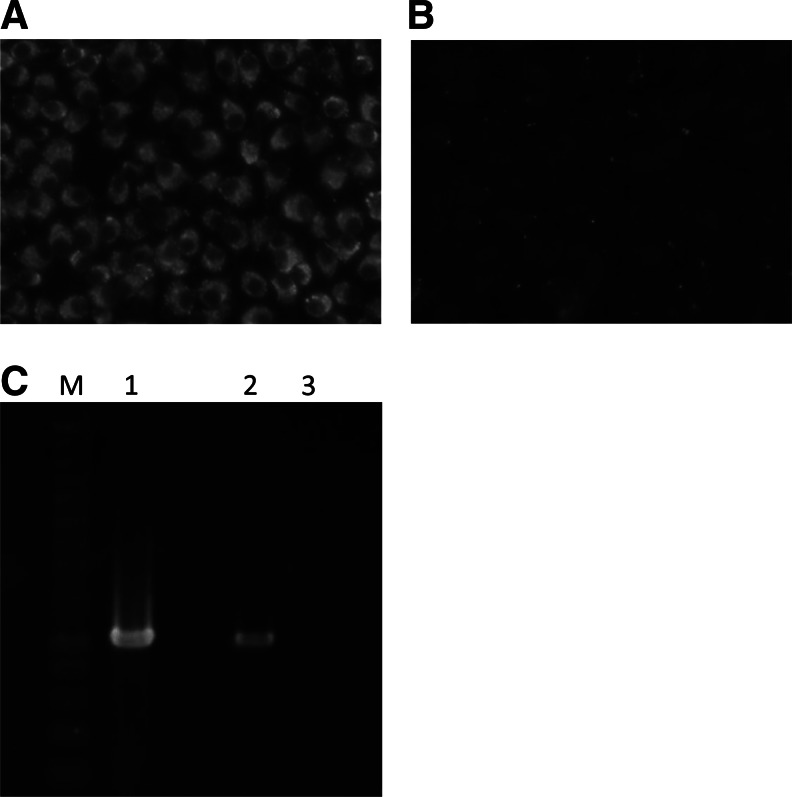
One suckling mouse became sick after inoculation with 1 group of spleen homogenates of an Apodemus speciosus captured in Hokuto. The brain homogenate of this mouse was inoculated into BHK cells to confirm the isolation of TBEV. Viral-specific antigens and a band were detected with an indirect IFA and RT-PCR, respectively (Fig. 2). Consequently, the isolate was identified as TBEV and designated Oshima 08-As.
FIG. 2.
Detection of tick-borne encephalitis virus (TBEV)-specific antigen and RNA. Baby hamster kidney (BHK) cells were inoculated with the brain homogenate of a sick mouse inoculated with the spleen homogenate of wild rodents. Inoculated (A) and mock-treated (B) cells were stained using anti-tick-borne flavivirus antibodies. (C) TBEV-specific products were amplified by RT-PCR from cells infected with the brain homogenate of the sick mouse (lane 1). TBEV-infected (lane 2) and mock-treated (lane 3) cells were used as positive and negative controls, respectively.
Genetic analysis of the isolated TBEV
The nucleotide sequence of the complete genome of Oshima 08-As was determined (accession no. AB753012). A phylogenetic tree of the TBEV strains is shown in Fig. 3. Oshima 08-As and the other strains isolated in Hokkaido from 1995 to 1996 (Takashima et al. 1997, Hayasaka et al. 2001) formed a cluster with 100% bootstrap support, and were classified as the Far Eastern subtype of TBEV.
The sequence of Oshima 08-As was compared with that of the Oshima 5–10 strain isolated from a dog in 1995 in Hokuto (Table 2) (Takashima et al. 1997). Thirty-six nucleotide differences resulted in 12 amino acid changes. All of the amino acid changes were located in the region encoding the nonstructural (NS) proteins.
Table 2.
