Abstract
Purpose
Bortezomib, a first generation proteasome inhibitor, induces an endoplasmic reticulum (ER) stress response which ultimately leads to dysregulation of intracellular Ca2+ and apoptotic cell death. This study investigated the role of the Ca2+-dependent enzyme, calpain, in bortezomib cytotoxicity. A novel therapeutic combination was evaluated in which HIV protease inhibitors were used to block calpain activity and enhance bortezomib cytotoxicity in myeloma cells in vitro and in vivo.
Methods
Bortezomib-mediated cell death was examined using assays for apoptosis (Annexin V staining), total cell death (trypan blue exclusion) and growth inhibition (MTT). The effects of calpain on Bortezomib-induced cytotoxicity were investigated using siRNA knockdown or pharmaceutical inhibitors. Enzyme activity assays and immunofluorescence analysis were used to identify mechanistic effects.
Results
Inhibition of the Ca2+ dependent cysteine protease calpain, either by pharmacologic or genetic means, enhances or accelerates bortezomib-induced myeloma cell death. The increase in cell death is not associated with an increase in caspase activity, nor is there evidence of greater inhibition of proteasome activity, suggesting an alternate, calpain-regulated mechanism of brtezomib-induced cell death. Bortezomib initiates an autophagic response in myeloma cells associated with cell survival. Inhibition of calpain subverts the cytoprotective function of autophagy leading to increased bortezomib-mediated cell death. Combination therapy with bortezomib and the calpain-blocking HIV protease inhibitor, nelfinavir, reversed bortezomib resistance and induced near-complete tumor regressions in a SCID mouse xenograft model of myeloma.
Keywords: autophagy, bortezomib, myeloma, calpain, nelfinavir
Introduction
The advent of proteasome inhibitors, most notably, bortezomib (PS-341/Velcade®) has provided new therapeutic options that have extended the overall survival of patients with multiple myeloma. Bortezomib (BZ) is a first-in-class dipeptide boronic acid proteasome inhibitor that has shown efficacy in the treatment of relapsed and newly diagnosed myeloma, both alone and in combination with other chemotherapeutic agents [1,2,3]. Despite these advances, the vast majority of patients with multiple myeloma will ultimately relapse with disease that is refractory to further treatment. Thus, identifying mechanisms of drug resistance and modulators of response will aid the design of new therapeutic strategies.
Our previous work demonstrated that bortezomib induces an ER stress response ultimately leading to mitochondrially-mediated Ca2+-dependent apoptotic death [4]. BZ treatment induced a rapid and transient elevation of Ca2+ and, conversely, inhibition of the mitochondrial Ca2+ uniporter conferred resistance to BZ. In the current study we define one Ca2+-dependent mechanism of BZ response and resistance in human multiple myeloma cells.
Resistance to BZ-mediated cell death has been associated with the stress response known as autophagy [5,6,7]. Autophagy is characterized by intracellular formation of a double membrane vesicle, the autophagosome. Effective autophagy requires fusion of the autophagosome with lysosomes, which then digest and recycle cellular components to maintain critical processes until homeostasis can be restored. The key determinants of the relationship between autophagy and apoptosis, and whether the outcome is cell death or cell survival are not well understood [8,9,10].
Calpains comprise a family 14 Ca2+ dependent cysteine proteases whose activation has been suggested to contribute to ER stress associated apoptosis [11,12]. Calpains have been implicated in the cleavage of a variety of substrates including cytoskeletal proteins, signal transduction molecules, and membrane receptors (reviewed in [13]. The typical calpains, μ- and m-calpain, are heterodimers with a small common regulatory unit, and a conserved catalytic subunit that requires Ca2+-dependent conformational change for proteolytic activity.
Because calpains have previously been implicated in cell death, and based on our work demonstrating that BZ mediated cell death is Ca2+ dependent, we hypothesized that inhibition of calpain activity would abrogate cell death induced by BZ. In contrast, we found that inhibition of calpain enhances BZ cytotoxicity, and our data suggest that this effect is related to inhibition of autophagic progression. We further demonstrate that clinically approved HIV protease inhibitors, which are known to inhibit calpains, significantly increase the cytotoxicity of bortezomib both in vitro and in vivo. These data suggest a new therapeutic strategy for treatment of myeloma patients that fail to respond to BZ or relapse following initial therapy in multiple myeloma and other B cell malignancies.
Materials and Methods
Cells and treatments
The RPMI 8226, MM.1S and NCI-H929 multiple myeloma cell lines were maintained in RPMI 1640 medium (Invitrogen, Carlsbad, CA) with 10% fetal bovine serum (Omega Scientific, Tarzana, CA), and 1 mM L-glutamine (Invitrogen, Carlsbad, CA). Beta-mercaptoethanol is also added to the media of the NCI-H929 cell line at a final concentration of 0.05 mM. Cell line identity was validated using short tandem repeat (STR) analysis [14] by the Human Origins Genotyping Laboratory (HOGL) at the University of Arizona. Bortezomib was generously provided by Millennium Pharmaceuticals (Cambridge MA). Calpain deficient cells were created using RNAi technology [15]. Retroviral or GFP-Lentiviral expression constructs expressing siRNA to the common catalytic subunit of calpain (CAPNS1) or non-silencing control siRNA sequences were obtained from Open Biosystems (Huntsville, AL). Stably infected cells were selected in 0.4 μg/ml puromycin. The calpain inhibitors z-LLY-FMK (Calp Inh IV) and PD150606 were obtained from EMD Biosciences (San Diego CA).
