Abstract
HNF4α, the master regulator of hepatocyte differentiation, has been recently shown to inhibit hepatocyte proliferation via unknown mechanisms. We investigated the mechanisms of HNF4α-induced inhibition of hepatocyte proliferation using a novel TAM-inducible, hepatocyte specific HNF4α knockdown mouse model. Hepatocyte specific deletion of HNF4α in adult mice resulted in increased hepatocyte proliferation with a significant increase in liver to body weight ratio. We determined global gene expression changes using Illumina HiSeq-based RNA sequencing, which revealed that, a significant number of up-regulated genes following deletion of HNF4α were associated with cancer pathogenesis, cell cycle control, and cell proliferation. The pathway analysis further revealed that c-Myc-regulated gene expression network was highly activated following HNF4α deletion. To determine whether deletion of HNF4α affects cancer pathogenesis, HNF4α knockdown was induced in mice treated with the known hepatic carcinogen diethylnitrosamine (DEN). Deletion of HNF4α significantly increased the number and size of DEN-induced hepatic tumors. Pathological analysis revealed that tumors in HNF4α deleted mice were well-differentiated hepatocellular carcinoma (HCC) and mixed HCC-cholangiocarcinoma. Analysis of tumors and surrounding normal liver tissue in DEN-treated HNF4α knockout mice showed significant induction in c-Myc expression. Taken together, deletion of HNF4α in adult hepatocytes results in increased hepatocyte proliferation and promotion of DEN-induced hepatic tumors secondary to aberrant c-Myc activation.
Introduction
Hepatocyte nuclear factor 4 alpha (HNF4α, NR2A1) is considered the master regulator of hepatocyte differentiation (1, 2). It plays an important role in the regulation of many hepatocyte specific genes including those involved in glycolysis, gluconeogenesis, ureagenesis, fatty acid metabolism, bile acid synthesis, drug metabolism, apolipoprotein synthesis, and blood coagulation (3-7). Because of its important role in liver development and homeostasis, disruption of HNF4α has been linked to various disorders of the liver including metabolic syndrome, type 2 diabetes, mature onset diabetes in the young (MODY), and hepatocellular carcinoma (8-12).
Recent studies suggest a novel role of HNF4α in the regulation of cell proliferation within multiple tissues including liver, pancreas, and kidney (3, 4, 10-17). It is known that HCC progression is associated with down regulation of HNF4α in rodents and humans (10) (12, 14, 16). More recently, Ning et al. have reported that over expression of HNF4α suppresses diethylnitrosamine (DEN)-induced HCC in rats (11). These data suggest that HNF4α may have the ability to inhibit hepatocyte proliferation within the liver; however, the mechanisms are yet to be determined.
Because of its fundamental role in liver development and homeostasis, whole body deletion of HNF4α results in an embryonic lethal phenotype (18). Liver-specific deletion of HNF4α under an albumin promoter-driven cre recombinase results in severe hepatic metabolic disruption and lethality between 6 and 8 weeks of age (4, 18). In these mice produced using constitutively active albumin-cre, HNF4α is deleted during early postnatal development making it difficult to decipher the effect of improper hepatic differentiation and aberrant hepatic proliferation on the observed phenotype. To overcome these issues, we developed an inducible knock-out of HNF4α where HNF4α is deleted in the mature mouse liver using a tamoxifen (TAM)-inducible cre recombinase (ERT2-Cre), first described by Bonzo et al. (17). Using this novel mouse model of hepatocyte specific HNF4α deletion in the adult liver combined with RNA sequencing mediated transcriptomics, we investigated the mechanism of HNF4α-mediated inhibition of hepatocyte proliferation. We also studied the significance of the role of HNF4α-mediated regulation of hepatocyte proliferation using a chemical carcinogenesis model. Our studies indicate that apart from its role in hepatic differentiation, HNF4α actively inhibits hepatocyte proliferation and plays a critical role in maintenance of hepatic homeostasis.
Materials and Methods
Animals, Treatments, and Tissue Collection
The HNF4αFl/Fl mice (provided by Dr. Frank Gonzalez of NCI-NIH) and the TAM-inducible Albumin cre mice (AlbCreERT2+, provided by Dr. Pierre Chambon, IGBMC-France) used in these studies have been previously described (4). The HNF4αFl/Fl, AlbCreERT2+ mice were produced by standard animal breeding and identified using PCR based genotyping of tail biopsies. All animals were housed in Association for Assessment and Accreditation of Laboratory Animal Care-accredited facilities at the University of Kansas Medical Center under a standard 12-h light/dark cycle with access to chow and water ad libitum. The Institutional Animal Care and Use Committee approved all of the studies.
