Abstract
The repair of large bone defects, such as segmental defects in the long bones of the limbs, is a challenging clinical problem. Our recent work has shown the ability to create porous scaffolds of silicate 13-93 bioactive glass by robocasting which have compressive strengths comparable to human cortical bone. The objective of this study was to evaluate the capacity of those strong porous scaffolds with a grid-like microstructure (porosity = 50%; filament width = 330 μm; pore width = 300 μm) to regenerate bone in a rat calvarial defect model. Six weeks postimplantation, the amount of new bone formed within the implants was evaluated using histomorphometric analysis. The amount of new bone formed in implants composed of the as-fabricated scaffolds was 32% of the available pore space (area). Pretreating the as-fabricated scaffolds in an aqueous phosphate solution for 1, 3, and 6 days, to convert a surface layer to hydroxyapatite prior to implantation, enhanced new bone formation to 46%, 57%, and 45%, respectively. New bone formation in scaffolds pretreated for 1, 3, and 6 days and loaded with bone morphogenetic protein-2 (BMP-2) (1 μg/defect) was 65%, 61%, and 64%, respectively. The results show that converting a surface layer of the glass to hydroxyapatite or loading the surface-treated scaffolds with BMP-2 can significantly improve the capacity of 13-93 bioactive glass scaffolds to regenerate bone in an osseous defect. Based on their mechanical properties evaluated previously and their capacity to regenerate bone found in this study, these 13-93 bioactive glass scaffolds, pretreated or loaded with BMP-2, are promising in structural bone repair.
Keywords: bone regeneration, bioactive glass scaffold, surface modification, bone morphogenetic protein-2, rat calvarial defect model
1. Introduction
The repair of large bone defects is a challenging clinical problem [1]. While contained bone defects are repairable with commercially-available, osteoconductive and osteoinductive filler materials [2, 3], there is no ideal biological solution to reconstitute structural bone loss, such as segmental defects in the long bones of the limbs. Available treatments such as bone allografts, autografts, porous metals, and bone cement have limitations related to costs, availability, longevity, donor site morbidity, and uncertain healing to host bone. Consequently, there is a great need for porous biocompatible implants that can replicate the structure and function of bone and have the requisite mechanical properties for reliable long-term cyclical loading during weight bearing.
As described previously [4–6], bioactive glasses have several attractive properties as a scaffold material for bone repair, such as their biocompatibility, ability to convert in vivo to hydroxyapatite (the mineral constituent of bone), and ability to bond strongly to hard tissue. Some bioactive glasses, such as the silicate glass designated 45S5, also have the ability to bond to soft tissue [5, 6]. Most previous studies have targeted bioactive glass scaffolds with relatively low strength three-dimensional (3D) architectures, such as strengths in the range of human trabecular bone (2–12 MPa) [7]. Recent studies have shown that silicate bioactive glass scaffolds (13-93 and 6P53B) created by solid freeform fabrication techniques such as freeze extrusion fabrication [8] and robocasting [9, 10] have compressive strengths (~140 MPa) comparable to human cortical bone (100–150 MPa) [7].
Our recent work showed that strong porous bioactive glass (13-93) scaffolds created using robocasting had excellent mechanical reliability (Weibull modulus = 12) and promising fatigue resistance under cyclic stresses far greater than normal physiological stresses [11], but the capacity of those strong porous bioactive glass (13-93) scaffolds to regenerate bone has not yet been studied. Our recent studies also showed that the elastic (brittle) mechanical response of the 13-93 bioactive glass scaffolds in vitro changed to an “elasto–plastic” response after implantation for longer than 2–4 weeks in vivo, as a result of soft and hard tissue growth into the pores of the scaffolds [11, 12]. However, concerns still remain about the low fracture toughness, flexural strength and torsional strength of the as-fabricated bioactive glass scaffolds.
In addition to material composition and microstructure [13], scaffold healing to bone in vivo can be markedly affected by other variables, such as surface composition and structure, the release of osteoinductive growth factors, and the presence (or absence) of living cells. Interconnected pores of size 100 μm are recognized as the minimum requirement for supporting tissue ingrowth [14], but pores of size 300 μm or larger may be required for enhanced bone ingrowth and capillary formation [15]. Surface modification of macroporous bioactive glass scaffolds have targeted the creation of fine pores (nanometers to a few microns in size) to modify the surface roughness and increase the surface area of the scaffolds [16–18]. Conversion of a surface layer to HA, by reaction in an aqueous phosphate solution, has been shown to improve the capacity of borate and silicate bioactive glass to support cell proliferation and differentiation in vitro [19]. Treatment of B2O3-doped silicate bioactive glass scaffolds with a fibrous microstructure in simulated body fluid (SBF) to create a rough surface layer of carbonated HA was shown to improve the capacity of the scaffolds to support cell proliferation in vitro and to enhance bone formation in vivo [20].
Osteoinductive growth factors such as bone morphogenetic protein-2 (BMP-2) and BMP-7 are well known to stimulate bone formation [21, 22]. However, the use of porous 3D bioactive glass scaffolds as delivery devices for growth factors has so far received little attention. In a recent study [23], the surfaces of three silicate bioactive glasses were functionalized by a silanization technique using 3-amino-propyl-triethoxysilane; then BMP-2 was immobilized on the glass surfaces. However, the release of the BMP-2 and the effect of the BMP-2 on bone regeneration in vivo were not studied.
Particles of 13-93 glass have a lower tendency to crystallize prior to appreciable sintering when compared to 45S5 glass [4]. Consequently, 13-93 glass particles can be more readily sintered into a dense and strong network. As described earlier, our recent work showed that strong porous 3D scaffolds of 13-93 glass, prepared with a grid-like microstructure using robocasting, had promising mechanical properties for loaded bone repair. The bioactivity of 13-93 glass and the capacity of 13-93 glass scaffolds with a “trabecular” and an “oriented” microstructure to support bone ingrowth in vivo were shown in our previous studies [12, 24].
The objective of the present study was to evaluate the capacity of those strong porous 13-93 bioactive glass scaffolds fabricated by robocasting to regenerate bone in an osseous defect model. The effects on bone regeneration of pretreating the scaffolds for various times in an aqueous phosphate solution, to convert the glass surface to hydroxyapatite (HA) prior to implantation, and loading the pretreated scaffolds with BMP-2 were studied. After implantation for 6 weeks in rat calvarial defects, new bone formation in the implants was evaluated using histomorphometric techniques and scanning electron microscopy.
2. Experiments
2.1 Preparation of bioactive glass (13-93) scaffolds
Scaffolds of 13-93 bioactive glass (composition 6Na2O, 12K2O, 5MgO, 20CaO, 53SiO2, 4P2O5; wt %) with a grid-like microstructure were prepared using a robotic deposition (robocasting) method, as described in our previous work [11]. Briefly, a slurry was prepared by mixing 40 vol% glass particles (~1 μm) with a 20 wt % aqueous Pluronic® F-127 solution in a planetary centrifugal mixer (ARE-310, THINKY U.S.A. Inc, Laguna Hills, CA, USA). Then the slurry was loaded into a robotic deposition device (RoboCAD 3.0, 3-D Inks, Stillwater, OK) and extruded through a syringe (tip diameter = 410 μm) onto an Al2O3 substrate to form a 3D scaffold. The extruded filaments were deposited at right angles to the filaments in the adjacent layer, with a center-to-center spacing between the filaments of 910 μm in the plane of deposition. After forming, the scaffolds were dried for 24 h at room temperature and heated for 2 h at 100 °C to remove any residual water. Then the scaffolds were heated slowly in flowing oxygen to 600 °C (heating rate = 0.5 °C/min, with isothermal holds for 2 h each at 150 °C, 200 °C, 250 °C, and 300 °C) to burn out the polymer processing aids, and sintered in air for 1 h at 700 °C (heating rate = 5 °C/min) to densify the glass filaments. The as-fabricated constructs were sectioned and ground into thin discs (4.6 mm in diameter × 1.5 mm), washed twice with deionized water and twice with ethanol, dried in air, and then sterilized by heating for 12 h at 250 °C.
