Abstract
Purpose
Chemotherapy-induced peripheral neuropathy is a major complication in the treatment of cancer, including multiple myeloma (MM). Patients may develop painful and nonpainful (e.g., numbness) neuropathy symptoms that impair function and often persist after therapy is terminated. This study tested the hypothesis that baseline subclinical neuropathy, as assessed by sensory thresholds, is related to the development of neuropathy symptoms (e.g., pain and numbness) in patients with MM undergoing treatment with chemotherapy.
Methods
Patients (N=56) who had undergone 2 or fewer cycles of induction therapy and who had no evident neuropathy were assessed using quantitative sensory tests to determine multiple-modality sensory thresholds. Patient-reported pain and numbness were assessed through induction therapy (16 weeks) via the MD Anderson Symptom Inventory. A subset of participants (n=15) continued reporting on their symptoms for an additional 16 weeks (“maintenance phase”).
Results
Patients with sharpness-detection deficits at baseline (n=11, 20% of sample) reported less-severe pain and numbness during induction therapy and less numbness during maintenance therapy (P<0.05). During the maintenance phase, patients with warmth-detection deficits (n=5, 38% of sample) reported more-severe pain and numbness, and those with skin-temperature deficits (n=7, 47% of maintenance sample) reported more-severe pain (P<0.05). These deficits were related to patient-reported difficulty walking, a common symptom of peripheral neuropathy.
Conclusions
Our results suggest that baseline subclinical sensory deficits may be related to a patient's risk for developing chemotherapy-induced peripheral neuropathy.
Keywords: bortezomib, peripheral neuropathy, quantitative sensory testing, patient reported outcome, multiple myeloma
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a common treatment-related toxicity affecting cancer patients, including patients with multiple myeloma (MM) [1,2]. CIPN includes painful symptoms (e.g., burning pain) and nonpainful symptoms (e.g., numbness, tingling) [3,4] that may result in distress, functional impairment, and treatment limitations (e.g., dose reductions, treatment agent changes). Agents known to improve MM outcomes, such as bortezomib and thalidomide, have also been associated with high rates of CIPN [5-7]. Although the mechanisms by which these agents induce neuropathy are unclear, the literature suggests that they can significantly reduce peripheral nerve-fiber density and function [8-10].
When patients with MM become symptomatic from their disease, induction therapy is generally initiated. During induction therapy, grade 2 or higher CIPN (based on the National Cancer Institute Common Terminology Criteria (NCI-CTC)) develops in approximately 30% of patients [2,11]. Once neuropathy develops, it may persist after treatment termination [8,12]. Additionally, because maintenance chemotherapy may improve outcomes [13-15], patients often undergo treatment for long durations and receive high cumulative doses.
Although a few genetic markers [16,17], comorbidities (e.g., diabetes) [18], and demographic characteristics (e.g., age) [19] are associated with higher CIPN rates in patients with MM, no method for determining an individual's risk has been established. Therefore, developing methods to determine a patient's risk of developing CIPN would be a significant advance. Increasing evidence suggests that patients with pretreatment neuropathy are at increased risk of developing CIPN [20-23]; however, treatment-related neuropathy symptoms also develop in patients without obvious baseline neuropathy. Recently, nerve conduction [23] and quantitative sensory testing (QST) [24,25] studies have detected subclinical sensory deficits in a surprisingly large percentage of patients with colorectal cancer or multiple myeloma prior to any therapy.
This study is, to the best of our knowledge, the first to explore the hypothesis that subclinical sensory deficits, detected by QST, are related to the development of CIPN symptoms, as reflected in patient report of pain and numbness. QST is a well-validated noninvasive battery of sensory-threshold tests (Table 1) designed to evaluate loss and gain of function in nerve fibers [26-28]. Specifically, this study classified patients as having a deficit or no deficit on these sensory threshold tests based upon data collected from healthy controls. The trajectory of patient reported pain and numbness, as assessed by the M.D. Anderson Symptom Inventory (MDASI), was evaluated by deficit category during induction therapy (week 0 to 16) and, in a subset of patients, during maintenance therapy (week 17 to 32).
Table 1.
Description of the QST measures
| Measure | Tool | Range | Primary Nerve Fibers Assessed |
|---|---|---|---|
| Touch detection threshold | Von Frey monofilaments | 0.008–300 grams | Aβ |
| Sharpness detection threshold | Blunted needle | 8–128 grams | Aδ |
| Thermal detection thresholds | Pelteir thermode, Medoc | 3.0–51. 5°C | Aδ and C |
| Manual dexterity | Grooved pegboard test | ≤300 seconds | Aβ |
| Skin temperature | Infrared thermistor | °C | – |
QST Quantitative Sensory Testing
Patients and Methods
Patients
Patients were recruited from the MM clinic at The University of Texas MD Anderson Cancer Center. Inclusion criteria included: 2 or fewer cycles of induction therapy at enrollment (baseline), no evident preexisting neuropathy (NCI-CTC score of 0), at least 18 years of age, and ability to use the telephone-based interactive voice response (IVR) system. The IVR system allows patients to rate their symptoms using a telephone keypad. Patients were monitored for up to 32 weeks. Control participants were recruited from institutional faculty and staff. All participants provided written informed consent. Procedures were approved by the MD Anderson Institutional Review Board.
Quantitative sensory testing
QST has been shown to be a sensitive assessment of neuropathic pain as well as subclinical sensory deficits [25-28]. Furthermore it has been shown to have good re-test reliability and, when administered by a trained examiner, good inter-observer reliability [26,27]. In our study, QST was conducted by a trained research coordinator at baseline and at the start of each cycle (approximately every 4 weeks), at 3 body locations: the tip of the index finger, thenar eminence, and volar surface of the forearm on the dominant limb. These sites were chosen based of the stocking-and-glove distribution of chronic bortezomib-related CIPN, wherein pain, numbness, and tingling are commonly reported in the glabrous skin of the hands and feet, but not in more-proximal hairy skin [8,29].
Touch-detection threshold was assessed using von Frey monofilaments in an up-down manner [8,30,31]. While the participant looked away from the testing site, filaments were applied until they bent slightly for 1 second. When the participant did not report detection of the stimulus, the next-higher-force filament was applied. Conversely, when the participant detected the stimulus, the next lower force filament was applied. The force produced by the first filament a patient reported detecting three times was recorded as their threshold.