Cell imaging and Analysis of Autophagic Induction
Monodansylcadaverine (MDC) was used to examine autophagy by live cell imaging [16]. RPMI 8226 myeloma cells were incubated with bortezomib for 4 hours. Amino acid deprivation by incubation in nutrient-free Earle’s Balanced Salt Solution (EBSS) for 4 hours was used as a positive control. Cells were then incubated with 50 μM MDC (Sigma, St. Louis, MO) at 37°C for 15 minutes and unincorporated dye was removed by washing 6x in prewarmed serum free media (SFM). For live cell imaging, cells were immobilized on 25 mm cover slips using Extracel Hydrogel™ (Glycosan Biosystems, Salt Lake City, UT). This matrix is a non-refractory gel composed of thiol-modified hyaluronate and gelatin, cross linked by polyethylene glycol diacrylate. Coverslips containing cells were placed in a 37°C chamber housed on the stage of an Olympus IX71 microscope, and incubated in Hank’s Balanced Salt Solution (HBSS). Autophagosome formation was analyzed with Image J (NIH, Bethesda, MD) [17] by two different measures. First, the number of acidic vesicles was counted in 15 fields, with 8–10 cells per field under each treatment condition. To account for variability in dye loading, and for the 3 dimensional nature of the live cell imaging, a second method was used in which the average intensity of 5 vesicles was normalized to the measured average cytosolic value. In both analyses, a minimum of 50 cells per experimental condition were analyzed, and statistical significance determined by Student’s t-test.
Protein extraction and Western blot analysis
Cells were lysed in M-Per mammalian lysis reagent (Thermo-Fisher) and proteins quantified by BCA assay (Bio-Rad). Extracts were separated on a 12% SDS poly-acrylamide gel and transferred to PVDF membrane (Millipore, Billerica, MA) for immunoblotting and analysis. Detection was done using ECL substrate (Pierce) and analysis was done by densitometry using Image J (NIH, Bethesda MD). The anti-LC3B antibody was from Cell Signaling (Danvers MA), anti-p62 was from Santa Cruz Biotechnology, (Santa Cruz CA), and the calpain small subunit antibody (CAPNS1) was from AbCam (Cambridge MA).
Cell viability assays
For apoptosis assays, myeloma cells were incubated with various doses of BZ with or without calpain inhibitors for 24 hours. Cells were washed and resuspended in Annexin-V binding buffer containing Alexa 488 conjugated Annexin-V (Invitrogen, Carlsbad, CA). The apoptotic fraction was analyzed by BD FACScan and Cell Quest Software (San Jose, CA). Total cell death was determined by trypan blue exclusion. Cells were incubated with drug as above, and live cells stained with trypan blue. Counts of live versus dead cells were done in triplicate, with a minimum of 100 cells counted per sample. MTT dye reduction was used to assay growth inhibition.
Enzyme Activity Assays
Cells were lysed and samples normalized for total protein. Substrate specific to caspase-3, caspase-8 or caspase-9 (Biovision, Mountain View, CA) was added and incubated at 37°C for 2 hrs, after which sample absorbance was evaluated at 405 nm. Mean absorbance readings were adjusted for background, and presented as a percent control of caspase activity of untreated control cells. To measure calpain activity, Ac-LLY-AFC substrate (Promega, Madison WI) was incubated with 100 μg of protein and calpain activity reaction buffer for one hour at 37°C. Fluorescence was measured on a Spectra-Max (Molecular Devices, Sunnyvale CA) fluorescent plate reader with excitation of 400 nm and emission of 505 nm. Calpain activity of treated cells was compared to untreated controls and expressed as a percent of untreated control. Proteasome activity was determined using a cell-based assay (Promega) in which the chymotrypsin-like, trypsin-like, and caspase-like protease activity is measured in intact cultured cells. Although the peptide substrate used is the same as that for calpain activity (Suc-LLY-aminoluciferin), the proteasome activity assay relies on endogenous cellular Ca2+ for activation, while the calpain assay utilizes additional Ca2+ in the reaction buffer for activation.
In vivo activity
All studies were done according to guidelines of the American Association for Laboratory Animal Care under protocols approved by the University of Arizona Institutional Animal Care and Use Committee. The MM.1S human multiple myeloma cell line was used to establish subcutaneous xenograft tumors in SCID mice. Animals were stratified into treatment groups and treatment initiated at 100 mm3 mean tumor volume. Bortezomib, 1.0 mg/kg, was administered by i.v. tail vein injection on days 1, 4, 8, 11. Two courses of nelfinavir (50 mg/kg) were administered by oral gavage daily for 5 days followed by 2 days off. The combination treatment group received both drugs as above. Mice were monitored for general health, body weight, and tumor volume, which was measured by caliper measure every third day. The study was terminated when tumor volumes exceeded 10% of total body weight.
Statistical Analysis
The in vitro experimental data were analyzed using an unpaired Student’s t-test and are expressed as mean ± standard error (SE) with p values of ≤0.05 considered statistically significant. In vivo studies were analyzed using area under the curve (AUC) for each mouse using the trapezoidal rule, and tumor regression models by fitting the least square regression line of tumor burden by day. The AUC was analyzed for each experiment by treatment group using ANOVA, and tumor regression was compared using the slope of the line.