2.2 Surface modification of scaffolds
Some of the as-fabricated scaffolds were modified prior to implantation by reacting them in an aqueous phosphate solution to convert a surface layer of the glass to an amorphous calcium phosphate (ACP) or hydroxyapatite (HA) material. In the surface modification process, the scaffolds were immersed for 1, 3, and 6 days in 0.25 M K2HPO4 solution at 60 °C and a starting pH = 12.0 (obtained by adding the requisite amount 2 M NaOH solution). The mass of the glass scaffolds to the volume of the K2HPO4 solution was kept constant at 1 g per 200 ml, and the system was stirred gently each day. These reaction conditions were based on our previous studies on the conversion of bioactive glasses to HA [25, 26] and the ability to enhance the dissolution rate of the silicate glass network at higher pH [27]. In general, the reaction conditions were selected to accelerate the conversion of 13-93 glass to HA because of the slow conversion of the glass in simulated body fluid (SBF) at the body temperature (37 °C) [4, 24]. After each reaction time, the scaffolds were removed from the solution, washed twice with deionized water, and twice with anhydrous ethanol to displace residual water from the scaffolds. The scaffolds were removed from the ethanol, dried for at least 24 h at room temperature, and stored in desiccator.
2.3 Characterization of converted surface layer
The surface-treated scaffolds were sputter-coated with Au/Pd and examined in a scanning electron microscope, SEM (S-4700; Hitachi, Tokyo, Japan), using an accelerating voltage of 15 kV and a working distance of 8 mm. Some surface-treated scaffolds were also mounted in epoxy resin, sectioned, polished to expose the cross-sections of the glass filaments, and examined in the SEM (S-4700; Hitachi). The thickness of the converted surface layer was determined from more than 15 measurements in the SEM images using the ImageJ software (National Institutes of Health, USA), and expressed as a mean value ± standard deviation (SD).
The converted surface layer was removed by vigorously shaking the scaffolds and used in determining its surface area and phase composition. Surface area measurements were made using nitrogen gas adsorption (Nova 2000e; Quantachrome, Boynton Beach, FL, USA). The volume of nitrogen adsorbed and desorbed at different gas pressures was measured and used to construct adsorption–desorption isotherms. Eleven points of the adsorption isotherm, which initially followed a linear trend implying monolayer formation of adsorbate, were fitted by the Brunauer–Emmett–Teller equation to determine the surface area.
The presence of crystalline phases in the converted surface layer was determined using X-ray diffraction (XRD) (D/mas 2550 v; Rigaku, The Woodlands, TX, USA). The material was ground into a powder and analyzed using Cu Kα radiation (λ = 0.15406 nm) at a scanning rate of 1.8 °/min in the 2θ range 10–80°.
2.4 Loading the pretreated scaffolds with BMP-2
Some of the pretreated scaffolds were loaded with BMP-2 prior to implantation. In the process, a solution of BMP-2 (Shenandoah Biotechnology Inc., PA, USA) in citric acid was prepared by dissolving 10 μg of BMP-2 in 100 μl sterile citric acid (pH = 3.0). Then 10 μl of the BMP-2 solution was pipetted on to each bioactive glass scaffold (4.6 mm in diameter × 1.5 mm). The amount of BMP-2 loaded into the scaffolds was equivalent to 1 μg per bone defect (or per implant) in the animal model. The BMP-loaded scaffolds were kept for 24 h in a refrigerator at 4 °C to dry them prior to implantation. For comparison, some of the as-fabricated scaffolds (no pretreatment in the phosphate solution) were also loaded with BMP-2 using the same procedure. The BMP-2 solution was totally incorporated within the pores of the scaffolds, and there was no visible evidence for any of the solution flowing out of the scaffolds.
2.5 Release profile of BMP-2 from the scaffolds in vitro
The release of BMP-2 from the scaffolds into a medium composed of equal volumes of fetal bovine serum (FBS) and phosphate-buffered saline (PBS) plus 1vol% penicillin was measured as a function of time in vitro. Each scaffold was immersed in 500 μl of the solution in a 2.0 ml microtube and kept at 37 °C in an incubator. Three replicates were used for each group at each time point. At selected times (1h, 8h, 1d, 3d, 7d, 14d), the solution was completely removed and replaced with fresh solution. The amount of BMP-2 released into the solution was measured using by an enzyme-linked immunosorbent assay (ELISA) kit (Pepro Tech, Rocky Hill, NJ, USA) according to the manufacturer’s instructions.
2.6 Animals and surgical procedure
All animal experimental procedures were approved by the Animal Care and Use Committee, Missouri University of Science and Technology, in compliance with the NIH Guide for Care and Use of Laboratory Animals (1985). Seven groups of scaffolds, described in Table I, were implanted in rat calvarial defects for 6 weeks. This implantation time was used because our previous studies had shown considerable bone regeneration in rat calvarial defects implanted with BMP-loaded hollow HA microspheres for the same time [28]. The implants were assigned randomly to the defects, but scaffolds with and without BMP-2 were not mixed in the same animal.
Table I.
Bioactive glass (13-93) scaffold groups used in this study
| Scaffold group | Pretreatment time in K2HPO4 solution (day) | BMP-2 loading | Number of Defects |
|---|---|---|---|
| As fabricated (0d) | - | - | 10 |
| 1d | 1 | - | 5 |
| 3d | 3 | - | 10 |
| 6d | 6 | - | 5 |
| 1d+BMP | 1 | 1μg/defect | 5 |
| 3d+BMP | 3 | 1μg/defect | 5 |
| 6d+BMP | 6 | 1μg/defect | 5 |
Thirty male Sprague Dawley rats (3 months old; weight = 350–400 g, Harlan Laboratories Inc., USA) were maintained in the animal facility for 2 weeks to become acclimated to diet, water and housing. The rats were anesthetized with a combination of ketamine (72 mg/kg) and xylazine (6 mg/kg) and maintained under anesthesia with ether gas in oxygen. The surgical site was shaved, scrubbed with iodine and draped. Using sterile instruments and aseptic technique, a cranial skin incision was sharply made in an anterior to posterior direction along the midline. The subcutaneous tissue, musculature and periosteum were dissected and reflected to expose the calvarium. Bilateral full-thickness defects 4.6 mm in diameter were created in the central area of each parietal bone using a saline-cooled trephine drill. The dura mater was not disturbed. The sites were constantly irrigated with sterile PBS to prevent overheating of the bone margins and to remove the bone debris. The bilateral defects were randomly implanted with 5 or 10 implants per group. The periosteum and skin were repositioned and closed using wound clips. All animals were given a dose of ketoprofen (3 mg/kg) intramuscularly and ~200 μl penicillin subcutaneously post-surgery. The animals were monitored daily for condition of the surgical wound, food intake, activity and clinical signs of infection. After 6 weeks, the animals were sacrificed by CO2 inhalation, and the calvarial defect sites with surrounding bone and soft tissue were harvested for subsequent evaluation.
2.7 Histologic processing
The calvarial samples, including the surgical sites with surrounding bone and tissue, were fixed in 10% buffered formaldehyde for 3 days, then transferred into 70% ethyl alcohol, and cut in half. Half of each sample was for paraffin embedding and the other half for methyl methacrylate embedding. The samples for paraffin embedding were de-siliconized by immersion for 2 h in 10% hydrofluoric acid, decalcified in 14% ethylenediaminetetraacetic acid (EDTA) solution for 4 weeks, dehydrated in a series of graded ethanol, and embedded in paraffin using routine histological techniques. Then the specimens were sectioned to 5 μm and stained with hematoxylin and eosin (H&E). The undecalcified samples were dehydrated in ethanol and embedded in PMMA. Sections were affixed to acrylic slides and ground down to 40 μm using a surface grinder (EXAKT 400CS, Norderstedt, Germany), and stained using the von Kossa technique. Transmitted light images of the stained sections were taken with an Olympus BX 50 microscope connected with a CCD camera (DP70, Olympus, Japan).
2.8 Histomophometric analysis
Histomorphometric analysis was carried out using optical images of the stained sections and the ImageJ software. The percent new bone formed in the defects was evaluated from the H&E stained sections. The entire defect area was determined as the area between the two defect margins, including the entire glass scaffold and the tissue within. The available pore area within the scaffold was determined by subtracting the area of the bioactive glass scaffold from the total defect area. The newly formed bone, fibrous tissue, and bone marrow-like tissue within the defect area were then outlined and measured. The area of each tissue was expressed as a percentage of the total defect area as well as a percentage of the available pore area within the scaffold.