Three-month-old male, HNF4αFl/Fl, AlbERT2-Cre+ mice were treated with TAM (6 μg/mouse, IP, referred as HNF4α-KO), or with vehicle alone (corn oil, IP, referred as Control) subcutaneously. To account for changes induced by TAM, three-month-old male, HNF4αFl/Fl, AlbERT2-Cre− mice were treated with TAM (6 μg/mouse, IP, referred as TAM Control). Mice were killed by cervical dislocation under isoflurane anesthesia, and livers were collected, 7 days post-injection. For the DEN-induced HCC protocol, male HNF4αFl/Fl, AlbERT2-Cre+ mice were injected, subcutaneously, with 15μg/g DEN (in 0.9% saline) at postnatal day 12-15 and allowed to grow until 8 months of age. At 8 months, these mice were divided into 2 groups and treated with either TAM (6μg/mouse) or corn oil, and sacrificed two month later at 10 months of age. Liver and serum samples were obtained and processed as described before (19). Liver and body weights of mice were noted at the time of sacrifice and used to determine liver/body weight ratios. Liver injury and function were determined by serum alanine aminotransferase (ALT), serum bilirubin and serum glucose levels measured using the Infinity™ ALT (GPT) and the Infinity™ Glucose kit (Thermo Scientific; Middletown, VA) according to the manufacturer’s protocol.
Western Blotting
RIPA extracts obtained from whole liver tissues were used for Western blot analysis and Western blots were performed using the previously described protocol (20). The antibodies used in this study are as follows- HNF4α, (1:1000; R&D Systems; Minneapolis, MN; Cat. # PP-H1415-00), Cyclin D1 (Cat. # 2978), c-Myc (Cat. # 5605), and β-Actin (Cat. # 4970) (1:1000; Cell Signaling; Danvers, MA).
Staining Procedures
Paraffin-embedded liver sections (4 μm thick) were used for H&E, PAS and immunohistochemical staining of PCNA as described before (20). After staining for PCNA, positive cells were quantified by counting four 40x fields per slide for each liver sample (n=3 per group). Fresh frozen sections (5 μm thick) were used to detect lipid accumulation by staining with Oil Red O and Ki-67 immunofluorescence as described before (19, 20). Apoptosis was measured using the In Situ Cell Death Detection Kit, TMR red (Roche Applied Science; Indianapolis, IN; Cat # 12156792910) according to the manufacturer’s protocol.
RNA Seq and Functional Analysis
Total RNA was isolated from liver tissue using the phenol/chloroform extraction protocol. Integrity of RNA was analyzed by the Microarray Core Facility at KUMC (Kansas City, KS) using an Agilent Bioanalyzer 2100 (Agilent Technologies; Santa Clara, CA).
We performed two separate and independent RNA-Seq experiments for the same treatment conditions, Cre+\Tamoxifen, Cre−\ Tamoxifen and Cre+\Corn Oil. In the first instance (Run1), the total processed RNA extracted from pooled mouse liver samples (3 mice per group) treated with Cre+\Tamoxifen, Cre−\ Tamoxifen and Cre+\Corn Oil was sequenced in an Illumina HiSeq 2000 sequencing machine (Illumina, San Diego, CA). The initial library of 10nM concentration for each of the three samples was split into two diluted concentrations of 5pM and 3pM and sequenced separately at a 2×100 bp paired-end resolution and the output of the sequencing runs combined for downstream analysis. In order to complement the initial RNA-Seq analysis, we ran a second RNA-seq experiment (Run2) on biological replicate samples (n=2) of mouse liver treated with Cre+\Tamoxifen, Cre−\ Tamoxifen and Cre+\Corn Oil. These samples were sequenced at a 50bp single-end resolution. The RNA-seq data obtained from both experiments was for the bioinformatics analysis. Reads from both experiments were mapped to the mouse reference genome (NCBI37/mm9) using TopHat v1.4.1 (Trapnell et al.). TopHat was run with default parameters and for Run1 with paired reads, a mate inner distance of −40 was set to accommodate the 260bp average fragment length. The binary output files generated by TopHat were passed to CuffDiff (21) for calculating differential gene expression. For samples from both runs, differential gene expression was first calculated for Cre+\Tamoxifen against Cre+\Corn Oil and Cre+\Tamoxifen against Cre−\ Tamoxifen. For each run, genes that were significantly differentially expressed in both these measures based on an absolute fold change of at least 1.5 and a q-value (the p-value adjusted for multiple hypothesis correction using the Benjamini-Hochberg procedure) less than or equal to 0.05 were selected. This data filtering resulted in 1096 genes from Run1 and 3436 genes from Run2. Of these 877 genes were common to both runs (right tailed Fisher’s exact test significance p-value <1E-100). These genes were uploaded to Ingenuity Pathways Analysis (IPA, Ingenuity Systems, version 7.6 (www.ingenuity.com) for gene set enrichment analysis. Out of the 877 genes uploaded, 864 genes qualified for analysis in IPA. The analysis was performed in IPA with default parameters. The RNA-seq data have been submitted to the Sequence Read Archive (SRA) of NCBI.