Results
Inhibition of calpain promotes cell death
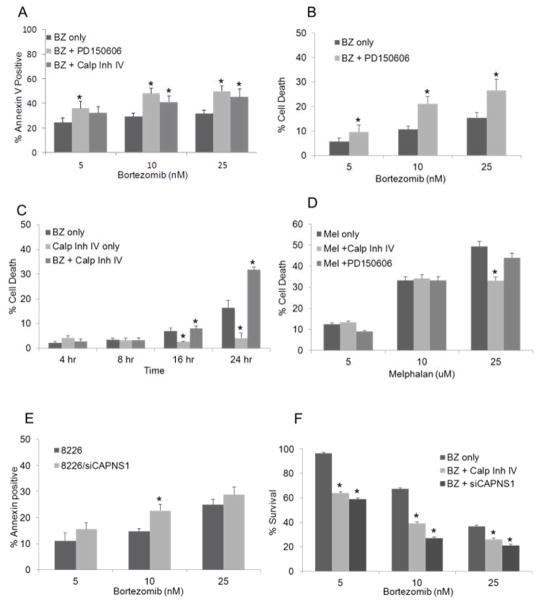
Our previous work demonstrated that dysregulation of intracellular calcium is a major determinant of BZ-induced cell death [4]. Because the Ca2+-dependent serine protease, calpain, has been reported to activate caspase-12 and initiate stress induced apoptosis [11,12], we investigated whether inhibition of calpain activity would protect myeloma cells from cell death. Unexpectedly, we found that co-treatment of myeloma cells with pharmacologic calpain inhibitors rendered the cells significantly more sensitive to BZ (Fig. 1). Inhibition of calpain activity with the tri-peptide Calp Inh IV or the non-peptide inhibitor PD150606 resulted in a significant increase in the cytotoxic activity of bortezomib with a near doubling of the affected fraction in cells treated with the combination of BZ and calpain inhibitor. Three independent methods of assessing drug activity were used: Annexin V staining, trypan blue exclusion, and MTT dye reduction. All three assays demonstrated enhanced cytotoxicity in cells incubated with BZ in the presence of calpain inhibitors. In contrast, inhibition of calpain did not confer greater sensitivity to non- Ca2+ dependent cytotoxic agents such as melphalan (Fig. 1d). Control experiments showed that in all assays calpain activity was reduced by greater than 50%, and that the inhibition of calpain alone was not significantly cytotoxic (induced less than 10% cell death). To exclude non-specific effects of pharmacologic inhibitors, we also used siRNA to eliminate expression of the common catalytic subunit of calpain, CAPNS1. Inhibition of the CAPNS1 protein expression by 65% induced a corresponding decrease in calpain activity by 41% (Suppl. Fig. 1). Similar to the effects seen with pharmacological inhibition of calpain, both apoptotic cell death and growth inhibition was increased in siCAPNS1 transfected cells at all concentrations of bortezomib (Fig. 1E and F).
Fig 1. Enhancement of BZ cytotoxicity by calpain inhibition.
RPMI 8226 myeloma cells were incubated with the indicated concentration of bortezomib in the presence or absence of 10 nM PD150606 or 10 μM Calpain Inhibitor IV. Apoptosis was analyzed by Annexin V-FITC staining and flow cytometry (A) or trypan blue exclusion (B) following 24 hours of drug exposure, or as a function of time (C). (D) Myeloma cells were incubated with the indicated concentration of melphalan or a combination of melphalan plus calpain inhibitors and analyzed by trypan blue exclusion. (E) Myeloma cells transduced with siCAPNS1 were incubated with BZ for 24 hours and examined for Annexin V staining by flow cytometry or by MTT dye reduction (F). All values are the mean of 3 independent experiments ± SE, and data is presented as (% treated−% control)/(100−% control) where the control is calpain inhibitor only. * significant vs. BZ only at p<0.05.
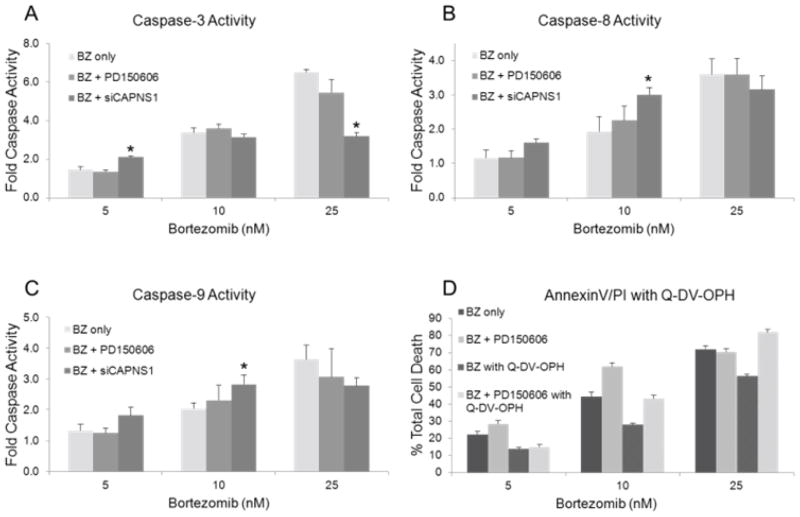
Despite an increase in total cell death under all conditions of calpain inhibition, the activity of caspases-3, 8 and 9 were not significantly elevated compared to myeloma cells treated with bortezomib alone (Fig. 2). In addition, co-treatment with the broad spectrum caspase inhibitor, Q-VD-OPh reduced the apoptotic fraction in all treatment groups, however, it did not counteract the enhanced cytotoxicity of calpain inhibitors with BZ (Fig. 2d). Control experiments demonstrated that the tri-peptide calpain inhibitor (Calp Inh IV) interfered with the pNA assay in cell free lysates. The non-peptide inhibitor, PD150606 did not effect caspase activity at concentrations up to 50 nM (data not shown).