2.9 Scanning electron microscopy
Unstained sections of the implants in PMMA were coated with carbon and examined using a field-emission scanning electron microscope (SEM) (S-4700; Hitachi) operating in the backscattered electron (BSE) mode. The specimens were examined at an accelerating voltage of 15 kV and a working distance of 12 mm.
2.10 Statistical analysis
The data are presented as a mean ± SD. Analysis for differences in new bone, fibrous tissue, and bone marrow-like tissue between groups was performed using one-way ANOVA with Tukey’s post hoc test [29]. Differences were considered significant for p < 0.05.
3. Results
3.1 Characteristics of bioactive glass scaffolds and converted surface layer
As fabricated, the scaffolds implanted in the rat calvarial defects had a grid-like microstructure (Fig. 1a), composed of almost fully dense bioactive glass filaments of diameter 330 ± 10 μm and pores of width 300 ± 10 μm in the plane of deposition (xy plane) and 150 ± 10 μm in the direction perpendicular to the deposition plane (z direction) (Fig. 1b). The porosity of the scaffolds, as measured using the Archimedes method, was 47 ± 1%.
Fig. 1.

(a) Optical image of 13–93 bioactive glass scaffold prepared by robocasting for implantation in rat calvarial defect. (b) Higher magnification SEM image of the scaffold showing the dense glass filaments and porous architecture in the plane of deposition (xy plane). Inset: SEM image in a plane perpendicular to the xy plane. The scaffolds had a porosity of 47 ± 1%, a pore width of 300 ±10 μm in the plane of deposition (xy plane) and 150 ± 10 μm in z direction.
After reaction of the scaffolds in the K2HPO4 solution, the smooth dense surface of the glass filaments became rough and porous (Fig. 2a), with a fine particulate morphology that was dependent on the reaction time. After reaction for 1 day, the converted surface layer consisted mainly of nearly spherical nanoparticles with some fine needle-like particles (Fig. 2b). With an increase in the reaction time to 3 days, the amount of needle-like particles increased (Fig. 2c), whereas after reaction for 6 days, the surface consisted predominantly of needle-like particles (Fig. 2d). The thickness of the converted layer on the surface of the glass was determined from the cross-section of the glass filaments in the scaffold (Fig. 2a inset). As the reaction time increased from 1 day to 6 days, the thickness of the converted layer increased from 2 μm to 13 μm, while the surface area increased from 19 m2/g to 47 m2/g (Table II).
Fig. 2.
(a) SEM image of a bioactive glass scaffold after reaction for 6 days in 0.25 M K2HPO4 solution (60 °C; pH = 12.0). Inset: cross section of a bioactive glass filament showing the thickness of the converted surface layer. (b)–(d) Higher magnification SEM images of the surface of the converted layer formed by reaction for (b) 1 day, (c) 3 days, and (d) 6 days in the phosphate solution.
Table II.
Thickness and specific surface area of the converted layer formed by reacting silicate (13-93) bioactive glass scaffolds for the times shown in 0.25 M K2HPO4 solution at 60°C and starting pH = 12.0.
| Immersion time (day) | Thickness (μm) | Specific surface area (m2/g) |
|---|---|---|
| 1 | 2 ± 1 | 19 ± 2 |
| 3 | 5 ± 2 | 30 ± 3 |
| 6 | 13 ± 2 | 47 ± 1 |
Figure 3 shows XRD patterns of the as-fabricated bioactive glass scaffold and the converted surface layer of the scaffolds after reaction times of 1, 3, and 6 days in the K2HPO4 solution. The pattern of the as-fabricated scaffold showed no measurable peaks; instead, it contained a broad band centered at ~30° 2θ, typical of an amorphous glass. In comparison, the pattern of the converted surface layer on the scaffold reacted for 1 day showed small peaks that corresponded to those of a reference HA (JCPDS 09-0423); as the reaction time increased from 1 day to 6 days, the number and intensity (height) of the peaks increased. The XRD pattern of the converted layer formed after the one-day reaction also showed a broad bump at ~22° 2θ in the vicinity of the major peak for the cristobalite phase of silica. The height of the bump gradually weakened with increasing reaction time. A similar bump has been observed in the XRD pattern of silicate 45S5 and 13-93 bioactive glass, and it has been attributed to the polymerization of silanol groups during the early stage of the conversion process, leading to the formation of a silica gel phase [30]. Apparently after the initial formation of a silica-rich layer on the glass, an ACP layer is formed which continued to grow and crystallize to HA with increasing reaction time, as described in detail elsewhere [4–6, 31].
Fig. 3.

X-ray diffraction patterns of the as-fabricated bioactive glass (13–93) scaffold, and the converted surface layer formed by reacting the bioactive glass for 1 day, 3 days, and 6 days in 0.25 M K2HPO4 solution (60°C; pH = 12.0). The diffraction peaks corresponding to a reference hydroxyapatite (JCPDS 09-0423) and the main cristobalite peak (JCPDS 39-1425) are indicated.
3.2 Release profile of BMP-2 in vitro
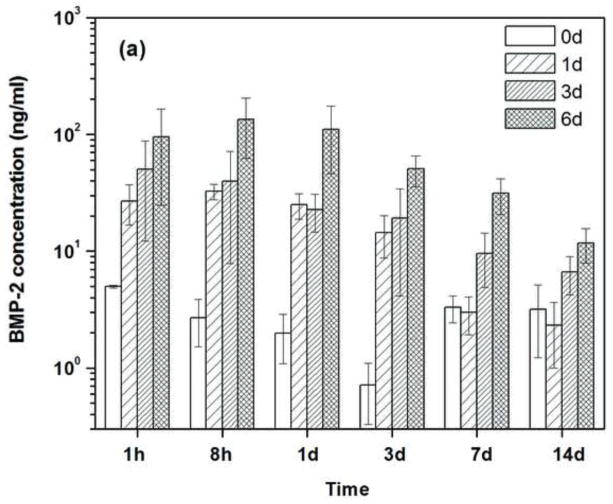
Figure 4a shows the amount of BMP-2 released from the scaffolds into the medium at each time interval. The amount of BMP-2 released from the as-fabricated scaffolds was small at all of the time intervals, and there was no significant difference among the values except for the three-day interval. The reason for the lower BMP-2 concentration at the three-day interval is currently unclear but it may be related to difficulties in measuring the low BMP-2 concentrations. The data in Fig. 4a were used to determine the cumulative amount of BMP-2 released into the medium as a function of time, which was expressed as a percent of the total amount of BMP-2 initially loaded into the scaffolds (Fig. 4b). The BMP-2 release profile from the pretreated scaffolds showed the same trend: a rapid burst release during day 1 was followed by a much slower release rate. However, at a given time, the amount of BMP-2 released into the medium increased with the duration of the pretreatment time (1–6 days) in the phosphate solution. In comparison, there was little release from the as-fabricated scaffolds (no surface treatment) that were loaded with BMP-2. After 14 days, the cumulative amount of BMP-2 released from the scaffolds that had been pretreated for 6 days (30%) was significantly higher than the amount released from the scaffolds pretreated for 3 days (~10%) or for 1 day (~7%). The amount of BMP-2 released from the as-fabricated scaffolds after the fourteen-day period was only ~1%.
Fig. 4.
(a) Amount of BMP-2 released a different time intervals from the as-fabricated scaffold (0d) and the scaffolds pretreated for 1day, 3 day and 6 days (1d; 3d; 6d; respectively) into a medium composed of FBS/PBS. Each scaffold was initially loaded with 1μg of BMP-2. (b) Cumulative amount of BMP-2 released from the scaffolds (as a fraction of the amount of BMP-2 initially loaded into the scaffolds) versus time.
3.3 Assessment of bone regeneration and integration of the implanted scaffolds
H&E and von Kossa stained sections of implants composed of the as-fabricated bioactive glass scaffolds and the scaffolds pretreated in the K2HPO4 solution for 1 day, 3 days, and 6 days which were implanted for 6 weeks in rat calvarial defects are shown in Fig. 5. The von Kossa positive area (dark stain) within the defect showed the presence of mineralized bone as well as HA (or calcium phosphate material) resulting from the pretreatment of the scaffolds or conversion in vivo. Because of the limited amount of hydroxyapatite formed in the pretreatment process and the slow conversion of the bioactive glass in vivo, the von Kossa positive area corresponded generally to the H&E stained areas.