Nucleotide Differences Between Oshima 08-As and Oshima 5–10
| |
|
Oshima-08-As |
Oshima-5–10 |
||
|---|---|---|---|---|---|
| Position | Gene | Nucleotide | Amino acida | Nucleotide | Amino acid |
| 73 | 5′-UTR | c | — | t | — |
| 431 | Core | c | Asp | t | Asp |
| 1730 | Env | t | Asp | c | Asp |
| 1847 | t | Val | a | Val | |
| 2631 | NS1 | g | Val | t | Leu |
| 3855 | NS2A | t | Leu | c | Leu |
| 4151 | c | Gly | t | Gly | |
| 4188 | a | Ile | g | Val | |
| 4731 | NS3 | c | Leu | t | Phe |
| 4968 | a | Arg | g | Gly | |
| 4982 | a | Ala | g | Ala | |
| 5213 | a | Lys | g | Lys | |
| 5323 | a | Asn | g | Ser | |
| 5753 | a | Gly | g | Gly | |
| 5768 | t | Cys | c | Cys | |
| 5988 | a | Ile | g | Val | |
| 6051 | a | Ser | g | Gly | |
| 6524 | NS4A | t | Val | c | Val |
| 6583 | g | Arg | a | Lys | |
| 7236 | NS4B | t | Leu | c | Leu |
| 7385 | g | Gly | a | Gly | |
| 7587, 7589 | g, t | Gly | a, c | Ser | |
| 7616 | c | Phe | t | Phe | |
| 7898 | NS5 | t | Val | g | Val |
| 7966 | a | Lys | g | Arg | |
| 8462 | t | Val | c | Val | |
| 8615 | g | Ser | a | Ser | |
| 9233 | g | Leu | a | Leu | |
| 10109 | c | Asn | t | Asn | |
| 10175 | c | Val | t | Val | |
| 10249 | a | Lys | g | Arg | |
| 10300 | c | Thr | a | Lys | |
| 10476 | 3′-UTR | a | — | g | — |
| 10823 | a | — | g | — | |
| 10843 | g | — | a | — | |
Different amino acids between Oshima 08-As and Oshima 5–10 are shown in bold type.
UTR, untranslated region.
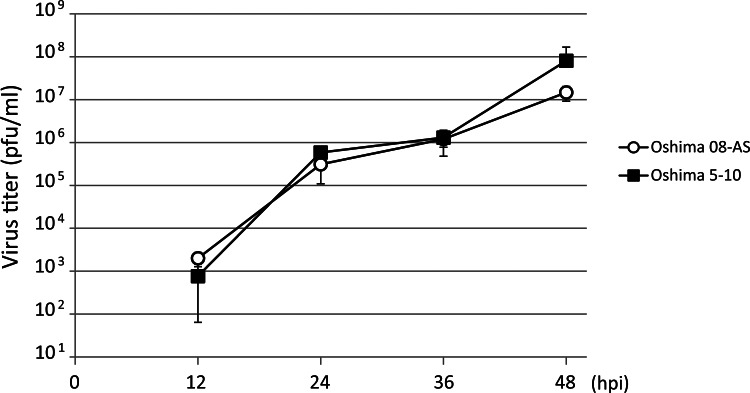
Growth properties of Oshima 08-As
The growth properties of Oshima 08-As were compared with those of Oshima 5–10 by monitoring the virus release after infection. BHK cells were infected with TBEV at a MOI of 0.01. Virus was harvested at 12-h intervals, and the yield was quantified using plaque assay (Fig. 4). The growth curves indicate similar growth properties for Oshima 08-As and Oshima 5–10.
FIG. 4.
Comparison of the growth curves of Oshima 08-As and Oshima 5–10. A monolayer of baby hamster kidney (BHK) cells was infected with each virus at a multiplicity of infection (MOI) of 0.01. At each time point, the medium was harvested and virus titers were determined using a plaque assay in BHK cells.