Sharpness-detection threshold was assessed by applying force to the skin using a blunted 30-gauge needle with pressure from weighted metal cylinders [8,30-32]. The needle was applied for 1 second while patients looked away from the testing site. Participants were instructed to describe each stimulus as touch, pressure, sharp, or pain. The weighted stimuli were presented in ascending order until the participant reported a sharp or painful sensation, at which point that weight was recorded. The threshold was defined as the average of three trials.
Skin temperature for each test site was determined through use of an infrared thermistor placed gently against the skin. Thermal thresholds were then assessed using a Peltier thermode (Medoc, Inc., Haifa, Israel). To assess a participant's warmth and hot detection thresholds, the skin was heated from 32°C (baseline temperature) at a rate of 0.3°C/second, with a cutoff of 51.5°C. To assess cool and cold detection thresholds, the skin was cooled from 32°C at a rate of -0.5°C/second, with a cutoff of 3°C. For both temperature sets, participants reported when they first perceived a temperature change (warm or cool) and when the change became painful (hot or cold). The thermode returned to 32°C when pain was reported. If a participant failed to report pain prior to the cutoff, the temperature was held for 10 seconds before it returned to baseline, and the cutoff temperature was recorded. The threshold was calculated by averaging 3 trials.
Manual dexterity was assessed by means of the grooved pegboard test [33]. Participants filled a 5-by-5 slotted pegboard in order across rows, first with their dominant hand and then with their nondominant hand. The time to complete the task was recorded. If the task was not completed within 5 minutes, this cutoff time was recorded.
Patients also completed a set of questionnaires at each QST session. Any areas of pain or sensory disturbance were drawn on a standardized body map. Those reporting sensory symptoms in the hands or feet rated their current and daily maximum pain on a visual analogue scale (VAS) with prompts ranging from “no pain” to “most imaginable.” Finally, sensory disturbances in the hands and feet were characterized by selecting from a standardized word descriptor list [8,29].
Patient-reported pain and numbness
Patient-reported pain and numbness were assessed using the multiply myeloma module of the MDASI, a multiple-symptom questionnaire sensitive to symptoms of disease and treatment [34,35]. The MM module of the MDASI has been shown to be valid, reliable method to assess disease and treatment-related symptom burden in patients with MM [35]. Within this scale patients rated the intensity of 13 core symptom items, 7 MM-specific symptom items, and 6 items of symptom interference with functioning (e.g., difficulty walking) within the past 24 hours on a 0 (“not present”) to 10 (“as bad as you can imagine”) scale. The MDASI can be completed within 5 minutes. The IVR system, which automatically called at patient-designated times, was the primary response mechanism. If the system failed to reach a patient, the research coordinator contacted the patient by phone or during a clinic visit. MDASI data were collected twice weekly during the first 12 weeks and once a week thereafter.
Data analysis
Patients were classified as “deficit” or “no deficit” for each test on their of baseline QST data. A detection threshold >2 standard deviations (SD) from control mean was considered a deficit for that modality. Patient-reported pain and numbness data were divided into induction (weeks 0-16) and maintenance (weeks 17-32) phases. To avoid potential confounds from autologous stem cell transplantation (ASCT), patients were dropped from induction-phase analysis when they began stem cell mobilization, and those who entered ASCT during the study were excluded from maintenance-phase analyses. The maintenance phase cohort included patients who continued on chemotherapy or watchful waiting after induction; limited sample size and changing treatment strategies prevented subdividing this group. Data are presented as means ± standard error. P-values less than 0.05 were considered significant, except when otherwise noted.
To determine whether QST deficits were associated with patient-reported MDASI pain and numbness during induction therapy and the maintenance phase, linear mixed models (LMM) were fit. These models controlled for time, age, diabetes, bortezomib use, and the number of therapy cycles. If a “deficit” or “no deficit” group contained fewer than 20% of study patients, the analysis was not conducted. After exclusions, there were 6 variables to assess during each phase; therefore, a significance level of 0.008 was set. Because difficulty walking is a significant functional issue associated with CIPN, we also fit LMM to determine if deficits associated with pain and numbness were associated with MDASI-reported difficulty walking.
As the MDASI assesses general pain and numbness, these scores may be influenced by non-neuropathy-related pain (e.g., bone pain). To verify that pain and numbness assessed by MDASI corresponded to sensory disturbances in areas expected to be affected by CIPN, we evaluated patient-reported data focused on the hands and feet that was collected during QST follow-up. Patients who reported moderate to severe pain (VAS score 4 on a 0 to 10 scale)[36] at least once were classified as having a “painful sensory disturbance,” whereas patients who reported numbness and tingling during more than 25% of QST sessions were classified as having a “nonpainful sensory disturbance.” Multivariate binary logistic regression models were conducted to determine if baseline QST deficits were independent prognostic factors for the development of sensory disturbances. Variables shown to be significant during univariate analyses were included.
Results
Patients
Patient characteristics are presented in Table 2. This study included data from 56 patients with an average age of 63.7 ± 1.5 years (range 36–86 years). All patients had a primary diagnosis of MM and most (89.3%) were receiving bortezomib-based induction therapy. One patient was on single-agent bortezomib, 18 were on bortezomib plus dexamethasone, and 6 were on lenolidomide plus dexamethasone. The remaining 31 patients were on bortezomib, dexamethasone, and other agents, such as: lenalidomide (n=16), cyclophosphamide (n=6), thalidomide (n=2), doxorubicin (n=2), melphalan (n=2), and vorinostat (n=2). A subset (n=15 patients) continued to the maintenance cohort. Control data were collected from 24 healthy volunteers, 14 men and 10 women, with an average age of 50.8 ± 2.8 years (range 31–83 years).
Table 2.