Fig. 5.
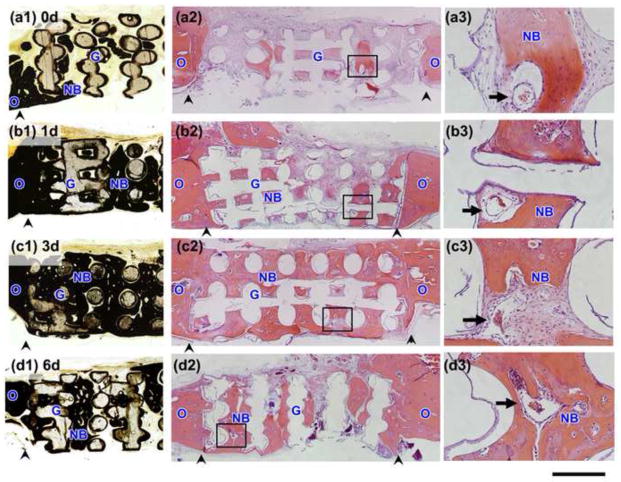
Von Kossa stained sections (a1–d1) and H&E stained sections (a2–d3) of rat calvarial defects implanted for 6 weeks with bioactive scaffolds as fabricated (0d) and pretreated for 1 day, 3 days, and 6 days in aqueous phosphate solution (1d; 3d; 6d; respectively). (a3)–(d3) are higher magnification images of the boxed areas in (a2)–(d2). Scale bar =1 mm for (a1–d2) and 200 μm for (a3–d3). G = bioactive glass; NB = new bone; O = old bone; arrows indicate blood vessels, arrowheads indicate the edges of old bone.
All implants showed the formation of new bone into the edges (periphery) of the implants (adjacent to the old bone), indicating good integration of the implants with the surrounding bone. New bone formation was observed mainly within the pores of the implants, and the amount of new bone formed was dependent on the pretreatment in the aqueous phosphate solution. Implants composed of the as-fabricated scaffolds showed a limited amount of new bone within the pores of the implants, predominantly in the form of “islands” (Fig. 5a1–a3). In comparison, the implants composed of the pretreated scaffolds, particularly the scaffolds pretreated for 3 days, showed a better capacity to support new bone formation (Fig. 5b1–d3). Blood vessels were observed within all of the implanted scaffolds in the defects (Fig. 5a3–d3).
Figure 6 shows H&E and von Kossa stained sections of the implants composed of scaffolds that were pretreated for 1day, 3 days, and 6 days, loaded with BMP-2 (1μg per defect), and implanted for 6 weeks in the rat calvarial defects. A considerable amount of new bone infiltrated the scaffolds and completely bridged the interface with old bone. When compared to the pretreated scaffolds described above (no BMP-2), these BMP-loaded implants showed a markedly larger amount of bone marrow-like tissue that was surrounded by new bone within the pore space of the implants (Fig. 6a3–c3).
Fig. 6.
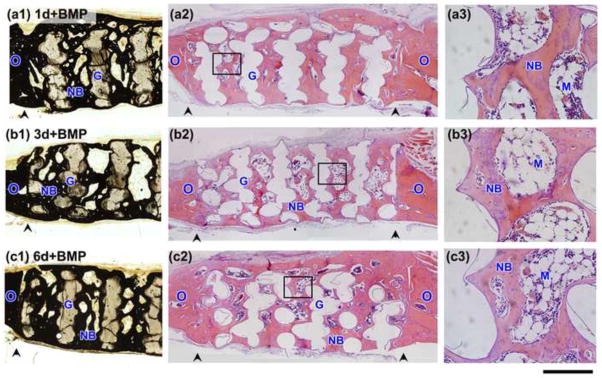
Von Kossa stained sections (a1–c1) and H&E stained sections (a2–c3) of rat calvarial defects implanted for 6 weeks with bioactive scaffolds pretreated for 1 day, 3 days, and 6 days in aqueous phosphate solution and loaded with BMP-2 (1 μg/defect) (denoted 1d + BMP; 3d+BMP; 6d+BMP, respectively). (a3)–(c3) are higher magnification images of the boxed areas in (a2)–(c2). Scale bar =1 mm for (a1–c2) and 200 μm for (a3–c3). G = bioactive glass; NB = new bone; O = old bone; M = bone marrow-like tissue, arrowheads indicate the edges of old bone.
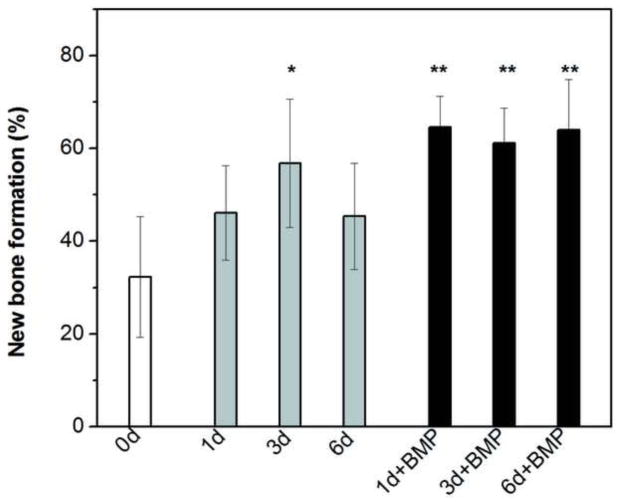
Since all the scaffolds had the same microstructure, the capacity of the scaffolds to regenerate bone was compared by normalizing the amount of new bone formed to the total pore space (area) of the scaffolds (Fig. 7, Table III). The amount of new bone formed in the as-fabricated scaffolds after the six-week implantation was 32 ± 13%. In comparison, the percent new bone formed in the scaffolds pretreated in the K2HPO4 solution for 1 day, 3 days, and 6 days was 46 ± 10%, 57 ± 14%, and 45 ± 11%, respectively. The amount of new bone formed in the scaffolds pretreated for 3 days was significantly higher than that in the as-fabricated scaffolds (p < 0.05). The amount of new bone in formed in the scaffolds pretreated for 1 day, 3 days, and 6 days and loaded with BMP-2 was 65 ± 7%, 61 ± 8%, and 64 ±11%, respectively; these values were significantly higher than the amount of new bone formed in the as-fabricated scaffolds and in the scaffolds pretreated for 1 and 3 days.
Fig. 7.
Percent new bone formed in rat calvarial defects implanted with scaffolds of 13-93 glass at 6 weeks postimplantation: as fabricated (0d); pretreated for 1 day, 3 days, and 6 days in aqueous phosphate solution (1d; 3d; 6d; respectively); pretreated 1 day, 3 days, and 6 days and loaded with BMP-2 (1 μg/defect) (1d+BMP; 3d+BMP; 6d+BMP, respectively). The new bone formed is shown as a percent of the available pore space in the scaffolds. (*significant difference compared to 0d; **significant difference compared to 0d, 1d, and 6d; p < 0.05).
Table III.