Fig 2. Effects of calpain inhibition on caspase activity in bortezomib induced cell death.
Myeloma cells were incubated with the indicated concentration of bortezomib in the presence or absence of 10 nM PD150606 or 10 μM Calpain Inhibitor IV for 24 hours. Cells were lysed and assayed for caspase-3 (A), caspase-8 (B) and caspase-9 (C) activity using pNA labeled tetrapeptides. Data shown are the mean of three independent experiments ± SE. * significant vs. BZ only at p < 0.05. (D) Myeloma cells were pretreated with the pan caspase inhibitor Q-VD-OPH for 30 minutes prior to drug treatment and analyzed for cell death by Annexin V/PI staining and flow cytometry. Values are the mean of 3 independent experiments, and data is presented as [(treated−%control)/(100−control)] where the control is calpain inhibitor only.
Bortezomib induces an early autophagic response
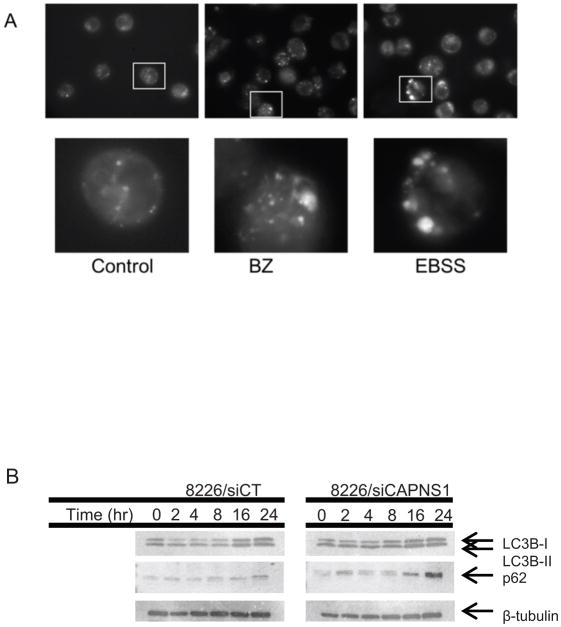
In addition to mediating cytotoxicity, calcium signaling has also been implicated in the regulation of autophagy. Therefore, we investigated the role of autophagy in bortezomib response or resistance. Autophagy has been described as both a cell survival response and a cell death mechanism depending on conditions and cell types [18]. Live cell imaging of myeloma cells treated with 10 nM BZ and stained with the lysosomotropic dye, monodansylcadaverine (MDC) demonstrated that bortezomib induces the formation of acidic vesicles characteristic of early autophagosomes within two hours (Fig. 3a). Because of the heterogeneity in the size and intensity of vesicles labeled with MDC, two approaches were used for quantitation. First, the mean number of MDC stained vesicles was determined by counting fifteen fields with a minimum of 8–10 cells each (Suppl. Fig. 2). Secondly, the mean ratio of lysosomal/cytoplasmic fluorescence intensity was compared from at least 50 individual cells from three different experiments. Both analytical approaches demonstrated a significant increase in vesicle formation in the bortezomib treated cells to approximately the same extent as the EBSS treatment, which is the positive control for inducing autophagy by nutrient deprivation. In accordance with other published reports [5,12,6], these data support an early autophagic response to BZ-induced proteasomal inhibition.
Fig 3. Accumulation of LC3BII and p62 in myeloma treated with BZ.
Myeloma cells transfected with siRNA to calpain (siCAPNS1) or a non-silencing control (siCT) were incubated with 10 nM BZ for the indicated time, harvested, and cell lysates examined for (A) LC3B-I and -II or p62 (B) expression by Western blot. B-tubulin is used as a loading control. See supplemental data for quantitation.
We next examined the effects of calpain inhibition on accumulation and activation of the autosomal protein LC3BII. Western blot analyses demonstrated an increase in the active form, LC3B-II, within 2 hours of treatment with 10 nM BZ. In contrast, myeloma cells that lack calpain are impaired in their autophagic response with very little evidence of LC3B- processing (Fig. 3 and Suppl. Fig. 2). In addition to the formation of autophagic vesicles, the process of autophagy requires the autophagosome to fuse with a lysosome for effective digestion of sequestered proteins. The polyubiquitin-binding cargo protein p62 (SQSTM1) is selectively degraded by autophagy and can be used to monitor autophagic protein degradation [19]. Western blot analysis of p62 over the course of drug treatment demonstrated a significant accumulation of p62 in cells that lack calpain compared to control cells with intact an autophagic response to BZ treatment.