Pathogenicity of Oshima 08-As
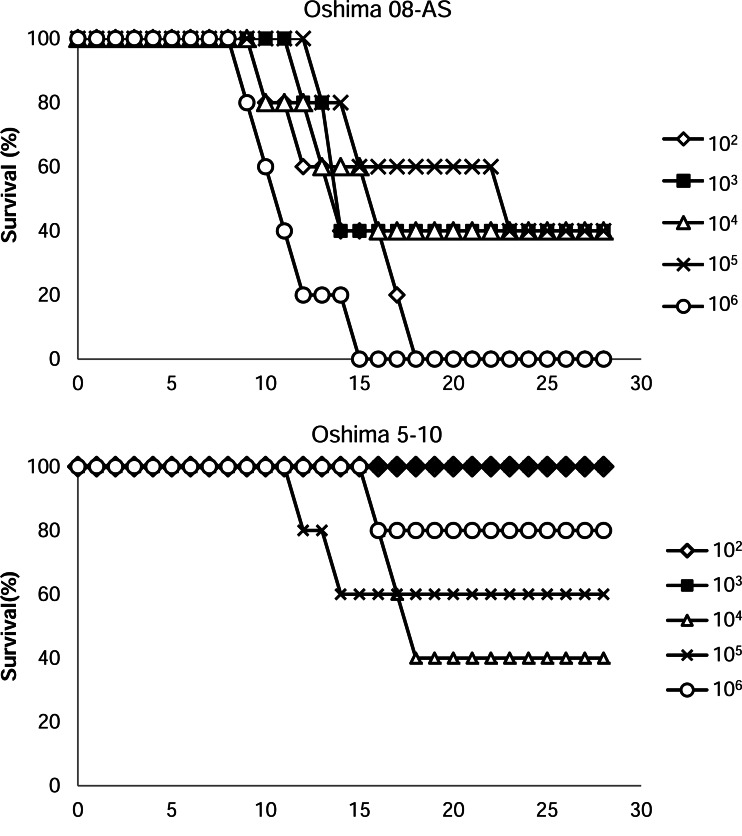
The pathogenicity of Oshima 08-As was examined in a mouse model. Mice were infected subcutaneously with serially diluted (102–106 pfu/mouse) Oshima 08-As or Oshima 5–10 and were monitored for 28 days (Fig. 5, Table 3). All mice infected with each dose of Oshima 08-As showed general signs of illness, such as a hunched posture, ruffled fur, and general malaise. Dose-independent morbidity (60–100%) was observed, and many dying mice infected with Oshima 08-As showed neurological signs, such as paralysis and loss of balance. Several mice died after a sharp decrease in body weight before displaying neurological signs. In contrast, lower mortality and morbidity were observed in the mice infected with Oshima 5–10. Several mice recovered after a slight weight loss.
FIG. 5.
The survival of mice inoculated with Oshima 08-As and Oshima 5–10. Mice were inoculated subcutaneously with 102 to 106 plaque-forming units (pfu) of each virus and were monitored for 28 days.
Table 3.
Mortality and Morbidity Following Subcutaneous Infection with Oshima 08-As and Oshima 5–10 in B6 Mice
| |
Oshima 08-AS |
Oshima 5–10 |
||||||
|---|---|---|---|---|---|---|---|---|
| Dose (pfu) | Morbiditya(%) | Mortality (%) | Day of onset (days) | Survival time (days) | Morbidity (%) | Mortality (%) | Day of onset (days) | Survival time (days) |
| 102 | 100 (5/5)b | 100 (5/5)c | 10.0±1.2 | 13.2±3.4 | 0 (0/5) | 0 (0/5) | — | — |
| 103 | 100 (5/5) | 60 (3/5) | 10.2±1.6 | 12.3±1.2 | 40 (2/5) | 0 (0/5) | 14.5±0.7 | — |
| 104 | 100 (5/5) | 60 (3/5) | 9.0±0.7 | 12.0±3.0 | 100 (5/5) | 60 (3/5) | 11.8±1.3 | 16.0±1.0 |
| 105 | 100 (5/5) | 60 (3/5) | 8.8±0.8 | 16.0±5.3 | 60 (3/5) | 40 (2/5) | 10.8±2.9 | 12.0±1.4 |
| 106 | 100 (5/5) | 100 (5/5) | 7.6±0.6 | 10.4±2.3 | 80 (4/5) | 20 (1/5) | 11.5±1.7 | 15 |
Morbidity of mice was estimated by >10% of weight loss.
Number of sick mice/number of infected mice.
Number of dead mice/number of infected mice.