Amount of new bone, fibrous tissue, and bone marrow-like tissue formed in bioactive glass (13-93) scaffolds implanted for 6 weeks in rat calvarial defects. The amount of each tissue is expressed as a percent of the available pore space (area) in the scaffolds and the total defect area.
| Scaffold group | New bone (%)
|
Fibrous tissue (%)
|
Bone marrow-like tissue (%)
|
|||
|---|---|---|---|---|---|---|
| Available area | Total area | Available pore area | Total area | Available area | Total area | |
| As fabricated (0d) | 32 ± 13 | 18 ± 8 | 62 ± 14 | 34 ± 8 | 1 ± 1 | 1 ± 1 |
| 1d | 46 ± 10 | 25 ± 5 | 45 ± 12 | 25 ± 7 | 2 ± 1 | 1 ± 1 |
| 3d | 57 ± 14 | 33 ± 10 | 35 ± 13 | 19 ± 7 | 3 ± 2 | 2 ± 1 |
| 6d | 45 ± 11 | 26 ± 8 | 48 ± 13 | 28 ± 8 | 2 ± 1 | 1 ± 1 |
| 1d+BMP | 65 ± 7 | 38 ± 4 | 14 ± 12 | 8 ± 7 | 13 ± 6 | 8 ± 3 |
| 3d+BMP | 61± 8 | 35 ± 3 | 7 ± 7 | 4 ± 4 | 22 ± 8 | 12 ± 4 |
| 6d+BMP | 64 ± 11 | 38 ± 6 | 15 ± 19 | 10 ± 12 | 16 ± 8 | 10 ± 5 |
3.4 Assessment of bone marrow and fibrous tissue formation
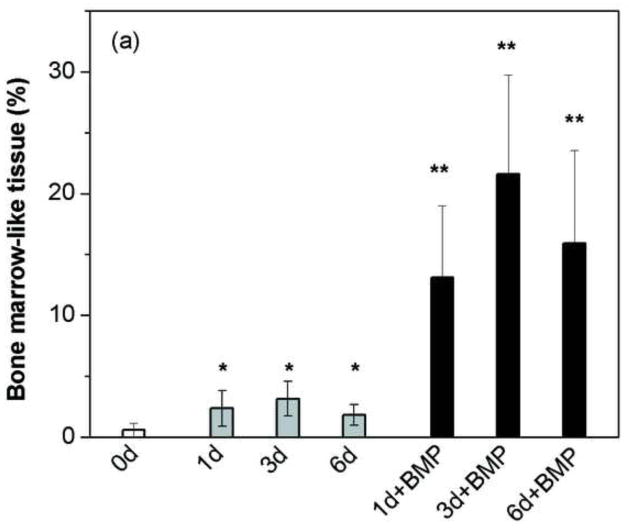
The amount of bone marrow-like tissue formed in the implants composed of the pretreated scaffolds loaded with BMP-2 was significantly higher than that in the scaffolds without BMP-2 (as-fabricated or pretreated in the phosphate solution) (Fig. 8a), but the amount of fibrous (soft) tissue was significantly lower (Fig. 8b). An interesting observation is that while the amount of new bone formed in the scaffolds pretreated for 3 days (57%) was not significantly different from that in the BMP-loaded scaffolds (61–65%), the type of tissue in the remainder of the pore space was very different. The remaining pore space in the scaffolds pretreated for 3 days was filled mainly with fibrous tissue whereas the remaining pore space in the BMP-loaded scaffolds was filled mainly with bone marrow-like tissue.
Fig. 8.
Percent bone marrow-like tissue (a) and fibrous tissue (b) formed in rat calvarial defects implanted with scaffolds of 13-93 glass at 6 weeks postimplantation: as fabricated (0d); pretreated for 1 day, 3 days, and 6 days in aqueous phosphate solution (1d; 3d; 6d; respectively); pretreated 1 day, 3 days, and 6 days and loaded with BMP-2 (1 μg/defect) (1d+BMP; 3d+BMP; 6d+BMP, respectively). (*significant difference compared to 0d; **significant difference compared to 0d, 1d, 3d and 6d; p < 0.05).
3.4 SEM evaluation of implants
Figure 9 shows back-scattered SEM images of the rat calvarial defects implanted with the bioactive glass scaffolds at 6 weeks. The contrast in the gray-scale images is an indication of differences in the calcium content [12]. The unconverted glass, the ACP/HA layer resulting from the pretreatment in the K2HPO4 solution and/or from the conversion of the glass in vivo, and new bone, all with a high calcium content, had a light-gray color, while the silica-rich layer formed in the early stage of the conversion of the glass was dark-gray. Lacunae within the bone, fibrous tissue, and bone marrow-like tissue were almost black. The glass filaments of the scaffolds consisted of three regions after implantation: an unconverted glass core (light-gray), a silica-rich layer (dark-gray) on the unconverted glass, and an ACP/HA surface layer (light-gray). The cracks in the scaffolds and the delamination of the ACP/HA layer from the scaffolds presumably resulted from capillary drying stresses during the sample preparation for SEM examination.
Fig. 9.
Back-scattered SEM images of rat calvarial defects implanted with bioactive glass scaffolds at 6 weeks postimplantation: (a), (b) as-fabricated scaffolds; (c), (d) scaffolds pretreated for 3 days in aqueous phosphate solution; (e), (f) scaffolds pretreated for 3 days in aqueous phosphate solution and loaded with BMP-2. (NB = new bone; G = bioactive glass). The approximate thickness of the HA surface layer on the pretreated scaffolds prior to implantation is shown in (d) and (f).
New bone formed during the six-week implantation did not appear to bond to the as-fabricated scaffolds (Fig. 9a, b). Instead, the new bone formed islands within the pores of the scaffolds and there were large gaps between the newly formed bone and the ACP/HA surface of the scaffold. In comparison, new bone appeared to bond firmly to the surface of the pretreated scaffolds and the pretreated scaffolds loaded with BMP-2 (Fig. 9c–f). The firm bonding to the surface of the pretreated scaffolds and the BMP-loaded scaffolds was found for all three pretreatment times (1 day, 3 days, and 6 days), but the images for the scaffolds pretreated for 1 day and 6 days are not included for the sake of brevity. Because of differences in new bone formation among scaffolds within the same group, the section in Fig. 9c (pretreated scaffold without BMP-2) showed a larger amount of new bone when compared to the section in Fig. 9e (pretreated scaffold with BMP-2). However, when all the sections within each group were considered, there was no significant difference in new bone formation between the two groups (Fig. 7).
For the scaffolds pretreated for 3 days in the K2HPO4 solution prior to implantation (without or with BMP-2) (Fig. 9d, f), the thickness of the HA layer (6 μm) after the six-week implantation was almost similar to the thickness (5 μm) prior to implantation (Table II). In comparison, the thickness of the HA layer formed on the as-fabricated scaffold during the six-week implantation was (20 μm), indicating that the conversion of the as-fabricated scaffold in vivo was much faster than that for the pretreated scaffold.
4. Discussion
Silicate 13-93 bioactive glass scaffolds similar to those used in this study were previously shown to have promising mechanical properties for the repair of loaded bone [11]. This study showed that pretreatment of those bioactive glass scaffolds in an aqueous phosphate solution to form an ACP or HA surface layer, or loading the pretreated scaffolds with BMP-2 markedly enhanced the capacity of the scaffolds to regenerate bone in an osseous defect. When compared to bioactive glass scaffolds with a variety of composition and microstructure reported in the literature, these pretreated or BMP-loaded scaffolds also showed the capacity to support faster bone regeneration in the same osseous defect model.
4.1 Bone regeneration in the as-fabricated bioactive glass scaffolds
The amount of new bone formed in the as-fabricated bioactive glass (13-93) scaffolds used in this study, determined as a fraction of the available pore area, was 32 ± 13% after the six-week implantation in the rat calvarial defects. For the same glass composition and in vivo model, the amount of new bone formed in scaffolds with an oriented microstructure (porosity = 50%; pore diameter = 100–150 μm) was 37 ± 8% at 12 weeks, while the amount of new bone formed in scaffolds with a trabecular microstructure (similar to dry human trabecular bone) (porosity = 80%; pore size = 100–500 μm) was 25 ± 12% at 12 weeks [12] (Table IV). Scaffolds with a fibrous microstructure, composed of thermally bonded short fibers (porosity = 50%; pore size = 50–500 μm), showed new bone formation equal to 8.5% of the total defect area at 12 weeks [32]. Since the porosity of the fibrous scaffolds was 50%, the amount of new bone estimated as a fraction of the pore area was 17%. These results indicate that the grid-like microstructure of the scaffolds used in this study has a better capacity to support bone regeneration when compared to the oriented, trabecular, and fibrous microstructures. The amount of new bone formed at 6 weeks in vivo was approximately the same or greater than that in the oriented, trabecular, or fibrous microstructure at for 12 weeks.
Table IV.