HIV protease inhibitors block calpain activity and enhance bortezomib cytotoxicity in vitro and in vivo
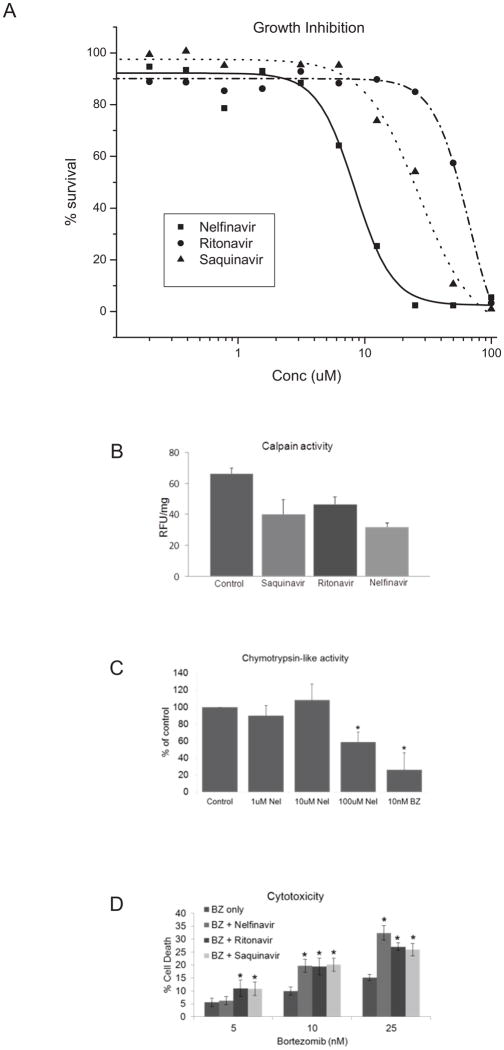
Previous studies have demonstrated that agents developed to block the HIV1-associated aspartyl protease have cross reactivity to the cysteine protease calpain [20,21]. To identify clinically applicable therapeutic combinations utilizing agents that inhibit calpain with bortezomib, we examined the activity of FDA approved HIV protease inhibitors in vitro and in vivo. Incubation of myeloma cells with 3 individual antiretroviral protease inhibitors produced concentration-dependent growth inhibition, with nelfinavir showing the greatest potency (Fig. 4a). At a concentration of 5 μM, each anti-HIV protease inhibitor was sufficient to inhibit calpain activity (Fig. 4b), but did not significantly affect chymotrypsin-like activity associated with proteasome inhibition (Fig. 4c), nor was there cell growth inhibition as a single agent (Fig. 4a). Myeloma cells treated with bortezomib in the presence of 5 μM nelfinavir, ritonavir, indinavir, or saquinavir each demonstrated a significant increase in cell death (Fig. 4d). Because nelfinavir does not inhibit 26S proteasome activity in vitro, the enhanced cytotoxicity is not likely due to an augmentation of proteasomal inhibition activity.
Fig 4. Cytotoxicity of BZ and HIV protease inhibitors in myeloma cells.
(A) Myeloma cells were incubated with increasing concentrations of HIV protease inhibitors for 72 hours and analyzed for growth inhibition by MTT assay. Data are presented as % of control, where the control is cells incubated with the highest concentration of vehicle (DMSO). (B) Myeloma cells were incubated with HIV protease inhibitors (5μM) for 16 hours and assayed for calpain activity. Data shown are the mean of three independent experiments with duplicate samples in each experiment. (C) Myeloma cells were incubated with the indicated concentration of nelfinavir for 2 hours and assayed for chymotrypsin-like activity using the Proteasome-Glo Luciferase assay. BZ is included as a positive control. Data shown are the mean of three independent experiments with triplicate samples in each experiment. (D) Myeloma cells were incubated for 16 hours with the indicated concentration of BZ in combination with HIV protease inhibitors (5 μM). Viability was assayed by trypan blue exclusion and data are presented as [(treated − control)/(100−control)]. Data shown are three independent experiments with a minimum of 100 cells counted per experiment.
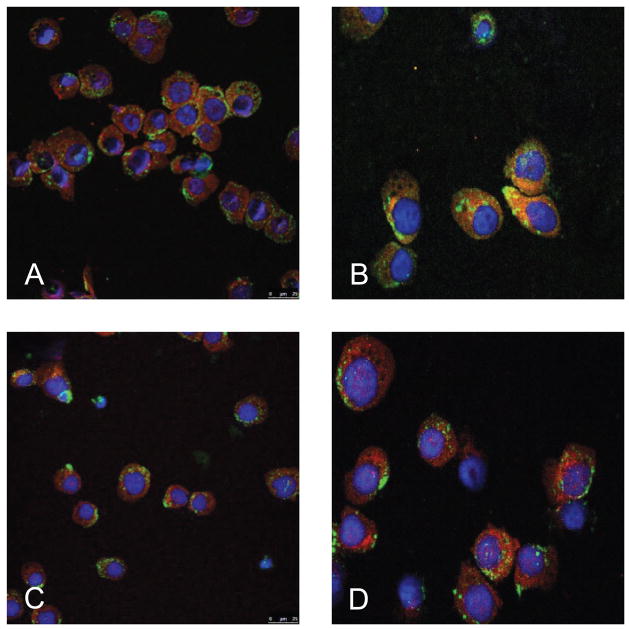
We further addressed the role of calpain inhibition by nelfinavir in autophagosome progression using co-immunofluorescent staining of the autophagosomal marker LC3II and the lysosomal marker LAMP-2 (Fig. 5). Cells incubated with BZ alone demonstrate co-localization of the LC3II (Alexa-647) with LAMP-2 (Alexa-488), presumably due to fusion of the autophagosome and lysosome. In contrast, the addition of nelfinavir reduces the degree of co-localization, suggesting impairment of the fusion process. These findings are consistent with an accumulation of non-functional autophagic vesicles in cells treated with nelfinavir, and suggest that inhibition of calpain inhibits the progression and digestive capacity of autophagy.
Fig 5. Immunofluorescent co-localization of LC3 and LAMP-2.