RNA-Seq/ChIP-Seq Comparative Analysis
We analyzed publically available ChIP-Seq data (SRA008281) from Hoffmen et al. (22) to obtain an unbiased whole genome mapping of Hnf4α binding sites in mouse. Sequences were aligned using Bowtie2 (ver 2.0.2) to the latest mouse reference genome (GRCm38/mm10) using default parameters (23). Peak detection was performed using the Model-based Analysis of ChIP-Seq (MACS) algorithm with the peak detection p-value cutoff set at 1e-5 (default) (24). This resulted in a set of 9281 significant (FDR less than 1 in a 100) Hnf4α binding sites. We searched for the Hnf4α consensus sequence within a 250 bp region from either side of the called peaks using a weight-matrix match with at least 80% similarity. The Hnf4α weight matrix obtained from the JASPAR database (25) was used as a surrogate to model Hnf4α binding sites. A substantial proportion (92%) of the highly enriched Hnf4α binding sites consisted of at least one Hnf4α consensus site. All identified Hnf4α binding sites were annotated with their closest up-stream, down-stream and overlapping genes using Ensembl gene annotations.
We looked at how many of the 877 putative Hnf4α perturbed genes identified in our experiment were among the putative Hnf4α target genes identified by the ChIP-Seq experiment. The significance of the overlap of genes between the two studies was calculated using the right tailed Fisher’s exact test. The statistic was calculated separately for genes with up-stream, down-stream and overlapping Hnf4α targets and in combination and is shown in supplementary table 2. The IPA analysis was also used to determine the most activated and most inhibited transcription factor gene networks using activation of Z-score criteria (described in supplementary material).
Statistical Analysis
For all experiments not associated with RNA sequencing, such as ALT measurements, results are expressed as mean ± standard deviation. Student’s T-test was applied to all analyses with a p-value <0.001 being considered significant.
Results
HNF4α Deletion Results in Increased Cell Proliferation
Treatment of HNF4αFl/Fl, AlbERT2-Cre+ mice with TAM resulted in deletion of HNF4α as demonstrated by Western blot analysis (Figure 1B). Data shows ~80-90% decrease in HNF4α protein level in the KO, as compared to controls, HNF4αFl/Fl AlbERT2-Cre+ treated with cornoil and HNF4αFl/Fl AlbERT2-Cre− treated with TAM was observed 7 days after TAM or corn oil injection. HNF4α deletion was also confirmed by immunohistochemical staining of paraffin embedded sections (data not shown).
Figure 1.
Deletion of HNF4α using TAM (TAM) driven albumin cre mice. (A) Scheme of HNF4α deletion using the HNF4αFl/Fl, AlbERT2-Cre+ mice. (B) Western blot analysis of HNF4α using nuclear proteins isolated from HNF4αFl/Fl, AlbERT2-Cre+ mice treated with either TAM or corn oil and HNF4αFl/Fl, AlbERT2-Cre− mice treated TAM. See Methods section for details. (C) Liver weight to body weight ratios (D) serum ALT levels, and (E) serum glucose levels of Control, HNF4α-KO and TAM Control mice seven days after either TAM or corn oil injection.