Comparison of new bone formed in scaffolds composed of silicate 13-93 glass with different microstructures after implantation in rat calvarial defects (4.0–4.6 mm in diameter × 1.5 mm). The amount of new bone is shown as a percent of the available pore space (area) in the scaffolds and the total defect area.
| Microstructure of scaffolds | Porosity (%) | Pore size (μm) | New bone (%)
|
Implantation time (weeks) | Reference | |
|---|---|---|---|---|---|---|
| Available pore area | Total area | |||||
| Grid-like | 47 | 300 × 300 × 150 | 32 ± 13 | 18 ± 8 | 6 | This study |
| Trabecular | 80 | 100–500 | 25 ± 12 | 19 ± 9 | 12 | [12] |
| Oriented | 50 | 100–150 | 37 ± 8 | 18 ± 3 | 12 | [12] |
| Fibrous | 50 | 50–500 | 17* | 8.5 ± 2 | 12 | [32] |
Estimated from the total defect area
The greater capacity of the grid-like microstructure to support bone infiltration may result from the uniform microstructure of interconnected pores with a favorable size. As described earlier, while interconnected pores of size 100 μm are recognized as the minimum requirement for supporting tissue ingrowth [14], pores of size ~300 μm or larger may be required for enhanced bone ingrowth and capillary formation [15]. In the grid-like microstructure used in this study, the pores are all interconnected, have the same size (width = 300 μm), and are not constricted at their necks. In comparison, the columnar pores in the oriented scaffolds had a diameter of only 100–150 μm and the interconnectivity between adjacent pores was limited [12], while the necks between adjacent pores in the trabecular and fibrous microstructures are commonly constructed. The greater capacity of the grid-like microstructure to support bone regeneration appeared to result from the better interconnectivity and uniformity of its pores and a pre size that is considered to be favorable for bone ingrowth.
The path of new bone infiltration into the grid-like scaffolds was also different from that in silicate 13-93 and borate bioactive glass scaffolds with the oriented and fibrous microstructures implanted in the same in vivo model. New bone infiltrated the grid-like scaffolds mainly from the edge (adjacent to old bone), indicating that new bone formation was mainly osteoconductive in nature, but some “islands” of new bone were also observed within the interior pores of the scaffold (Fig. 5a2). In comparison, while bone formation in the oriented and fibrous scaffolds was also mainly osteoconductive, new bone formed mainly on the dural side of the implant with little infiltration into the edge [12,]. New bone infiltration into trabecular 13-93 bioactive glass scaffolds was found predominantly at the periphery of the defect. Differences in the path of the new bone infiltration into the scaffolds appear to be dependent on the size and interconnectivity of the pores. Larger pores with better pore interconnectivity, such as those in the grid-like microstructure used in this study, appear to support greater bone infiltration from the edge of the scaffold.
4.2 Bone regeneration in pretreated bioactive glass scaffolds
Although the grid-like microstructure created in this study showed a greater capacity to support new bone formation than the trabecular, oriented or fibrous microstructure, the as-fabricated state of the glass may not be the most ideal condition for optimum bone regeneration. As-fabricated, bioactive glass scaffolds prepared by sintering melt-derived glass particles often have a dense smooth surface (Fig. 1) which provides a low surface area for adsorption of proteins and limits the amount of proteins that can be loaded into the scaffolds by adsorption. The surface of silicate bioactive glass such as 13-93, hydrated in aqueous media, commonly has a high density of negatively-charged silanol (Si–O−) groups which can form strong electrostatic interactions and hydrogen bonds with adsorbed proteins. Desorption of proteins from the glass surface can be difficult [33–35], and denaturing of the proteins can be significant [34,36]. In the present study, these problems associated with the as-fabricated glass were overcome by converting a thin surface layer of the glass to ACP or HA in an aqueous phosphate solution. The ACP/HA surface layer, with a high-surface-area mesoporous structure, could better support the adsorption and delivery of proteins such as BMP-2 [28, 37].
A remarkable finding of the present study was that when compared to the as-fabricated scaffolds, the surface-treated scaffolds significantly enhanced new bone formation without any additional osteogenic factors. New bone formation in the implanted scaffolds that had been pretreated for 3 days in the phosphate solution (57%) was approximately twice that in the as-fabricated scaffolds (32%), and the new bone almost completely bridged the defect within 6 weeks (Fig. 5). Apparently the pretreatment conferred superior osteoconductivity or osteoinductive-like properties to the scaffold, but the mechanism is at present unclear.
It is known that an HA surface layer with a chemical and structural similarity to the surface layer of the pretreated scaffold can also form on the as-fabricated bioactive glass scaffold upon implantation in vivo. However, a major difference is that while the HA surface layer is present immediately upon implantation of the pretreated scaffold, it takes some time before the HA layer is formed on the as-fabricated scaffold [4–6]. Thin-film XRD showed the formation of an HA surface layer on 13-93 glass within 7 days in SBF [24]. While the conversion is faster in vivo [4], a time period of a few days might still be required for the formation of the HA layer on the as-fabricated scaffold. Consequently, the pretreated scaffold might be able to play an active role at the defect site, by interacting with cells, tissues, and biomolecules such as endogeneous BMP-2, immediately upon implantation whereas the as-fabricated scaffold might not. Important initial interactions might not occur at the defect site implanted with the as-fabricated scaffolds, and it is possible that the outcome might be less successful later when the HA layer is formed. A dependence of the healing outcome on the intervention treatment time has also been observed in other studies [38, 39]. For example, the time at which exogenous BMP-2 is administered was shown to markedly influence bone healing at a fracture site [38]. Administration of BMP-2 at day 0 or at day 4 post-facture resulted in the augmentation of periosteal callus formation, bone mineral content, and superior biomechanical properties when compared to the administration of BMP-2 at a later time (day 8).
A possible interaction that could have an important influence on bone healing is that between the surface of the implant and the exogenous BMP-2 released by the local cells. Upon implantation, the surface of the as-fabricated bioactive glass reacts with the physiological fluid to initially form a silica-gel surface layer [4–6]. It is possible that this silica-gel layer could irreversibly denature proteins such as BMP-2, resulting in a loss of protein bioactivity [40]. A higher amount of the silica-gel, with a negative charge at the physiological pH, could lower the release of BMP-2 or increase the tendency for denaturing the BMP-2. The results for the BMP-2 release profile from the scaffolds in vitro (Fig. 3) appear to support this suggestion, but further studies are required to provide a clearer understanding. The scaffold pretreated for 6 days in the phosphate solution, showing little evidence for a silica-gel phase in the XRD pattern (Fig. 3), released a significantly larger amount of BMP-2 when compared to the scaffolds pretreated for 1 day or 3 days and the as-fabricated scaffold.
Another factor that could have an effect on the capacity of the scaffolds to support new bone formation in vivo is their degradation (or conversion) rate to HA (or ACP). The presence of the converted HA surface layer on the pretreated scaffolds lead to a reduction in their degradation rate and in the release of ions from the glass phase into the medium when compared to the as-fabricated scaffolds [26, 41]. SEM images of the pretreated implants showed little change in the thickness of the HA layer during the six-week implantation in vivo (Fig. 9b, c). In comparison, the thickness of HA layer on the as-fabricated implants grew to 20 μm (Fig. 9a). Degradation and conversion of the as-fabricated glass is initially rapid, resulting in a marked change in the local pH and osmolarity [26, 42, 43]. Cell viability could be adversely affected initially [44], and this could have an effect on subsequent cellular and tissue reactions, and eventually on eventually on bone formation.
4.3 Bone regeneration in the pretreated bioactive glass scaffolds loaded with BMP-2
Loading the pretreated scaffolds with BMP-2 (1 μg/defect) significantly enhanced new bone formation in the defects when compared to the as-fabricated scaffold (Fig. 7). The percent new bone formed in the BMP-loaded implants was not affected by their different surface treatments despite the large difference in the BMP-2 release profile in vitro between the scaffolds pretreated for 1 day and 3 days and the scaffolds pretreated for 6 days (Fig. 4b). While the release of BMP-2 from the scaffolds in vivo is expected to be different from that in vitro, it is possible that the amount of BMP-2 released from all three pretreated scaffolds might be above the threshold required to stimulate bone formation in vivo.