H929 myeloma cells were incubated with DPBS (A), 10 nM BZ (B), 5 μM Nelfinavir (C), or a combination of 10 nM BZ and 5 μM nelfinavir (D) for 16 hours. Cells were then immobilized on glass slides, fixed in formalin and stained for analysis on a Leica 5 confocal microscope. LC3 is stained with Alexa-647 (red) and LAMP-2 is stained with Alexa-488 (green).
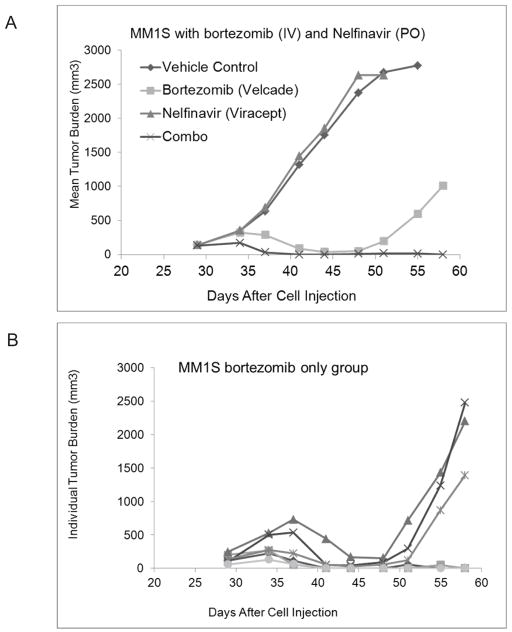
Finally, we used a xenograft model of multiple myeloma to determine if the combination of bortezomib and nelfinavir was active in vivo. SCID mice were inoculated with MM.1S myeloma cells and allowed to establish subcutaneous tumors. Treatment was initiated when the mean tumor volume was approximately 100 mm3. As shown in figure 6a, mice that received the combination of BZ plus nelfinavir demonstrated complete tumor regression. In the BZ only group, all animals initially responded with tumor regression, but 50% later relapsed with the tumor growth rate reverting to that of the untreated control group (Fig. 6b). Importantly, nelfinavir alone had no anti-tumor activity in vivo. The combination regimen was well tolerated, with mean weight loss in the BZ only group of 14%, compared to18% in the combination group. In both groups, the maximum weight loss was seen on day 12 following the final dose of BZ. Further, the animals did not appear distressed, and the weight loss fully recovered within 6 days for the BZ only group, and 9 days for the combination group. Statistical analysis using either AUC or tumor regression models demonstrated statistically significant differences between BZ only vs. the combination of BZ and nelfinavir, as well as vehicle control.
Fig 6. Tumor inhibition of nelfinavir and BZ in vivo.
Xenograft tumors of MM.1S myeloma cells were established in SCID mice by subcutaneous inoculation. Animals were stratified into groups with equivalent tumor volumes and treatment initiated when the mean tumor volume was 100 mm3. Drugs were administered as follows: BZ, 1 mg/kg, days 1, 4, 8, 11(IV); Nelfinavir, 50 mg/kg days 1–5 (PO) or in combination. Tumors volumes were measured every 3 days, and animals monitored for weight loss and general well-being. (A) Data are the mean tumor volume of n=6 animals per group. (B) BZ only treatment group. Each line represents one animal.
Discussion
Several recent studies have shown that proteasome inhibitors used in cancer chemotherapy can initiate an autophagic response, and autophagy has been proposed as a mechanism of drug resistance [5,7,22,23]. Autophagy is induced by various cellular stresses including nutrient or growth factor deprivation, oxidative stress, and dysregulation of intracellular Ca2+ among others. And although autophagy is well recognized as a cytoprotective mechanism, the presence of autophagic vesicles in dying cells initially provided the basis for the definition of a caspase-independent, autophagy-associated cell death, or type II cell death [24]. Cells with deficiencies in apoptosis, either through genetic mutation or deletion, or pharmaceutical inhibition, ultimately die with morphologic evidence of autophagy, and it has been suggested that type II cell death represents failed or defective autophagy. Our data with BZ support this hypothesis, and suggest that Ca2+ dependent mediators such as calpain are key regulators of autophagic progression that mitigate survival versus cell death.
Our previous work demonstrated that inhibition of mitochondrial Ca2+transport abrogated the cytotoxic activity of bortezomib in multiple myeloma cells [4]. These data indicated a requirement for a Ca2+-dependent pathway in cell death, leading us to investigate the role of calpain in caspase activation. Contrary to our hypothesis, inhibition of calpain did not reduce or prevent bortezomib cytotoxicity. Rather, calpain inhibition enhanced BZ-mediated cell death. These data are compatible with the work of Li et al, in which they demonstrate that inhibition of calpain induces apoptosis a priori in the Eμ-myc transgenic model of B-cell lymphoma [25]. Tumor cells from these animals have constitutively active calpain, and it is not unreasonable to hypothesize that calpain activity is promoting autophagy in the transformed cells and conferring a survival advantage.
The role of calpain in autophagy is not a new concept. Demarchi et al. previously demonstrated that calpain deficient mouse embryonic fibroblasts have defects in autophagy, and treatment with etoposide, ceramide or serum free culture led to rapid cell death [26]. Similarly, Madden et al. demonstrated that pan-caspase inhibition induced autophagy in mouse fibrosarcoma cells, and that caspase-independent cell death only occurred when calpain was concurrently inhibited [27]. We have identified a similar effect in myeloma cells treated with bortezomib, where inhibition of calpain prevents the completion of autophagy and accelerates programmed cell death.