Patient demographics (N=56)
| Characteristic | n | (%) |
|---|---|---|
| Age – years, mean (SD) | 63.7 (11.6) | |
| Gender | ||
| Male | 34 | (60.7) |
| Female | 22 | (39.3) |
| Race | ||
| White | 41 | (73.2) |
| Other | 15 | (26.8) |
| Diabetes | ||
| Yes | 47 | (83.9) |
| No | 9 | (16.1) |
| Marital status | ||
| Married | 43 | (76.8) |
| Not married | 13 | (23.2) |
| Education level | ||
| High school or less | 11 | (19.6) |
| Greater than high school | 45 | (80.4) |
| Chemotherapy regimen | ||
| Bortezomib-based | 50 | (89.3) |
| No bortezomib | 6 | (10.7) |
| Chemotherapy cycles at baseline | ||
| <1 cycle | 13 | (23.2) |
| 1-2 cycles | 43 | (76.8) |
| Total chemotherapy cycles | ||
| <5 cycles | 41 | (73.2) |
| 5 cycles | 15 | (26.8) |
SD Standard Deviation
Sensory deficits predict MDASI pain and numbness
The LMM analyses revealed that, during induction, higher levels of MDASI pain were reported by patients with diabetes than by those without diabetes (3.3 ± 0.5 vs 1.9 ± 0.3, P=0.006) and by patients treated with bortezomib than by those treated without bortezomib (3.4 ± 0.26 v 1.8 ± 0.62, P=0.016). A review of clinic notes indicated that 19.6% of patients in this study (n=11) had a break from chemotherapy, dose reduction, or change in treatment due to developing neuropathy. Therefore, it is not surprising that patients who received more cycles of therapy (>4 cycles) reported less numbness during induction. In our sample, age was not significantly related to the development of pain or numbness.
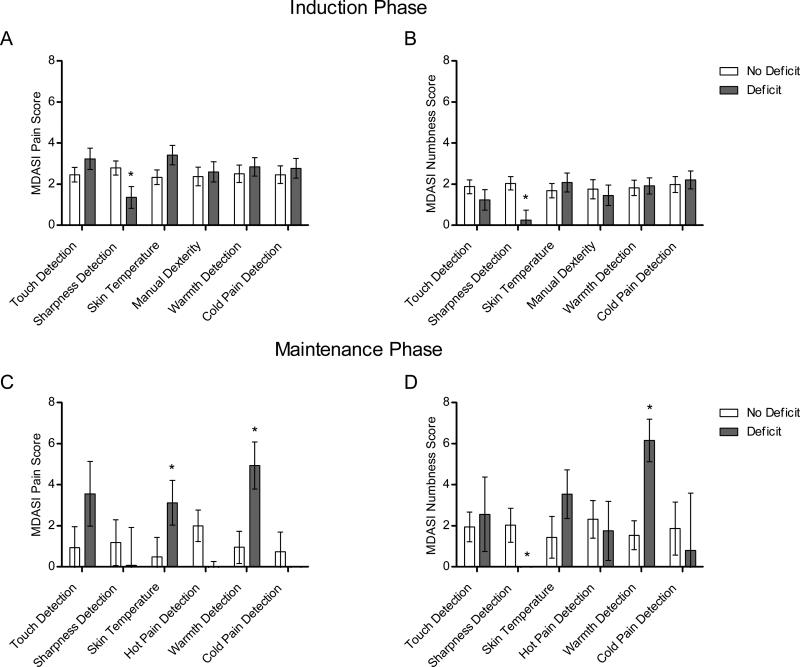
Baseline QST deficits were observed in at least 20% of patients for all measures except hot (6.8%) and cool (7.5%) detection. The LMM results during induction therapy are summarized in Figure 1A and 1B. The only variable that exhibited a significant effect (p < 0.008) during induction therapy was sharpness detection. Specifically, baseline sharpness-detection deficits were negatively associated with MDASI pain (P=0.004) and numbness (P< 0.001), such that patients with normal sharpness-detection thresholds (n=44, 80.0%) reported more severe pain (2.8 ± 0.3 vs 1.4 ± 0.5; Figure 2A) and numbness (2.0 ± 0.3 vs 0.2 ± 0.5; Figure 3A). There was a nonsignificant trend (P=0.01) for skin temperature, suggesting that patients with lower-than-normal skin temperature (n =17, 30.9%) may have more-severe pain than patients without skin-temperature deficits (Figure 2E).
Figure 1.
Patient-reported pain and numbness by QST baseline deficit category. A, Pain reported during induction therapy. B, Numbness reported during induction therapy. C, Pain reported during maintenance therapy. D, Numbness reported during maintenance therapy. *Asterisks indicate P < 0.008.
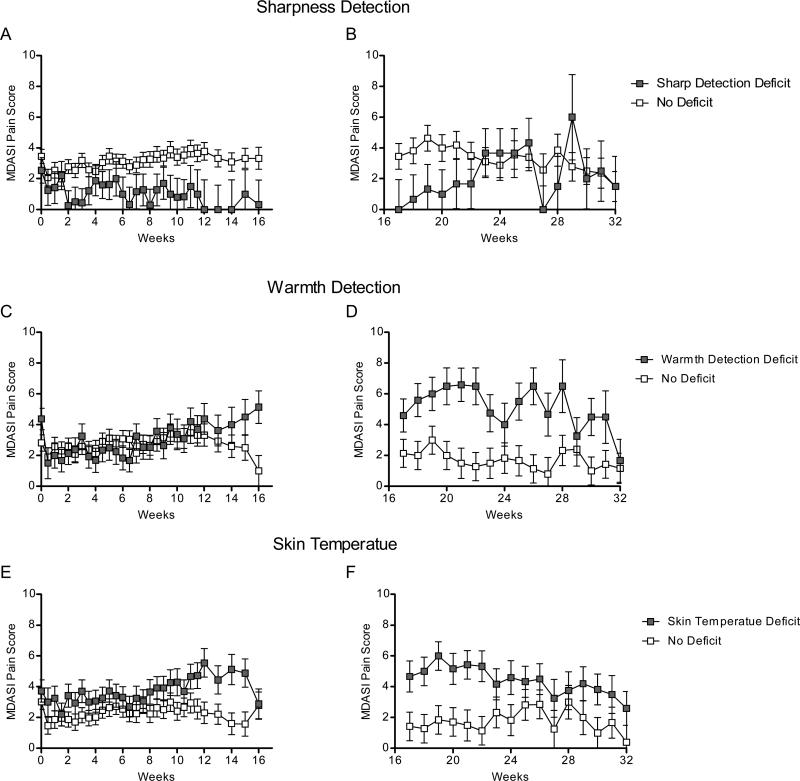
Figure 2.
Pain reported in: A, patients with (n=11) and without (n=44) sharpness detection deficits during induction therapy ; B, patients with (n=3) and without (n=12) sharpness detection deficits during maintenance therapy; C, patients with (n=20) and without (n=30) warmth detection deficits during induction therapy ; D, patients with (n=5) and without (n=8) warmth detection deficits during maintenance therapy ; E, patients with (n=17) and without (n=38) skin temperature deficits during induction therapy ; and F, patients with (n=7) and without (n=8) skin temperature deficits during maintenance therapy.