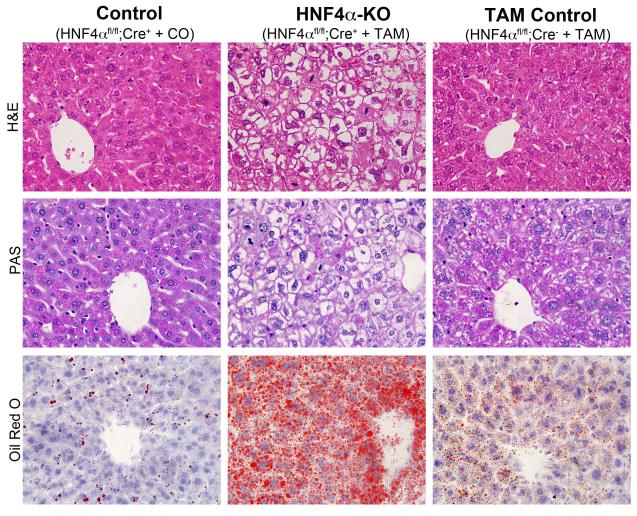
Deletion of HNF4α resulted in significant increase in liver to body weight ratio (Fig. 1C) but did not result in significant liver injury as indicated by serum ALT and glucose concentrations (Figure 1D and E). Staining of liver sections indicated that there was no cell death or inflammation following deletion of HNF4α. There was no apparent apoptosis, necrosis, or infiltration of immune cells, all which are hallmark signs of injury (Figure 2, H&E). Also, we did not observe an increase in TUNEL positive cells following deletion of HNF4α Figure 3D). However, the hepatocytes exhibited extensive vacuolization giving them ‘empty’ appearance. Further analysis indicated a significant decrease in hepatic glycogen accumulation and a significant increase in lipid accumulation demonstrated by PAS and Oil Red O staining, respectively after HNF4α deletion (Figure 2, PAS and Oil Red O). Finally, deletion of HNF4α resulted in a dramatic increase in cell proliferation as demonstrated by an ~20% increase in the amount of PCNA positive cells (Figure 3A and B). These data were corroborated by Ki-67 staining (Figure 3C).
Figure 2.
Histopathological analysis of livers after HNF4α deletion. Representative photographs of paraffin embedded liver sections from the livers of Control, HNF4α-KO, and TAM Control livers seven days after either TAM or corn oil treatment were used to stain for H&E (upper panel), and PAS for glycogen staining (middle panel). Frozen sections from the same samples were used for Oil Red O staining (lower panel). All images are 600x.
Figure 3.
Increased cell proliferation after HNF4α deletion. (A) Representative photomicrographs of PCNA immunohistochemistry and (B) bar graph showing percentage of PCNA positive cells in Control, HNF4α-KO, and TAM Control livers seven days after either TAM or corn oil treatment. Frozen sections from same liver were used to stain for Ki-67 for detection of cell proliferation (C) and TUNEL assay for detection of apoptosis (D).
HNF4α Deletion Results in Increased Pro-Mitogenic Gene Expression
High-throughput sequencing generated 117, 179, and 136 million reads for the Cre+\TAM, Cre−\ TAM and Cre+\Corn Oil samples respectively. Of these, TopHat was able to map 103, 163 and 121 million reads to the mouse reference genome respectively. Further statistics on the quality of the RNA-Seq data is provided in supplemental table 1.
Deletion of HNF4α resulted in the down regulation of many genes known to be involved in hepatocyte function, such as xenobiotic metabolism, cholesterol metabolism, coagulation, bile acid synthesis, etc. (Table 1). Interestingly, many of the up-regulated genes are known to be involved in the cell cycle and cancer (Table 2). A complete list of gene expression changes can be found in supplemental table 6.
Table 1.