Calpain substrates include structural proteins, signal transduction molecules and membrane receptors [13]. The mechanisms regulating calpain activity and specificity are not well understood. For example, it is not clear why calpain activity appears to have a cell death promoting effect under some conditions, and a cell death inhibiting effect in others. Our data suggest that the distinction may be a kinetic effect, and that calpain activity may participate in the later stages of autophagy. Because calpain is involved in vesicular fusion events [28,29] it is attractive to speculate that calpain inhibition prevents the fusion of the autophagosome with the lysosome. In our system, autophagy is initiated by proteasome inhibition. Previous studies, as well as our unpublished data, have demonstrated that co-treatment of proteasome inhibitors with agents that block the initiation of autophagy do not enhance cell death, but rather have an antagonistic effect [7]. In contrast, agents such as Bafilomycin A, which prevents lysosome-autophagosome fusion, is particularly effective in synergizing with bortezomib in mouse embryonic fibroblasts [30]. Taken together, these studies suggest that once the cell has initiated autophagy as a survival mechanism, it becomes more vulnerable to disruption of the process and can rapidly initiate caspase-independent cell death.
Alterations in calpain activity have been implicated in a number of disease conditions, including, diabetes, neurological disorders such as Alzheimer’s disease and cancer [31]. Many calpain inhibitors under development are peptide analogues that mimic the physiological peptide inhibitor, calpastatin. These agents are characterized by their lack of specificity and cross reactivity with other cysteine proteases, a feature that may be advantageous in some therapeutic settings. The HIV protease is an aspartyl protease, and two FDA approved HIV protease inhibitors, indinavir and ritonavir, have previously been reported to directly inhibit calpain activity [21,20]. These agents have demonstrated antitumor activity in the NCI 60 cell line panel both in vitro and in vivo [32] however, therapeutically active plasma levels would require significant dose escalation over the current doses used in anti-retroviral therapy. Our data demonstrate the feasibility of a combination strategy that provides anti-myeloma activity for BZ and HIV protease inhibitors even at protease inhibitor levels below the established Cmax. Additionally, calpain inhibition may provide a potential biomarker to determine the biologically effective dose. The novel combination of two FDA-approved agents could be rapidly translated to the clinic with a potential immediate impact on the treatment of patients with refractory myeloma.
Supplementary Material
Acknowledgments
This work was supported by the Multiple Myeloma Research Foundation (MMRF), and the National Institutes of Health/National Cancer Institute [CA23074, CA017094] Bortezomib (PS-341/Velcade®) was generously provided by Millennium Pharmaceuticals Inc.
Footnotes
Disclosures: None:
References
- 1.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, Maas J, Pien CS, Prakash S, Elliott PJ. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 1999;59:2615–2622. [PubMed] [Google Scholar]
- 2.Schenkein D. Proteasome inhibitors in the treatment of B-cell malignancies. Clin Lymphoma. 2002;3:49–55. doi: 10.3816/clm.2002.n.011. [DOI] [PubMed] [Google Scholar]
- 3.Kane RC, Bross PF, Farrell AT, Pazdur R. Velcade: U.S. FDA approval for the treatment of multiple myeloma progressing on prior therapy. Oncologist. 2003;8:508–513. doi: 10.1634/theoncologist.8-6-508. [DOI] [PubMed] [Google Scholar]
- 4.Landowski TH, Megli CJ, Nullmeyer KD, Lynch RM, Dorr RT. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005;65:3828–3836. doi: 10.1158/0008-5472.CAN-04-3684. [DOI] [PubMed] [Google Scholar]
- 5.Milani M, Rzymski T, Mellor HR, Pike L, Bottini A, Generali D, Harris AL. The role of ATF4 stabilization and autophagy in resistance of breast cancer cells treated with Bortezomib. Cancer Res. 2009;69:4415–4423. doi: 10.1158/0008-5472.CAN-08-2839. [DOI] [PubMed] [Google Scholar]
- 6.David E, Kaufman JL, Flowers CR, Schafer-Hales K, Torre C, Chen J, Marcus AI, Sun SY, Boise LH, Lonial S. Tipifarnib sensitizes cells to proteasome inhibition by blocking degradation of bortezomib-induced aggresomes. Blood. 2010;116:5285–5288. doi: 10.1182/blood-2010-03-272393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoang B, Benavides A, Shi Y, Frost P, Lichtenstein A. Effect of autophagy on multiple myeloma cell viability. Mol Cancer Ther. 2009;8:1974–1984. doi: 10.1158/1535-7163.MCT-08-1177. [DOI] [PubMed] [Google Scholar]
- 8.Klionsky DJ. Autophagy: from phenomenology to molecular understanding in less than a decade. Nat Rev Mol Cell Biol. 2007;8:931–937. doi: 10.1038/nrm2245. [DOI] [PubMed] [Google Scholar]