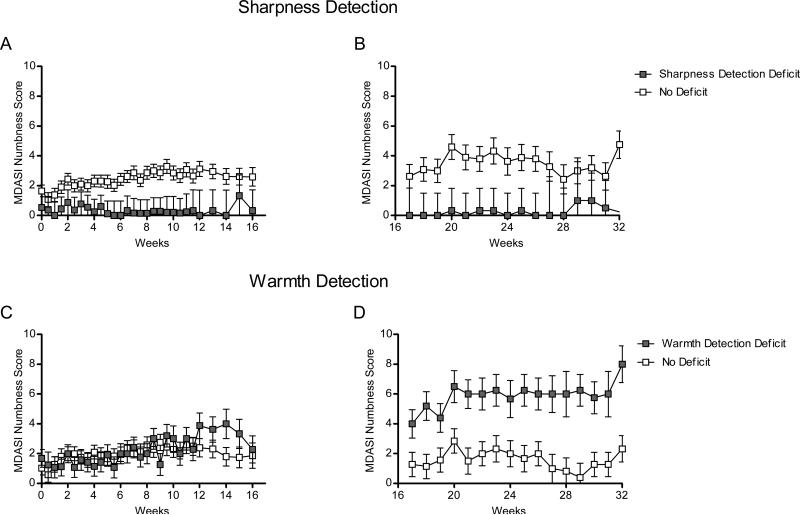
Figure 3.
Numbness reported in: A, patients with (n=11) and without (n=44) sharpness detection deficits during induction therapy; B, patients with (n=3) and without (n=12) sharpness detection deficits during maintenance therapy; C, patients with (n=5) and without (n=8) warmth detection deficits during maintenance therapy; D, patients with (n=5) and without (n=8) warmth detection deficits during maintenance therapy.
During the maintenance phase (Figure 1C and 1D), skin temperature (P=0.005; Figure 2F) and warmth-detection (P<0.001; Figure 2D) deficits were associated with patient-reported MDASI pain. Patients with skin-temperature deficits (n =7, 47% of maintenance sample) reported more-severe pain than did patients with normal skin temperature (3.1 ± 1.1 vs 0.5 ± 0.9), and patients with warmth-detection deficits (n =5, 38% of sample) reported more-severe pain than did patients without this deficit (4.9 ± 1.1 vs 1.0 ± 0.8). Warmth-detection deficits were associated with more-severe numbness (6.2 ± 1.0 vs 1.6 ± 0.7, P<0.001; Figure 3D), whereas sharpness-detection deficits were associated with less-severe numbness (P<0.001; Figure 3B).
Given that deficits in sharpness and warmth detection were associated with both pain and numbness, we sought to determine if their combination would provide more information. Although the difference between sharpness-detection groups emerged within a few weeks, the difference between warmth-detection groups did not emerge until approximately week 12 (Figure 2C and 3C). Therefore, we examined the interaction between sharpness-detection and warmth-detection deficits from weeks 12 to 32 using LMM. A significant interaction between baseline sharpness-detection and warmth-detection deficits was observed for pain (P=0.002) and numbness (P=0.003). Specifically, patients with sharpness-detection deficits but without warmth-detection deficits (n =4) reported virtually no pain (0.0 ± 0.50) or numbness (0.0 ± 1.29), whereas patients with warmth-detection deficits but without sharpness-detection deficits (n =8) reported, on average, moderate [36] pain (4.73 ± 0.23) and numbness (5.06 ± 0.46).
Sensory deficits predict sensory disturbances in hands and feet
As MDASI pain and numbness items are not necessarily specific to neuropathy, these data was corroborated by analyzing patient reported data specific to the anticipated site of neuropathy (i.e., hands and feet) collected during follow-up QST testing (Table 3). Multivariate models, which included only items shown to be significant during univariate analyses, for painful and nonpainful sensory disturbances were conducted. For painful sensory disturbances (n=12), baseline warmth-detection and skin-temperature deficits were included, and for nonpainful sensory disturbances (n=26) baseline sharpness-detection and warmth-detection deficits were included. Results indicated warmth-detection (OR: 4.9, P=0.036) and skin-temperature (OR: 5.0, P=0.031) deficits were independent prognostic factors for the development of painful sensory disturbances. Additionally, sharpness-detection (OR: 0.1, P=0.032) and warmth-detection (OR: 4.8, P=0.021) deficits were independent prognostic factors for the development of nonpainful sensory disturbances.
Table 3.
Results of binary logistic regression analyses to determine QST variables predictive of painful and nonpainful sensory disturbances in hands and feet
| Painful sensory disturbance | Nonpainful sensory disturbance | |||||
|---|---|---|---|---|---|---|
| n=12 (21.4%) | n=26 (46.4%) | |||||
| Odds Ratio | 95% Confidence Interval | P | Odds Ratio | 95% Confidence Interval | P | |
| Univariate analyses | ||||||
| Touch | 2.1 | 0.6-8.1 | 0.263 | 1.7 | 0.5-5.6 | 0.354 |
| Skin temperature | 4.2 | 1.1- 16.0 | 0.035 | 2.4 | 0.8-7.6 | 0.134 |
| Sharpness | 0.0 | -- | 0.999 | 0.1 | 0.0 to 0.7 | 0.021 |
| Hot pain | 3.3 | 0.4-26.9 | 0.275 | 3.8 | 0.4-40.3 | 0.268 |
| Warmth | 4.5 | 1.3-17.9 | 0.033 | 3.4 | 1.0-11.0 | 0.043 |
| Coolness | 1.9 | 0.3-13.1 | 0.507 | 3.3 | 0.5-20.9 | 0.210 |
| Cold pain | 0.7 | 0.1-2.9 | 0.590 | 0.9 | 0.3-3.2 | 0.927 |
| Manual dexterity | 1.1 | 0.3-4.6 | 0.918 | 0.9 | 0.3-2.7 | 0.811 |
| Multivariate analyses | ||||||
| Sharpness | -- | -- | -- | 0.1 | 0.0-0.8 | 0.032 |
| Warmth | 4.9 | 1.1-21.3 | 0.036 | 4.8 | 1.3-18.3 | 0.021 |
| Skin temperature | 5.0 | 1.2-22.0 | 0.031 | -- | -- | -- |
QST Quantitative Sensory Testing
Sensory deficits predict difficulty walking
Given that neuropathy can result in significant difficulty walking, we sought to determine if variables that impact pain and numbness also affect patient reported difficulty walking as assessed by the MDASI. Patients with sharpness-detection deficits reported less interference with walking than did patients without sharpness-detection deficits during induction (1.5 ± -0.16 vs 3.7 ± 0.11, P=0.038), but not during maintenance (1.9 ± 0.37 vs 4.5 ± 0.25, P=0.144). More-severe interference with walking was reported during maintenance by patients with skin-temperature deficits (5.1 ± 0.35 vs 2.8 ± 0.20, P=0.009) and warmth-detection deficits (6.9 ± 0.94 vs 2.4 ± 0.22, P<0.001) than by those without.