The amount of new bone formed in the BMP-loaded implants was not significantly greater than that in the implants pretreated for 3 days (no BMP-2) but it was significantly greater than that in implants pretreated for 1 day and 6 days (Fig. 7). Presumably because of the effectiveness of the three-day pretreatment alone in enhancing bone formation, little effect of the BMP-2 loading can actually be seen. Comparing the tissue formed in the defects, the new bone formed in the implants pretreated for 3 days appeared to be more lamellar, while the new bone in the BMP-loaded implants appeared to be more woven. Furthermore, there was significantly more bone marrow-like tissue and less fibrous tissue in the BMP-loaded implants when compared to pretreated implants (Fig. 8). Marrow-rich bone is known to be a typical outcome of BMP-2 induced bone growth [45–47], but the mechanism of formation is unclear. The lack of mature and hypertrophic cartilage-like tissue is an indication that the bone was formed by a membraneous mechanism.
The BMP-2 loading (1 μg/defect) used in this study was based on the results of previous studies reported in the literature for the use of BMP-2 in stimulating bone healing [48, 49]. While that amount of BMP-2 was effective for enhancing bone regeneration in the bioactive glass scaffolds used in this study, the optimum amount is not clear. In a previous study to evaluate the effect of BMP-2 loading on the capacity of three-dimensional poly (lactic-co-glycolic acid) (PLGA) scaffolds to regenerate bone in rat calvarial defects (5 mm in diameter), a BMP-2 dose greater than 120 ng/mm3 was required for bridging the defect after a six-week implantation period [50]. In comparison, the amount of BMP-2 used in this study (1 μg/defect) was equivalent to a loading of ~ 60 ng/mm3, which indicates that a much lower concentration of BMP-2 was required to bridge a similar defect using the pretreated bioactive glass scaffolds.
The BMP-loaded bioactive glass scaffolds appear to be more appropriate for treating larger critical sized bone defects. Nevertheless, based on the significant bone formation in the defects implanted with the pretreated scaffolds without BMP-2, it is also reasonable to consider the use of the pretreated scaffolds, particularly the scaffolds pretreated for 3 days, in some bone repair situations. Taking into account the mechanical properties of similar scaffolds observed in a recent study [11], these bioactive glass scaffolds could be considered for applications in loaded bone repair. The scaffolds are currently being evaluated for their capacity to repair segmental bone defects in animal models.
5. Conclusions
Scaffolds of 13-93 bioactive glass prepared with a grid-like microstructure by robocasting (porosity ~50%; filament width = 330 μm; pore width = 300 μm) showed the capacity to support the formation of new bone in rat calvarial defects. Pretreatment of the as-fabricated scaffolds for 3 days in a K2HPO4 solution (60 °C; pH = 12), to convert a thin surface layer (5 μm) of the glass to a high-surface-area hydroxyapatite or amorphous calcium phosphate material prior to implantation, significantly enhanced the capacity of the scaffolds to support new bone formation. Loading the pretreated scaffolds with BMP-2 (1 μg/defect) was also effective for enhancing new bone formation. The capacity of the BMP-loaded scaffolds to enhance bone regeneration was independent of the pretreatment time (1–6 days) in the K2HPO4 solution. Pretreatment of silicate bioactive glass scaffolds in an aqueous phosphate solution or loading the pretreated scaffolds with BMP-2 can provide an effective approach for enhancing the capacity of the scaffolds to support bone regeneration and integration in osseous defects. Taking into account the mechanical properties of similar scaffolds observed in a previous study, these 13-93 scaffolds, pretreated or loaded with BMP-2, are promising in structural bone repair.
Acknowledgments
This work was supported by the National Institutes of Health, National Institute of Arthritis, Musculoskeletal and Skin Diseases (NIAMS), Grant # 1R15AR056119-01. The authors thank Mo-Sci Corp., Rolla, MO for the bioactive glass used in this work.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cancedda R, Giannoni P, Mastrogiacomo M. A tissue engineering approach to bone repair in large animal models and in clinical practice. Biomaterials. 2007;28:4240–50. doi: 10.1016/j.biomaterials.2007.06.023. [DOI] [PubMed] [Google Scholar]
- 2.Giannoudis PV, Dinopoulos H, Tsiridis E. Bone substitutes: An update. Injury. 2005;36:S20–S7. doi: 10.1016/j.injury.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 3.Laurencin C, Khan Y, El-Amin SF. Bone graft substitutes. Expert Rev Med Devices. 2006;3:49–57. doi: 10.1586/17434440.3.1.49. [DOI] [PubMed] [Google Scholar]
- 4.Rahaman MN, Day DE, Bal BS, Fu Q, Jung SB, Bonewald LF. Bioactive glass in tissue engineering. Acta Biomater. 2011;7:2355–73. doi: 10.1016/j.actbio.2011.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hench LL. Bioceramics. J Am Ceram Soc. 1998;81:1705–28. [Google Scholar]
- 6.Hench LL. The story of Bioglass®. J Mater Sci Mater Med. 2006;17:967–78. doi: 10.1007/s10856-006-0432-z. [DOI] [PubMed] [Google Scholar]
- 7.Fu Q, Saiz E, Rahaman MN, Tomsia AP. Bioactive glass scaffolds for bone tissue engineering: state of the art and future perspectives. Mater Sci Eng C. 2011;31:1245–56. doi: 10.1016/j.msec.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang TS, Rahaman MN, Doiphode ND, Leu MC, Bal BS, Day DE, et al. Porous and strong bioactive glass (13–93) scaffolds fabricated by freeze extrusion technique. Mater Sci Eng C. 2011;31:1482–9. [Google Scholar]
- 9.Deliormanli AM, Rahaman MN. Direct-write assembly of silicate and borate bioactive glass scaffolds for bone repair. J Euro Ceram Soc. 2012;32:3637–46. [Google Scholar]
- 10.Fu Q, Saiz E, Tomsia AP. Direct ink writing of highly porous and strong glass scaffolds for load-bearing bone defects repair and regeneration. Acta Biomater. 2011;7:3547–54. doi: 10.1016/j.actbio.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X, Rahaman MN, Hilmas GE. Mechanical properties of bioactive glass (13-93) scaffolds fabricated by robotic deposition for structural bone repair. Acta Biomater. 2013 doi: 10.1016/j.actbio.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu X, Rahaman MN, Fu Q. Bone regeneration in strong porous bioactive glass (13-93) scaffolds with an oriented microstructure implanted in rat calvarial defects. Acta Biomater. 2013;9:4889–98. doi: 10.1016/j.actbio.2012.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Place ES, Evans ND, Stevens MM. Complexity in biomaterials for tissue engineering. Nat Mater. 2009;8:457–70. doi: 10.1038/nmat2441. [DOI] [PubMed] [Google Scholar]
- 14.Hulbert SF, Young FA, Mathews RS, Klawitter JJ, Talbert CD, Stelling FH. Potential of ceramic materials as permanently implantable skeletal prostheses. J Biomed Mater Res. 1970;4:433–56. doi: 10.1002/jbm.820040309. [DOI] [PubMed] [Google Scholar]
- 15.Karageorgiou V, Kaplan D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials. 2005;26:5474–91. doi: 10.1016/j.biomaterials.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Marques A, Jain H, Kiely C, Song K, Kiely C, Almeida R. Nano/macroporous monolithic scaffolds prepared by the sol–gel method. J Sol-Gel Sci Technol. 2009;51:42–7. [Google Scholar]
- 17.Moawad HMM, Jain H. Creation of nano–macro-interconnected porosity in a bioactive glass–ceramic by the melt-quench-heat-etch method. J Am Ceram Soc. 2007;90:1934–6. [Google Scholar]