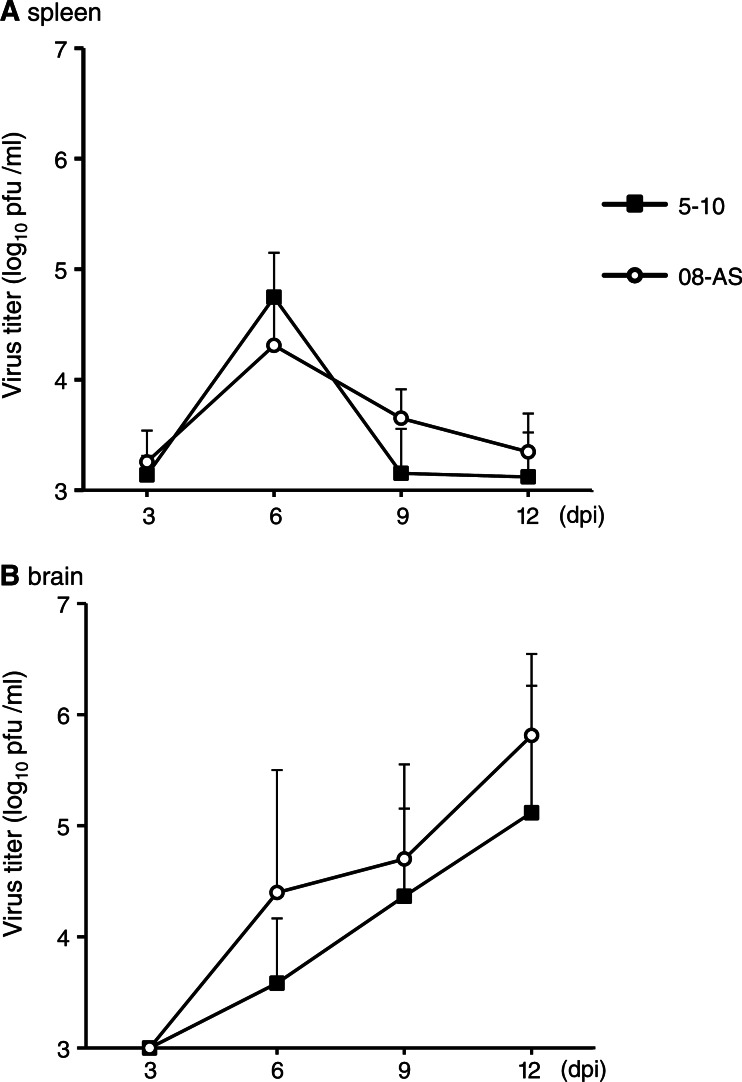
To examine the correlation between disease development and viral replication in organs, the viral loads in the blood, spleen, and brain were compared in mice inoculated with 1000 pfu of Oshima 08-As and Oshima 5–10 (Fig. 6). Viremia was barely observed in both of the mice infected with each virus. Virus was detected in the serum of 1 mouse each infected with Oshima 08-As (4.8×104 pfu/mL) or Oshima 5–10 (2.9×104 pfu/mL) at 3 days postinfection (dpi). Similar transient multiplication in the spleen was observed in the mice infected with Oshima 08-As and Oshima 5–10 (Fig. 6A). In the brain, the virus was detected from 6 dpi and multiplied until 12 dpi (Fig. 6B). Although statistically significant difference was not observed, the virus titer was higher in the mice infected with Oshima 08-As than in those infected with Oshima 5–10 at each time postinfection.
FIG. 6.
Virus replication in organs. Mice were infected with 1000 pfu of Oshima 08-As or Oshima 5–10. Virus titers in spleen (A) and brain (B) at the indicated days after infection were determined using plaque assays. Error bars represent the standard deviation (SD) (n=3).