Discussion
On the basis of literature suggesting preexisting neuropathy increases the risk for CIPN [20-23], we hypothesized that subclinical peripheral sensory deficits may be sensitive indicators of CIPN risk. Prior to the start of chemotherapy a significant proportion of patients in our study displayed subclinical peripheral sensory deficits [24]. These deficits are likely related to disease-mediated factors (e.g., amyloidosis, compression injuries, autoimmune mechanism) [37,38]. Our results suggest that baseline peripheral sensory deficits detected through objective QST measures may indicate increased risk for the development of CIPN, as assessed by patient-reported pain and numbness. This important potential insight will no doubt be the focus of follow-up work given the limited sample size in this study. Nevertheless, this work could have fundamental implications for the care a broad range of cancer patients in that the CIPN is a major side effect associated with all the frontline chemotherapy drugs used to treat the most common types of cancers.
Within our study, a subset of QST measures was associated with the development of pain and numbness. Specifically, patients with impaired sharpness-detection reported less pain and numbness during induction and less numbness during the maintenance phase than did patients without sharpness-detection deficits. Further, patients with impaired warmth-detection were more likely to report pain and numbness during the maintenance phase than were patients without warmth-detection deficits. Additionally, those who had skin-temperature deficits were more likely to report pain during the maintenance phase than were patients without temperature deficits. These deficits were also related to patient-reported symptom interference with walking. The independent predictive value of these deficits was corroborated by questionnaires focused on body areas sensitive to the development of CIPN (i.e., hands and feet).
The association between sharpness-detection deficits and neuropathy symptoms emerged within the first few weeks. However, the association between warmth-detection and skin-temperature deficits and neuropathy symptoms emerged after several cycles of therapy. Using sharpness-detection and warmth-detection deficits together, we established low-risk and high-risk CIPN profiles. This suggests that combining QST performance data may allow for a more-accurate assessment of an individual's risk.
Given that temperature-detection and sharpness-detection deficits are mediated by small peripheral nerve fibers, our data suggest that subclinical small-fiber (C and A ) neuropathy may predispose individuals to CIPN. Both C and A fibers can respond to thermal stimuli; however, the warmth-detection thresholds within this study were below the detection threshold for the type-1 A fibers found in glabrous skin [39]. Therefore, our findings suggest that baseline C-fiber function may be associated with development of pain and numbness. A fibers are associated with sharpness detection [40]. Our data suggests that patients with sharpness-detection deficits were at lower risk for developing CIPN. As impairment in A function has previously been observed within areas of ongoing pain in bortezomib-treated patients [29], the mechanism underlying this potential effect is unclear and requires further investigation. One possibility is that patients with normal baseline sharpness detection enter therapy with more A fibers than do patients with deficits; once therapy is initiated, treatment-related toxicity would presumably affect greater number of fibers in these normal patients, resulting in increased pain. It is also possible that tests for sharpness detection uniquely identify a more-stoic patient population who are less willing to report sharp pain during QST testing or pain and numbness during treatment.
This study is an initial exploratory study and, as such, has several limitations, particularly the small sample size and paucity of treatment-naïve patients at baseline. A multicenter study consisting of large, treatment naïve population and an assessment of baseline fiber density is needed to validate this predictive model and to determine if these results are related to subclinical small-fiber neuropathy. Further, studies are also needed to determine whether these results are generalizable to other cancer types and treatments.
These findings suggest that chemotherapy-induced neuropathy may be related to deficits in primary afferent fibers. Furthermore, it suggests that simple measures of nerve function may be able to be used clinically to determine a patient's neuropathy risk prior to the start of chemotherapy. As treatment strategies that reduce neuropathy risk have been identified (e.g., spaced treatment [41], low doses [42,43], and using alternative treatment agents [44] or routes of administration [45]), establishing a risk level would allow physicians and patients to have more information as they select a treatment plan. Implementing these alternatives for at-risk patients may improve patient quality of life and result in better treatment outcomes as a result of reduced need for toxicity-related breaks in therapy.
Acknowledgments
The authors wish to acknowledge Jackie Joy and Venus Ilagan for symptom and clinical data collection, Tina Peters and Tony Perez for QST data collection, Gary Mobley and Katherine Gilmore for data management, and Jeanie F. Woodruff, ELS for editorial assistance.
Financial Support: This work was supported by the National Cancer Institute at the US National Institutes of Health (CA124787 and NS046606). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Footnotes
Financial Disclosures: None.