Down-regulated Genes following Deletion of HNF4α in adult mouse liver
| Gene Name | Gene Symbol | Fold Change | P-value |
|---|---|---|---|
| UDP Glucuronosyltransferase 2 Family, Polypeptide B4 |
Ugt2b1 | −159.92 | <1.00E-20 |
| Apolipoprotein A-II | Apoa2 | −54.52 | <1.00E-20 |
| Coagulation Factor XII | F12 | −22.06 | <1.00E-20 |
| Cytochrome P450, Family 8, Subfamily B, Polypeptide 1 |
Cyp8b1 | −25.47 | <1.00E-20 |
| Apolipoprotein A-IV | Apoa4 | −3.93 | <1.00E-20 |
| Sulfotransferase Family, Cytosolic, 1A, Phenol- Preferring, Member 1 |
Sult1a1 | −11.72 | <1.00E-20 |
| Claudin 2 | Cldn2 | −4.18 | 6.08E-12 |
| Apolipoprotein B | Apob | −7.13 | 1.15E-14 |
| Claudin 1 | Cldn1 | −4.79 | <1.00E-20 |
| Cytochrome P450, Family 7, Subfamily B, Polypeptide 1 |
Cyp7b1 | −13.68 | <1.00E-20 |
Table 2.
Up-regulated Genes following Deletion of HNF4α in adult mouse liver
| Gene Name | Gene Symbol | Fold Change | q-value |
|---|---|---|---|
| Cyclin B1 | Ccnb1 | 58.75 | <1.00E-20 |
| Cell Division Cycle Homolog 25 C |
Cdc25c | 21.07 | 7.96E-05 |
| Cyclin-Dependent Kinase Inhibitor 3 |
Cdkn3 | 144.66 | 2.28E-03 |
| Cell Division Cycle 20 Homolog |
Cdc20 | 74.59 | <1.00E-20 |
| Antigen Identified by Antibody Ki-67 |
Ki-67 | 23.71 | <1.00E-20 |
| Polo-Like Kinase 1 |
Plk1 | 12.90 | 1.71E-14 |
| Cyclin B2 | Ccnb2 | 21.79 | <1.00E-20 |
| Cyclin A2 | Ccna2 | 20.89 | <1.00E-20 |
| Epithelial Cell Transforming Sequence 2 |
Ect2 | 20.53 | <1.00E-20 |
| Cell Division Cycle Associated 3 |
Cdca3 | 13.16 | <1.00E-20 |
| Cell Division Cycle Associated 8 |
Cdca8 | 8.02 | 1.82E-10 |
| Early Growth Response 1 |
Egr1 | 5.44 | <1.00E-20 |
| Myelocytomatosis Viral Oncogene Homolog |
Myc | 3.84 | <1.00E-20 |
RNA-Seq/ChIP-Seq comparative analysis revealed that of the total gene changes observed following deletion of HNF4α (877), ~53% of these (462) contained a putative HNF4α binding site within 50 kb of the transcriptional start site (TSS) (supplementary table 2). Further, ~45% (395) contained a putative HNF4α binding site within 10 kb of the TSS.
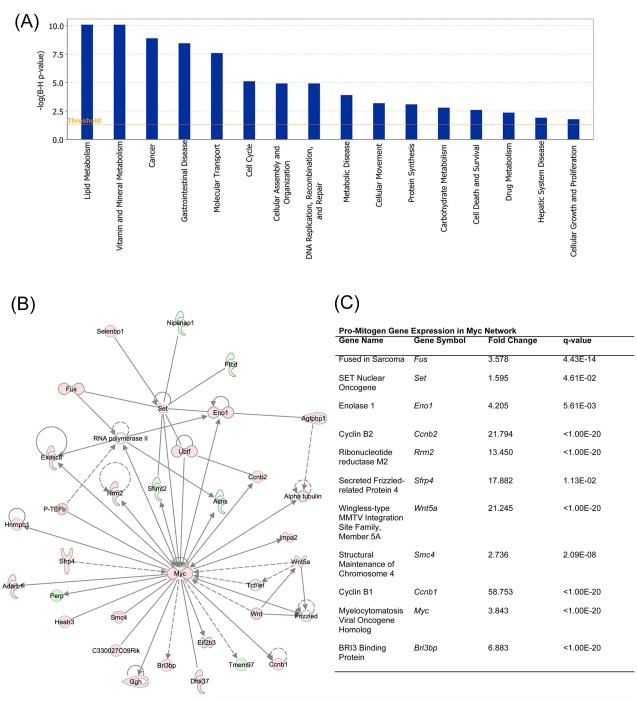
To identify patterns in gene expression changes, we utilized Ingenuity Pathway Analysis (IPA, Ingenuity® Systems, www.ingenuity.com). Functional analysis of gene expression changes revealed genes to be involved with cancer pathogenesis to be one of the most significant groups of genes to be changed (Figure 4A). Other groups of genes found to be significantly changed include gene involved in cell cycle, cellular growth and proliferation, and lipid metabolism (Figure 4A and 4B). IPA further revealed changes in major transcription factor activity following HNF4α deletion (supplementary table 3 and 4). The c-Myc-regulated gene expression network showed the most significant changes in gene expression that correlate with activation of c-Myc following HNF4α deletion (Figure 3B and C). This includes several genes involved in cell proliferation including ccnb1, ccnb2, fus, and set oncogene. Also, many other transcription factor networks known to be involved in cell proliferation and cancer were significantly activated (supplementary table 3). As expected, gene network associated with HNF4α was inhibited (regulation z-score −5.0, supplementary table 4). Other factors inhibited include CDKN1A (p21), Smarcb1, Tob1, and CDKN2A (p16), all of which have been shown to be associated with cancer pathogenesis.