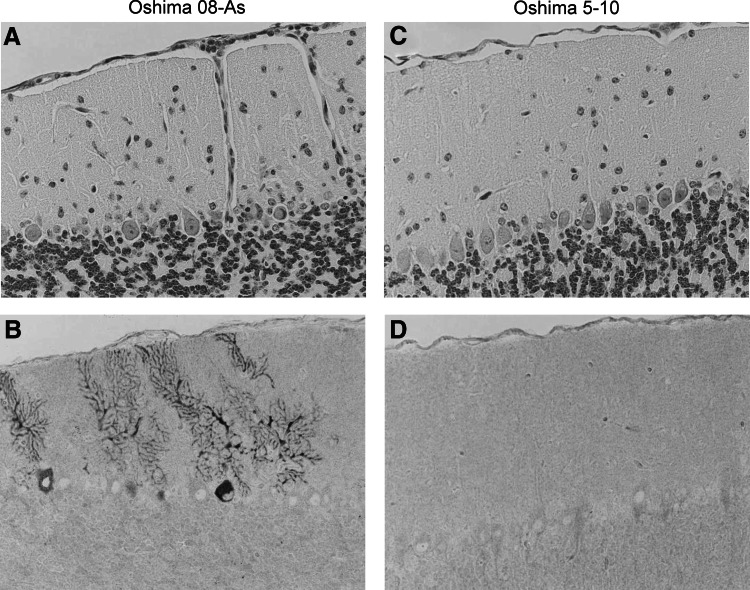
The histopathological features of the infected mice were examined following infection with 1000 pfu of Oshima 08-As or Oshima 5–10 at 10 dpi. Meninges inflammation and perivascular cuffing were observed in the brains of all mice (4/4) infected with Oshima 08-As (Fig. 7A). Viral antigens were detected in neuronal cells (Fig. 7B), and the virus titer in the brain was 3.8×106 pfu/mL. In contrast, minimal inflammatory reaction and few viral antigen-positive cells were observed in the brains infected with Oshima 5–10 (Fig. 7C, D). The viral load in the brain was 4.2×105 pfu/mL. These results indicated that Oshima 08-As is more virulent than Oshima 5–10.
FIG. 7.
Histopathological features in the brain at 10 days post-infection. B6 mice were infected with 103 pfu of the Oshima 08-As (A and B) or Oshima 5–10 (C and D) strain. TBEV antigens were detected using E protein-specific antibodies (lower columns: B and D).
Discussion
It has been shown that rodents can be used a useful indicator of the circulation of TBEV in an area (Achazi et al. 2011, Knap et al. 2012). In this study, TBEV was isolated from A. speciosus captured in Hokuto in 2008. One virus was isolated from 34 wild rodents (2.9%). This ratio of isolation was similar to that in a survey conducted in the same area in 1995–1996 (1.2%: 2/169) (Takeda et al. 1999). The proportion of rodents seropositive for TBEV in Hokuto was 12.4% in 1995–1996 and 10.4% in 2008 (Takeda et al. 1999, Yoshii et al. 2011). These data demonstrated that TBEV has been endemic in wild rodents and ticks in Hokuto.
The complete sequence analysis revealed 36 nucleotide differences resulting in 12 amino acid changes between the Oshima 08-As and Oshima 5–10 strains. These differences were consistent with the average synonymous substitution rate estimated previously in the Far Eastern subtype of TBEV (2.9×10−4 per site per year) (Hayasaka et al. 1999). All of the amino acid changes were located in the NS proteins. The genetic stability of the viral structural proteins may be important for transmission in wild rodents and ticks.
In the mouse model, Oshima 08-As was more virulent than Oshima 5–10 isolated in 1995. Although the multiplication in the blood and peripheral organs was similar for both strains, the multiplication in the brain was slightly higher in Oshima 08-As than in Oshima 5–10. These data imply that Oshima 08-As was more neuroinvasive than Oshima 5–10 or that Oshima 08-As replicates more efficiently in neural cells. Early inflammatory responses were observed with the viral multiplication in brain infected with Oshima 08-As, compared with the mice infected with Oshima 5–10. In our previous study, systemic inflammatory and stress responses were involved in fatal infection following the subcutaneous infection of mice with the Oshima 5–10 strain (Hayasaka et al. 2009). The induction of the systemic inflammatory and stress responses by viral multiplication might be involved in the greater virulence of Oshima 08-As. It has been shown that naturally occurring mutations affect the pathogenic properties of circulating TBEV (Gritsun et al. 2003, Ruzek et al. 2008, Ruzek et al. 2009). Only 12 amino acid differences exist between Oshima 08-As and Oshima 5–10, but none of them are previously reported amino acid residues that affect the virulence of TBEV. The identification of critical residues that affect the virulence might contribute to the understanding regarding the pathogenicity of the Oshima strains.