References
- 1.Aghajanian C, Soignet S, Dizon DS, Pien CS, Adams J, Elliott PJ, Sabbatini P, Miller V, Hensley ML, Pezzulli S, Canales C, Daud A, Spriggs DR. A phase I trial of the novel proteasome inhibitor PS341 in advanced solid tumor malignancies. Clin Cancer Res. 2002;8(8):2505–2511. [PubMed] [Google Scholar]
- 2.Jagannath S, Durie BG, Wolf J, Camacho E, Irwin D, Lutzky J, McKinley M, Gabayan E, Mazumder A, Schenkein D, Crowley J. Bortezomib therapy alone and in combination with dexamethasone for previously untreated symptomatic multiple myeloma. Br J Haematol. 2005;129(6):776–783. doi: 10.1111/j.1365-2141.2005.05540.x. doi:10.1111/j.1365-2141.2005.05540.x. [DOI] [PubMed] [Google Scholar]
- 3.Loprinzi CL, Reeves BN, Dakhil SR, Sloan JA, Wolf SL, Burger KN, Kamal A, Le-Lindqwister NA, Soori GS, Jaslowski AJ, Novotny PJ, Lachance DH. Natural history of paclitaxel-associated acute pain syndrome: prospective cohort study NCCTG N08C1. J Clin Oncol. 2011;29(11):1472–1478. doi: 10.1200/JCO.2010.33.0308. doi:JCO.2010.33.0308 [pii] 10.1200/JCO.2010.33.0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tofthagen C. Patient perceptions associated with chemotherapy-induced peripheral neuropathy. Clin J Oncol Nurs. 2010;14(3):E22–28. doi: 10.1188/10.CJON.E22-E28. doi:758N375251807W57 [pii] 10.1188/10.CJON.E22-E28. [DOI] [PubMed] [Google Scholar]
- 5.Chaudhry V, Cornblath DR, Polydefkis M, Ferguson A, Borrello I. Characteristics of bortezomib- and thalidomide-induced peripheral neuropathy. J Peripher Nerv Syst. 2008;13(4):275–282. doi: 10.1111/j.1529-8027.2008.00193.x. doi:JNS193 [pii] 10.1111/j.1529-8027.2008.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prince HM, Adena M, Smith DK, Hertel J. Efficacy of single-agent bortezomib vs. single-agent thalidomide in patients with relapsed or refractory multiple myeloma: a systematic comparison. Eur J Haematol. 2007;79(2):93–99. doi: 10.1111/j.1600-0609.2007.00886.x. doi:EJH886 [pii] 10.1111/j.1600-0609.2007.00886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson PG, Briemberg H, Jagannath S, Wen PY, Barlogie B, Berenson J, Singhal S, Siegel DS, Irwin D, Schuster M, Srkalovic G, Alexanian R, Rajkumar SV, Limentani S, Alsina M, Orlowski RZ, Najarian K, Esseltine D, Anderson KC, Amato AA. Frequency, characteristics, and reversibility of peripheral neuropathy during treatment of advanced multiple myeloma with bortezomib. J Clin Oncol. 2006;24(19):3113–3120. doi: 10.1200/JCO.2005.04.7779. doi:JCO.2005.04.7779 [pii] 10.1200/JCO.2005.04.7779. [DOI] [PubMed] [Google Scholar]
- 8.Boyette-Davis JA, Cata JP, Zhang H, Driver LC, Wendelschafer-Crabb G, Kennedy WR, Dougherty PM. Follow-up psychophysical studies in bortezomib-related chemoneuropathy patients. J Pain. 2011;12(9):1017–1024. doi: 10.1016/j.jpain.2011.04.008. doi:10.1016/j.jpain.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cavaletti G, Gilardini A, Canta A, Rigamonti L, Rodriguez-Menendez V, Ceresa C, Marmiroli P, Bossi M, Oggioni N, D'Incalci M, De Coster R. Bortezomib-induced peripheral neurotoxicity: a neurophysiological and pathological study in the rat. Exp Neurol. 2007;204(1):317–325. doi: 10.1016/j.expneurol.2006.11.010. doi:S0014-4886(06)00617-0 [pii] 10.1016/j.expneurol.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhry V, Cornblath DR, Corse A, Freimer M, Simmons-O'Brien E, Vogelsang G. Thalidomide-induced neuropathy. Neurology. 2002;59(12):1872–1875. doi: 10.1212/01.wnl.0000037480.59194.85. [DOI] [PubMed] [Google Scholar]
- 11.San Miguel JF, Schlag R, Khuageva NK, Dimopoulos MA, Shpilberg O, Kropff M, Spicka I, Petrucci MT, Palumbo A, Samoilova OS, Dmoszynska A, Abdulkadyrov KM, Schots R, Jiang B, Mateos MV, Anderson KC, Esseltine DL, Liu K, Cakana A, van de Velde H, Richardson PG. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359(9):906–917. doi: 10.1056/NEJMoa0801479. doi:359/9/906 [pii] 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 12.Cavaletti G, Jakubowiak AJ. Peripheral neuropathy during bortezomib treatment of multiple myeloma: a review of recent studies. Leuk Lymphoma. 2010;51(7):1178–1187. doi: 10.3109/10428194.2010.483303. doi:10.3109/10428194.2010.483303. [DOI] [PubMed] [Google Scholar]
- 13.Attal M, Harousseau JL, Leyvraz S, Doyen C, Hulin C, Benboubker L, Yakoub Agha I, Bourhis JH, Garderet L, Pegourie B, Dumontet C, Renaud M, Voillat L, Berthou C, Marit G, Monconduit M, Caillot D, Grobois B, Avet-Loiseau H, Moreau P, Facon T. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood. 2006;108(10):3289–3294. doi: 10.1182/blood-2006-05-022962. doi:blood-2006-05-022962 [pii] 10.1182/blood-2006-05-022962. [DOI] [PubMed] [Google Scholar]
- 14.Mateos MV, Oriol A, Martinez-Lopez J, Gutierrez N, Teruel AI, de Paz R, Garcia-Larana J, Bengoechea E, Martin A, Mediavilla JD, Palomera L, de Arriba F, Gonzalez Y, Hernandez JM, Sureda A, Bello JL, Bargay J, Penalver FJ, Ribera JM, Martin-Mateos ML, Garcia-Sanz R, Cibeira MT, Ramos ML, Vidriales MB, Paiva B, Montalban MA, Lahuerta JJ, Blade J, Miguel JF. Bortezomib, melphalan, and prednisone versus bortezomib, thalidomide, and prednisone as induction therapy followed by maintenance treatment with bortezomib and thalidomide versus bortezomib and prednisone in elderly patients with untreated multiple myeloma: a randomised trial. Lancet Oncol. 2010;11(10):934–941. doi: 10.1016/S1470-2045(10)70187-X. doi:S1470-2045(10)70187-X [pii] 10.1016/S1470-2045(10)70187-X. [DOI] [PubMed] [Google Scholar]