- 9.Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–293. doi: 10.1016/j.molcel.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007 doi: 10.1038/nrc2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oubrahim H, Chock PB, Stadtman ER. Manganese(II) induces apoptotic cell death in NIH3T3 cells via a caspase-12-dependent pathway. J Biol Chem. 2002;277:20135–20138. doi: 10.1074/jbc.C200226200. [DOI] [PubMed] [Google Scholar]
- 12.Tan Y, Wu C, De Veyra T, Greer PA. Ubiquitous calpains promote both apoptosis and survival signals in response to different cell death stimuli. J Biol Chem. 2006;281:17689–17698. doi: 10.1074/jbc.M601978200. [DOI] [PubMed] [Google Scholar]
- 13.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 14.Masters JR, Thomson JA, Daly-Burns B, Reid YA, Dirks WG, Packer P, Toji LH, Ohno T, Tanabe H, Arlett CF, Kelland LR, Harrison M, Virmani A, Ward TH, Ayres KL, Debenham PG. Short tandem repeat profiling provides an international reference standard for human cell lines. Proc Natl Acad Sci USA. 2001;98:8012–8017. doi: 10.1073/pnas.121616198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinez J, Patkaniowska A, Urlaub H, Luhrmann R, Tuschl T. Single-stranded antisense siRNAs guide target RNA cleavage in RNAi. Cell. 2002;110:563–574. doi: 10.1016/s0092-8674(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 16.Biederbick A, Kern HF, Elsasser HP. Monodansylcadaverine (MDC) is a specific in vivo marker for autophagic vacuoles. Eur J Cell Biol. 1995;66:3–14. [PubMed] [Google Scholar]
- 17.Abramoff MD, Magalhaes PJ, Ram SJ. Image Processing with ImageJ. Biophotonics International. 2004;11:36–42. [Google Scholar]
- 18.Debnath J, Baehrecke EH, Kroemer G. Does autophagy contribute to cell death? Autophagy. 2005;1:66–74. doi: 10.4161/auto.1.2.1738. [DOI] [PubMed] [Google Scholar]
- 19.Komatsu M, Ichimura Y. Physiological significance of selective degradation of p62 by autophagy. FEBS, Lett. 2010;584:1374–1378. doi: 10.1016/j.febslet.2010.02.017. [DOI] [PubMed] [Google Scholar]
- 20.Wan W, DePetrillo PB. Ritonavir inhibition of calcium-activated neutral proteases. Biochem Pharmacol. 2002;63:1481–1484. doi: 10.1016/s0006-2952(02)00907-3. [DOI] [PubMed] [Google Scholar]
- 21.Ghibelli L, Mengoni F, Lichtner M, Coppola S, De Nicola M, Bergamaschi A, Mastroianni C, Vullo V. Anti-apoptotic effect of HIV protease inhibitors via direct inhibition of calpain. Biochem Pharmacol. 2003;66:1505–1512. doi: 10.1016/s0006-2952(03)00505-7. [DOI] [PubMed] [Google Scholar]
- 22.Ding WX, Ni HM, Gao W, Yoshimori T, Stolz DB, Ron D, Yin XM. Linking of autophagy to ubiquitin-proteasome system is important for the regulation of endoplasmic reticulum stress and cell viability. Am J Pathol. 2007;171:513–524. doi: 10.2353/ajpath.2007.070188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogata M, Hino S, Saito A, Morikawa K, Kondo S, Kanemoto S, Murakami T, Taniguchi M, Tanii I, Yoshinaga K, Shiosaka S, Hammarback JA, Urano F, Imaizumi K. Autophagy is activated for cell survival after endoplasmic reticulum stress. Mol Cell Biol. 2006;26:9220–9231. doi: 10.1128/MCB.01453-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsujimoto Y, Shimizu S. Another way to die: autophagic programmed cell death. Cell Death Differ. 2005;12(Suppl 2):1528–34. 1528–1534. doi: 10.1038/sj.cdd.4401777. [DOI] [PubMed] [Google Scholar]
- 25.Li H, Nepal RM, Martin A, Berger SA. Induction of apoptosis in Emu-myc lymphoma cells in vitro and in vivo through calpain inhibition. Exp Hematol. 2012;40:548–563. doi: 10.1016/j.exphem.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Demarchi F, Bertoli C, Copetti T, Tanida I, Brancolini C, Eskelinen EL, Schneider C. Calpain is required for macroautophagy in mammalian cells. J Cell Biol. 2006;20(175):595–605. doi: 10.1083/jcb.200601024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Madden DT, Egger L, Bredesen DE. A calpain-like protease inhibits autophagic cell death. Autophagy. 2007;3:519–522. doi: 10.4161/auto.4052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans JS, Turner MD. Emerging functions of the calpain superfamily of cysteine proteases in neuroendocrine secretory pathways. J Neurochem. 2007;103:849–859. doi: 10.1111/j.1471-4159.2007.04815.x. [DOI] [PubMed] [Google Scholar]
- 29.Grumelli C, Berghuis P, Pozzi D, Caleo M, Antonucci F, Bonanno G, Carmignoto G, Dobszay MB, Harkany T, Matteoli M, Verderio C. Calpain activity contributes to the control of SNAP-25 levels in neurons. Mol Cell Neurosci. 2008;39:314–323. doi: 10.1016/j.mcn.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 30.Foti C, Demarchi F, Brancolini C. Inhibitors of the ubiquitin-proteasome system are not all alike: identification of a new necrotic pathway. Autophagy. 2009;5:543–545. doi: 10.4161/auto.5.4.8169. [DOI] [PubMed] [Google Scholar]
- 31.Storr SJ, Carragher NO, Frame MC, Parr T, Martin SG. The calpain system and cancer. Nat Rev Cancer. 2011;11:364–374. doi: 10.1038/nrc3050. [DOI] [PubMed] [Google Scholar]
- 32.Gills JJ, Lopiccolo J, Tsurutani J, Shoemaker RH, Best CJ, Abu-Asab MS, Borojerdi J, Warfel NA, Gardner ER, Danish M, Hollander MC, Kawabata S, Tsokos M, Figg WD, Steeg PS, Dennis PA. Nelfinavir, A lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis in vitro and in vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.