Our results indicated that naturally occurring virulent TBEV strains co-circulate among attenuated strains in endemic areas of Japan, as shown in the study by Ruzek et al. (2008). Although no human TBE cases have been reported in Japan since 1993, our study clearly demonstrated that TBEV is silently circulating in Hokkaido. It is possible that inhabitants in the endemic area may have been infected by attenuated TBEV strains and acquired immunity against TBEV.
In summary, we isolated TBEV strain Oshima 08-As from a wild rodent captured in Hokuto in 2008 and showed that a few naturally occurring mutations affect the virulence of the endemic Oshima strains. These results are important for monitoring TBEV to evaluate the epidemiological risk in the endemic area of Hokkaido.
Acknowledgment
This work was supported by Grants-in-Aid for Scientific Research (22780268 and 21405035) and the Global COE Program from the Ministry of Education, Culture, Sports, Sciences and Technology of Japan, and Health Sciences Grants for Research on Emerging and Re-emerging Infectious Disease from the Ministry of Health, Labour and Welfare of Japan.
Author Disclosure Statement
No competing financial interests exist for this paper.
References
- Achazi K. Ruzek D. Donoso-Mantke O. Schlegel M, et al. Rodents as sentinels for the prevalence of tick-borne encephalitis virus. Vector Borne Zoonotic Dis. 2011;11:641–647. doi: 10.1089/vbz.2010.0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaskovic D. Pucekova G. Kubinyi L. Stupalova S. Oravcova V. An epidemiological study of tick-borne encephalitis in the Tribec region: 1953–63. Bull World Health Organ. 1967;36(Suppl 1):89–94. [PMC free article] [PubMed] [Google Scholar]
- Ecker M. Allison SL. Meixner T. Heinz FX. Sequence analysis and genetic classification of tick-borne encephalitis viruses from Europe and Asia. J Gen Virol. 1999;80:179–185. doi: 10.1099/0022-1317-80-1-179. [DOI] [PubMed] [Google Scholar]
- Gritsun TS. Frolova TV. Pogodina VV. Lashkevich VA, et al. Nucleotide and deduced amino acid sequence of the envelope gene of the Vasilchenko strain of TBE virus; comparison with other flaviviruses. Virus Res. 1993;27:201–209. doi: 10.1016/0168-1702(93)90082-x. [DOI] [PubMed] [Google Scholar]
- Gritsun TS. Venugopal K. Zanotto P.M. Mikhailov , et al. Complete sequence of two tick-borne flaviviruses isolated from Siberia and the UK: Analysis and significance of the 5′ and 3′-UTRs. Virus Res. 1997;49:27–39. doi: 10.1016/s0168-1702(97)01451-2. [DOI] [PubMed] [Google Scholar]
- Gritsun TS. Frolova TV. Zhankov AI. Armesto M, et al. Characterization of a siberian virus isolated from a patient with progressive chronic tick-borne encephalitis. J Virol. 2003;77:25–36. doi: 10.1128/JVI.77.1.25-36.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayasaka D. Suzuki Y. Kariwa H. Ivanov L, et al. Phylogenetic and virulence analysis of tick-borne encephalitis viruses from Japan and far-Eastern Russia. J Gen Virol. 1999;80(Pt 12):3127–3135. doi: 10.1099/0022-1317-80-12-3127. [DOI] [PubMed] [Google Scholar]
- Hayasaka D. Ivanov L. Leonova GN. Goto A, et al. Distribution and characterization of tick-borne encephalitis viruses from Siberia and far-eastern Asia. J Gen Virol. 2001;82:1319–1328. doi: 10.1099/0022-1317-82-6-1319. [DOI] [PubMed] [Google Scholar]
- Hayasaka D. Nagata N. Fujii Y. Hasegawa H, et al. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology. 2009;390:139–150. doi: 10.1016/j.virol.2009.04.026. [DOI] [PubMed] [Google Scholar]