- 15.Palumbo A, Bringhen S, Rossi D, Cavalli M, Larocca A, Ria R, Offidani M, Patriarca F, Nozzoli C, Guglielmelli T, Benevolo G, Callea V, Baldini L, Morabito F, Grasso M, Leonardi G, Rizzo M, Falcone AP, Gottardi D, Montefusco V, Musto P, Petrucci MT, Ciccone G, Boccadoro M. Bortezomib-melphalan-prednisone-thalidomide followed by maintenance with bortezomib-thalidomide compared with bortezomib-melphalan-prednisone for initial treatment of multiple myeloma: a randomized controlled trial. J Clin Oncol. 2010;28(34):5101–5109. doi: 10.1200/JCO.2010.29.8216. doi:JCO.2010.29.8216 [pii] 10.1200/JCO.2010.29.8216. [DOI] [PubMed] [Google Scholar]
- 16.Favis R, Sun Y, van de Velde H, Broderick E, Levey L, Meyers M, Mulligan G, Harousseau JL, Richardson PG, Ricci DS. Genetic variation associated with bortezomib-induced peripheral neuropathy. Pharmacogenet Genomics. 2011;21(3):121–129. doi: 10.1097/FPC.0b013e3283436b45. doi:10.1097/FPC.0b013e3283436b45. [DOI] [PubMed] [Google Scholar]
- 17.Johnson DC, Ramos C, Szubert AJ, Gregory WM, Child JA, Davies FE, Durie BG, Van Ness BG, Morgan GJ. Genetic Variation in ADME Genes Is Associated with Thalidomide Related Peripheral Neuropathy in Multiple Myeloma Patients. ASH Annual Meeting Abstracts. 2008;112(11):1675. [Google Scholar]
- 18.Badros A, Goloubeva O, Dalal JS, Can I, Thompson J, Rapoport AP, Heyman M, Akpek G, Fenton RG. Neurotoxicity of bortezomib therapy in multiple myeloma: a single-center experience and review of the literature. Cancer. 2007;110(5):1042–1049. doi: 10.1002/cncr.22921. doi:10.1002/cncr.22921. [DOI] [PubMed] [Google Scholar]
- 19.Mateos MV, Hernandez JM, Hernandez MT, Gutierrez NC, Palomera L, Fuertes M, Diaz-Mediavilla J, Lahuerta JJ, de la Rubia J, Terol MJ, Sureda A, Bargay J, Ribas P, de Arriba F, Alegre A, Oriol A, Carrera D, Garcia-Larana J, Garcia-Sanz R, Blade J, Prosper F, Mateo G, Esseltine DL, van de Velde H, San Miguel JF. Bortezomib plus melphalan and prednisone in elderly untreated patients with multiple myeloma: results of a multicenter phase 1/2 study. Blood. 2006;108(7):2165–2172. doi: 10.1182/blood-2006-04-019778. doi:blood-2006-04-019778 [pii] 10.1182/blood-2006-04-019778. [DOI] [PubMed] [Google Scholar]
- 20.Bruna J, Ale A, Velasco R, Jaramillo J, Navarro X, Udina E. Evaluation of pre-existing neuropathy and bortezomib retreatment as risk factors to develop severe neuropathy in a mouse model. J Peripher Nerv Syst. 2011;16(3):199–212. doi: 10.1111/j.1529-8027.2011.00346.x. doi:10.1111/j.1529-8027.2011.00346.x. [DOI] [PubMed] [Google Scholar]
- 21.Cavaletti G, Bogliun G, Marzorati L, Zincone A, Piatti M, Colombo N, Franchi D, La Presa MT, Lissoni A, Buda A, Fei F, Cundari S, Zanna C. Early predictors of peripheral neurotoxicity in cisplatin and paclitaxel combination chemotherapy. Ann Oncol. 2004;15(9):1439–1442. doi: 10.1093/annonc/mdh348. doi:10.1093/annonc/mdh348. [DOI] [PubMed] [Google Scholar]
- 22.Dimopoulos MA, Mateos MV, Richardson PG, Schlag R, Khuageva NK, Shpilberg O, Kropff M, Spicka I, Palumbo A, Wu KL, Esseltine DL, Liu K, Deraedt W, Cakana A, Van De Velde H, San Miguel JF. Risk factors for, and reversibility of, peripheral neuropathy associated with bortezomib-melphalan-prednisone in newly diagnosed patients with multiple myeloma: subanalysis of the phase 3 VISTA study. Eur J Haematol. 2011;86(1):23–31. doi: 10.1111/j.1600-0609.2010.01533.x. doi:10.1111/j.1600-0609.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- 23.Lanzani F, Mattavelli L, Frigeni B, Rossini F, Cammarota S, Petro D, Jann S, Cavaletti G. Role of a preexisting neuropathy on the course of bortezomib-induced peripheral neurotoxicity. J Peripher Nerv Syst. 2008;13(4):267–274. doi: 10.1111/j.1529-8027.2008.00192.x. doi:JNS192 [pii] 10.1111/j.1529-8027.2008.00192.x. [DOI] [PubMed] [Google Scholar]
- 24.He Z, Boyette-Davis JA, Shah ND, Thomas SK, Vichaya EG, Wang XS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Cleeland CS, Dougherty PM. Subclinical peripheral neuropathy is a common finding in multiple myeloma patients prior to chemotherapy. In Preparation. [DOI] [PMC free article] [PubMed]
- 25.Boyette-Davis JA, Eng C, Wang XS, Cleeland CS, Wendelschafer-Crabb G, Kennedy WR, Simone DA, Zhang H, Dougherty PM. Subclinical peripheral neuropathy is a common finding in colorectal cancer patients prior to chemotherapy. Clin Cancer Res. 2012;18(11):3180–3187. doi: 10.1158/1078-0432.CCR-12-0205. doi:1078-0432.CCR-12-0205 [pii] 10.1158/1078-0432.CCR-12-0205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felix ER, Widerstrom-Noga EG. Reliability and validity of quantitative sensory testing in persons with spinal cord injury and neuropathic pain. Journal of rehabilitation research and development. 2009;46(1):69–83. [PubMed] [Google Scholar]
- 27.Geber C, Klein T, Azad S, Birklein F, Gierthmuhlen J, Huge V, Lauchart M, Nitzsche D, Stengel M, Valet M, Baron R, Maier C, Tolle T, Treede RD. Test-retest and interobserver reliability of quantitative sensory testing according to the protocol of the German Research Network on Neuropathic Pain (DFNS): a multi-centre study. Pain. 2011;152(3):548–556. doi: 10.1016/j.pain.2010.11.013. doi:10.1016/j.pain.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 28.Krumova EK, Geber C, Westermann A, Maier C. Neuropathic pain: is quantitative sensory testing helpful? Current diabetes reports. 2012;12(4):393–402. doi: 10.1007/s11892-012-0282-7. doi:10.1007/s11892-012-0282-7. [DOI] [PubMed] [Google Scholar]