- 18.Chen QZ, Rezwan K, Armitage D, Nazhat SN, Boccaccini AR. The surface functionalization of 45S5 Bioglass®-based glass-ceramic scaffolds and its impact on bioactivity. J Mater Sci Mater Med. 2006;17:979–87. doi: 10.1007/s10856-006-0433-y. [DOI] [PubMed] [Google Scholar]
- 19.Marion NW, Liang W, Reilly GC, Day DE, Rahaman MN, Mao JJ. Borate glass supports the in vitro osteogenic differentiation of human mesenchymal stem cells. Mech Adv Mater Struct. 2005;12:239–46. [Google Scholar]
- 20.Miguel BS, Kriauciunas R, Tosatti S, Ehrbar M, Ghayor C, Textor M, et al. Enhanced osteoblastic activity and bone regeneration using surface-modified porous bioactive glass scaffolds. J Biomed Mater Res A. 2010;94A:1023–33. doi: 10.1002/jbm.a.32773. [DOI] [PubMed] [Google Scholar]
- 21.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from the laboratory to the clinic, part I (basic concepts) J Tissue Eng Regen Med. 2008;2:1–13. doi: 10.1002/term.63. [DOI] [PubMed] [Google Scholar]
- 22.Bessa PC, Casal M, Reis RL. Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery) J Tissue Eng Regen Med. 2008;2:81–96. doi: 10.1002/term.74. [DOI] [PubMed] [Google Scholar]
- 23.Verne E, Vitale-Brovarone C, Bui E, Bianchi CL, Boccaccini AR. Surface functionalization of bioactive glasses. J Biomed Mater Res A. 2009;90:981–92. doi: 10.1002/jbm.a.32153. [DOI] [PubMed] [Google Scholar]
- 24.Fu Q, Rahaman MN, Brown RF, Bal BS, Day DE. Mechanical and in vitro performance of 13-93 bioactive glass scaffolds prepared by a polymer foam replication technique. Acta Biomater. 2008;4:1854–64. doi: 10.1016/j.actbio.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 25.Fu H, Rahaman MN, Day DE. Effect of process variables on the microstructure of hollow hydroxyapatite microspheres prepared by a glass conversion method. J Am Ceram Soc. 2010;93:3116–23. doi: 10.1111/j.1551-2916.2010.03833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yao A, Wang D, Huang W, Fu Q, Rahaman MN, Day DE. In vitro bioactive characteristics of borate-based glasses with controllable degradation behavior. J Am Ceram Soc. 2007;90:303–6. [Google Scholar]
- 27.Bunker BC. Molecular mechanisms for corrosion of silica and silicate glasses. J Non-Cryst Solids. 1994;179:300–8. [Google Scholar]
- 28.Fu H, Rahaman MN, Brown RF, Day DE. Evaluation of bone regeneration in implants composed of hollow HA microspheres loaded with transforming growth factor β1 in a rat calvarial defect model. Acta Biomater. 2013;9:5718–27. doi: 10.1016/j.actbio.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Montgomery DC. Design and analysis of experiments. 7. New York: Wiley; 2009. [Google Scholar]
- 30.Filgueiras MRT, La Torre G, Hench LL. Solution effects on the surface reactions of three bioactive glass compositions. J Biomed Mater Res. 1993;27:1485–93. doi: 10.1002/jbm.820271204. [DOI] [PubMed] [Google Scholar]
- 31.Martin RA, Twyman H, Qiu D, Knowles JC, Newport RJ. A study of the formation of amorphous calcium phosphate and hydroxyapatite on melt quenched Bioglass® using surface sensitive shallow angle X-ray diffraction. J Mater Sci Mater Med. 2009;20:883–8. doi: 10.1007/s10856-008-3661-5. [DOI] [PubMed] [Google Scholar]
- 32.Bi L, Jung S, Day D, Neidig K, Dusevich V, Eick D, et al. Evaluation of bone regeneration, angiogenesis, and hydroxyapatite conversion in critical-sized rat calvarial defects implanted with bioactive glass scaffolds. J Biomed Mater Res A. 2012;100A:3267–75. doi: 10.1002/jbm.a.34272. [DOI] [PubMed] [Google Scholar]
- 33.Messing RA. Molecular inclusions. Adsorption of macromolecules on porous glass membranes. J Am Chem Soc. 1969;91:2370–1. [Google Scholar]
- 34.Lobel KD, Hench LL. In vitro protein interactions with a bioactive gel–glass. J Sol-Gel Sci Tech. 1996;7:69–76. [Google Scholar]
- 35.Lobel KD, Hench LL. In vitro adsorption and activity of enzymes on reaction layers of bioactive glass substrates. J Biomed Mater Res. 1998;39:575–9. doi: 10.1002/(sici)1097-4636(19980315)39:4<575::aid-jbm11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 36.Snyder LR. Principles of Adsorption Chromatography. New York: Marcel Dekker; 1968. [Google Scholar]
- 37.Fu H, Rahaman MN, Day DE, Brown RF. Hollow hydroxyapatite microspheres as a device for controlled delivery of proteins. J Mater Sci Mater Med. 2011;22:579–91. doi: 10.1007/s10856-011-4250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murnaghan M, McIlmurray L, Mushipe MT, Li G. Time for treating bone fracture using rhBMP-2: a randomised placebo controlled mouse fracture trial. J Orthop Res. 2005;23:625–31. doi: 10.1016/j.orthres.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Chao EY, Inoue N. Biophysical stimulation of bone fracture repair, regeneration and remodelling. Eur Cell Mater. 2003;6:72–85. doi: 10.22203/ecm.v006a07. [DOI] [PubMed] [Google Scholar]
- 40.Messing RA. Insoluble papain prepared by adsorption on porous glass. Enzymologia. 1970;38:39–42. [PubMed] [Google Scholar]
- 41.Brown RF, Rahaman MN, Dwilewicz AB, Huang W, Day DE, Li Y, Bal BS. Conversion of borate glass to hydroxyapatite and its effect on proliferation of MC3T3-E1 cells. J Biomed Mater Res Part A. 2009;88A:392–400. doi: 10.1002/jbm.a.31679. [DOI] [PubMed] [Google Scholar]
- 42.Huang W, Day DE, Kittiratanapiboon K, Rahaman MN. Kinetics and mechanisms of the conversion of silicate (45S5), borate, and borosilicate glasses to hydroxyapatite in dilute phosphate solutions. J Mater Sci Mater Med. 2006;17:583–9. doi: 10.1007/s10856-006-9220-z. [DOI] [PubMed] [Google Scholar]
- 43.Fu Q, Rahaman MN, Fu H, Liu X. Silicate, borosilicate, and borate bioactive glass scaffolds with controllable degradation rates for bone tissue engineering applications, I: preparation and in vitro degradation. J Biomed Mater Res Part A. 2010;95A:164–71. doi: 10.1002/jbm.a.32824. [DOI] [PubMed] [Google Scholar]
- 44.Mather JP, Roberts PE. Introduction to cell and tissue culture: theory and technique. New York: Plenum Press; 1998. [Google Scholar]
- 45.Hayashi C, Hasegawa U, Saita Y, Hemmi H, Hayata T, Nakashima K, et al. Osteoblastic bone formation is induced by using nanogel-crosslinking hydrogel as novel scaffold for bone growth factor. J Cell Physiol. 2009;220:1–7. doi: 10.1002/jcp.21760. [DOI] [PubMed] [Google Scholar]
- 46.Hong S-J, Kim C-S, Han D-K, Cho I-H, Jung U-W, Choi S-H, et al. The effect of a fibrin-fibronectin/β-tricalcium phosphate/recombinant human bone morphogenetic protein-2 system on bone formation in rat calvarial defects. Biomaterials. 2006;27:3810–6. doi: 10.1016/j.biomaterials.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 47.Chung Y-I, Ahn K-M, Jeon S-H, Lee S-Y, Lee J-H, Tae G. Enhanced bone regeneration with BMP-2 loaded functional nanoparticle–hydrogel complex. J Control Release. 2007;121:91–9. doi: 10.1016/j.jconrel.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 48.Lee J-H, Kim C-S, Choi K-H, Jung U-W, Yun J-H, Choi S-H, et al. The induction of bone formation in rat calvarial defects and subcutaneous tissues by recombinant human BMP-2, produced in Escherichia coli. Biomaterials. 2010;31:3512–9. doi: 10.1016/j.biomaterials.2010.01.075. [DOI] [PubMed] [Google Scholar]
- 49.Marden LJ, Hollinger JO, Chaudhari A, Turek T, Schaub RG, Ron E. Recombinant human bone morphogenetic protein-2 is superior to demineralized bone matrix in repairing craniotomy defects in rats. J Biomed Mater Res A. 1994;28:1127–38. doi: 10.1002/jbm.820281003. [DOI] [PubMed] [Google Scholar]
- 50.Cowan CM, Aghaloo T, Chou YF, Walder B, Zhang X, Soo C, et al. MicroCT evaluation of three-dimensional mineralization in response to BMP-2 doses in vitro and in critical sized rat calvarial defects. Tissue Eng. 2007;13:501–12. doi: 10.1089/ten.2006.0141. [DOI] [PubMed] [Google Scholar]