- Heinz FX. Collett MS. Purcell RH. Gould EA, et al. Family Flaviviridae. In: Frauquet CM, editor; Bishop DHL, editor; Carstens E, editor. Virus Taxonomy. 7th International Committee for the Taxonomy of Viruses. San Diego: Academic; 2000. pp. 859–878. [Google Scholar]
- Knap N. Korva M. Dolinsek V. Sekirnik M, et al. Patterns of tick-borne encephalitis virus infection in rodents in Slovenia. Vector Borne Zoonotic Dis. 2012;12:236–242. doi: 10.1089/vbz.2011.0728. [DOI] [PubMed] [Google Scholar]
- Korenberg EI. Kovalevskii YV. Main features of tick-borne encephalitis eco-epidemiology in Russia. Zentralbl Bakteriol. 1999;289:525–539. doi: 10.1016/s0934-8840(99)80006-1. [DOI] [PubMed] [Google Scholar]
- Lindgren E. Gustafson R. Tick-borne encephalitis in Sweden and climate change. Lancet. 2001;358:16–18. doi: 10.1016/S0140-6736(00)05250-8. [DOI] [PubMed] [Google Scholar]
- Nagata N. Iwata N. Hasegawa H. Fukushi S, et al. Participation of both host and virus factors in induction of severe acute respiratory syndrome (SARS) in F344 rats infected with SARS coronavirus. J Virol. 2007;81:1848–1857. doi: 10.1128/JVI.01967-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzek D. Gritsun TS. Forrester NL. Gould EA, et al. Mutations in the NS2B and NS3 genes affect mouse neuroinvasiveness of a Western European field strain of tick-borne encephalitis virus. Virology. 2008;374:249–255. doi: 10.1016/j.virol.2008.01.010. [DOI] [PubMed] [Google Scholar]
- Ruzek D. Salat J. Palus M. Gritsun TS, et al. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology. 2009;384:1–6. doi: 10.1016/j.virol.2008.11.023. [DOI] [PubMed] [Google Scholar]
- Takashima I. Morita K. Chiba M. Hayasaka D, et al. A case of tick-borne encephalitis in Japan and isolation of the virus. J Clin Microbiol. 1997;35:1943–1947. doi: 10.1128/jcm.35.8.1943-1947.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T. Ito T. Chiba M. Takahashi K, et al. Isolation of tick-borne encephalitis virus from Ixodes ovatus (Acari: Ixodidae) in Japan. J Med Entomol. 1998;35:227–231. doi: 10.1093/jmedent/35.3.227. [DOI] [PubMed] [Google Scholar]
- Takeda T. Ito T. Osada M. Takahashi K, et al. Isolation of tick-borne encephalitis virus from wild rodents and a seroepizootiologic survey in Hokkaido, Japan. Am J Trop Med Hyg. 1999;60:287–291. doi: 10.4269/ajtmh.1999.60.287. [DOI] [PubMed] [Google Scholar]
- Thompson JD. Gibson TJ. Plewniak F. Jeanmougin F, et al. The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:4876–4882. doi: 10.1093/nar/25.24.4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachev SE. Demina TV. Dzhioev YP. Kozlova IV, et al. Genetic studies of tick-borne encephalitis virus strains from Western and Eastern Siberia. In: Ruzek D, editor. Flavivirus Encephalitis. InTech; 2011. pp. 235–254. [Google Scholar]
- Wallner G. Mandl CW. Kunz C. Heinz FX. The flavivirus 3′-noncoding region: Extensive size heterogeneity independent of evolutionary relationships among strains of tick-borne encephalitis virus. Virology. 1995;213:169–178. doi: 10.1006/viro.1995.1557. [DOI] [PubMed] [Google Scholar]
- Yoshii K. Konno A. Goto A. Nio J, et al. Single point mutation in tick-borne encephalitis virus prM protein induces a reduction of virus particle secretion. J Gen Virol. 2004;85:3049–3058. doi: 10.1099/vir.0.80169-0. [DOI] [PubMed] [Google Scholar]
- Yoshii K. Mottate K. Omori-Urabe Y. Chiba Y, et al. Epizootiological study of tick-borne encephalitis virus infection in Japan. J Vet Med Sci. 2011;73:409–412. doi: 10.1292/jvms.10-0350. [DOI] [PubMed] [Google Scholar]