- 29.Cata JP, Weng HR, Burton AW, Villareal H, Giralt S, Dougherty PM. Quantitative sensory findings in patients with bortezomib-induced pain. J Pain. 2007;8(4):296–306. doi: 10.1016/j.jpain.2006.09.014. doi:10.1016/j.jpain.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 30.Dougherty PM, Cata JP, Burton AW, Vu K, Weng HR. Dysfunction in multiple primary afferent fiber subtypes revealed by quantitative sensory testing in patients with chronic vincristine-induced pain. J Pain Symptom Manage. 2007;33(2):166–179. doi: 10.1016/j.jpainsymman.2006.08.006. doi:10.1016/j.jpainsymman.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 31.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1-2):132–142. doi: 10.1016/j.pain.2004.01.021. doi:10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs PN, Campbell JN, Meyer RA. Secondary hyperalgesia persists in capsaicin desensitized skin. Pain. 2000;84(2-3):141–149. doi: 10.1016/s0304-3959(99)00194-3. [DOI] [PubMed] [Google Scholar]
- 33.Ruff RM, Parker SB. Gender- and age-specific changes in motor speed and eye-hand coordination in adults: normative values for the Finger Tapping and Grooved Pegboard Tests. Percept Mot Skills. 1993;76(3 Pt 2):1219–1230. doi: 10.2466/pms.1993.76.3c.1219. [DOI] [PubMed] [Google Scholar]
- 34.Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, Engstrom MC. Assessing symptom distress in cancer patients: the M.D. Anderson Symptom Inventory. Cancer. 2000;89(7):1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. doi:10.1002/1097-0142(20001001)89:7<1634::AID-CNCR29>3.0.CO;2-V [pii] [DOI] [PubMed] [Google Scholar]
- 35.Jones D, Vichaya EG, Wang XS, Williams LA, Shah ND, Thomas SK, Johnson VE, Champlin RE, Cleeland CS, Mendoza TR. Validation of the M. D. Anderson symptom inventory multiple myeloma module. Journal of hematology & oncology. 2013;6(1):13. doi: 10.1186/1756-8722-6-13. doi:10.1186/1756-8722-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Palos GR, Mendoza TR, Mobley GM, Cantor SB, Cleeland CS. Asking the community about cutpoints used to describe mild, moderate, and severe pain. J Pain. 2006;7(1):49–56. doi: 10.1016/j.jpain.2005.07.012. doi:S1526-5900(05)00829-1 [pii] 10.1016/j.jpain.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 37.Dispenzieri A, Kyle RA. Neurological aspects of multiple myeloma and related disorders. Best Pract Res Clin Haematol. 2005;18(4):673–688. doi: 10.1016/j.beha.2005.01.024. doi:S1521-6926(05)00025-3 [pii] 10.1016/j.beha.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Drappatz J, Batchelor T. Neurologic complications of plasma cell disorders. Clin Lymphoma. 2004;5(3):163–171. doi: 10.3816/clm.2004.n.022. [DOI] [PubMed] [Google Scholar]
- 39.Treede RD, Meyer RA, Raja SN, Campbell JN. Evidence for two different heat transduction mechanisms in nociceptive primary afferents innervating monkey skin. J Physiol. 1995;483(Pt 3):747–758. doi: 10.1113/jphysiol.1995.sp020619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cain DM, Khasabov SG, Simone DA. Response properties of mechanoreceptors and nociceptors in mouse glabrous skin: an in vivo study. J Neurophysiol. 2001;85(4):1561–1574. doi: 10.1152/jn.2001.85.4.1561. [DOI] [PubMed] [Google Scholar]
- 41.Bringhen S, Larocca A, Rossi D, Cavalli M, Genuardi M, Ria R, Gentili S, Patriarca F, Nozzoli C, Levi A, Guglielmelli T, Benevolo G, Callea V, Rizzo V, Cangialosi C, Musto P, De Rosa L, Liberati AM, Grasso M, Falcone AP, Evangelista A, Cavo M, Gaidano G, Boccadoro M, Palumbo A. Efficacy and safety of once-weekly bortezomib in multiple myeloma patients. Blood. 2010;116(23):4745–4753. doi: 10.1182/blood-2010-07-294983. doi:blood-2010-07-294983 [pii] 10.1182/blood-2010-07-294983. [DOI] [PubMed] [Google Scholar]
- 42.Jagannath S, Barlogie B, Berenson J, Siegel D, Irwin D, Richardson PG, Niesvizky R, Alexanian R, Limentani SA, Alsina M, Adams J, Kauffman M, Esseltine DL, Schenkein DP, Anderson KC. A phase 2 study of two doses of bortezomib in relapsed or refractory myeloma. Br J Haematol. 2004;127(2):165–172. doi: 10.1111/j.1365-2141.2004.05188.x. doi:BJH5188 [pii] 10.1111/j.1365-2141.2004.05188.x. [DOI] [PubMed] [Google Scholar]
- 43.Offidani M, Corvatta L, Marconi M, Malerba L, Mele A, Olivieri A, Brunori M, Catarini M, Candela M, Capelli D, Montanari M, Rupoli S, Leoni P. Common and rare side-effects of low-dose thalidomide in multiple myeloma: focus on the dose-minimizing peripheral neuropathy. Eur J Haematol. 2004;72(6):403–409. doi: 10.1111/j.1600-0609.2004.00238.x. doi:10.1111/j.1600-0609.2004.00238.x. [DOI] [PubMed] [Google Scholar]
- 44.Rajkumar SV, Hayman SR, Lacy MQ, Dispenzieri A, Geyer SM, Kabat B, Zeldenrust SR, Kumar S, Greipp PR, Fonseca R, Lust JA, Russell SJ, Kyle RA, Witzig TE, Gertz MA. Combination therapy with lenalidomide plus dexamethasone (Rev/Dex) for newly diagnosed myeloma. Blood. 2005;106(13):4050–4053. doi: 10.1182/blood-2005-07-2817. doi:2005-07-2817 [pii] 10.1182/blood-2005-07-2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moreau P, Pylypenko H, Grosicki S, Karamanesht I, Leleu X, Grishunina M, Rekhtman G, Masliak Z, Robak T, Shubina A, Arnulf B, Kropff M, Cavet J, Esseltine D-L, Feng H, Girgis S, van de Velde H, Deraedt W, Harousseau J-L. Subcutaneous versus intravenous administration of bortezomib in patients with relapsed multiple myeloma: a randomised, phase 3, non-inferiority study. The Lancet Oncology. 2011;12(5):431–440. doi: 10.1016/S1470-2045(11)70081-X. [DOI] [PubMed] [Google Scholar]