Figure 4.
Gene expression change in livers following HNF4α deletion. Global gene expression analysis was conducted using Illumina-based RNA sequencing as described in Methods. The data was used for Ingenuity Pathway Analysis. (A) Bar graph showing top categories of genes changed following HNF4α deletion. (B) c-Myc Gene network showing various genes either up regulated (red) or down regulated (green) in HNF4α-KO mice as compared to Control. (C) Table showing up regulated pro-mitogenic genes within the c-Myc-regulated gene network.
HNF4α Deletion Results in Increased Progression of DEN-induced Hepatocellular Carcinoma
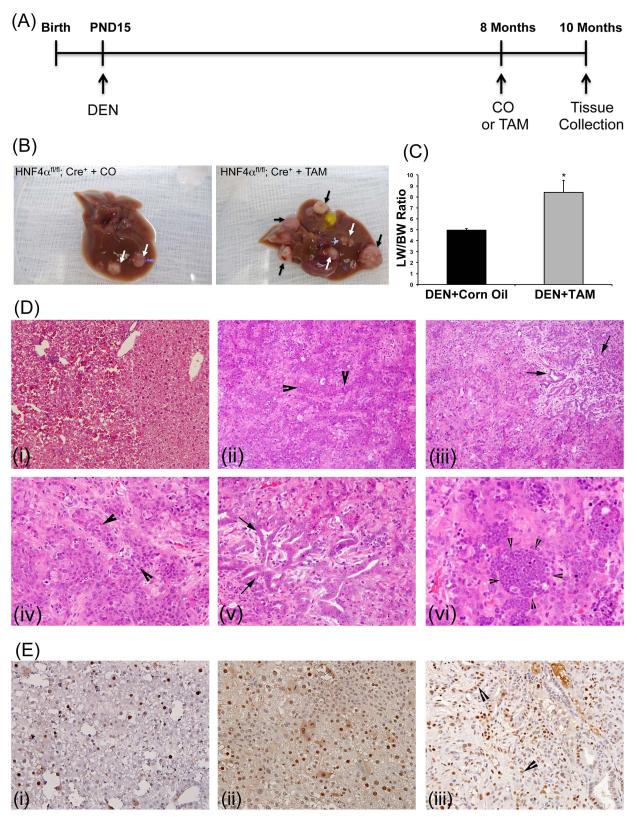
To determine effect of HNF4α deletion on hepatic tumor progression we utilized DEN-induced HCC model. HNF4αFl/Fl, AlbERT2-Cre+ mice were treated with known hepatic carcinogen, DEN, at post-natal day 15 and then treated with TAM (HNF4α-KO) or corn oil (control) at 8 months of age followed by tissue collection 2 months later at 10 months of age (Figure 4A). Deletion of HNF4α only for a 2 month period resulted in increased HCC progression demonstrated by an increase in tumor number and size (supplementary table 6 and Figure 4B, arrows), along with an ~ 2-fold increase in liver/body weight ratio (supplementary table 6and Figure 4C). HNF4α-KO livers display advanced tumor morphology and significantly increased proliferation when compared to control livers via H&E (Figure 4D) and PCNA staining, respectively (Figure 4E). The Control mice treated with DEN exhibited mainly regenerative nodules and a few high grade dysplastic nodules with few early-stage HCCs. In contrast, the HNF4α-KO mice treated with DEN exhibited extensive dyspastic nodules, HCCs (Figure 4D-ii and iv), and tumors with mixed HCC-cholangiocarcinoma morphology (Figure 4D-iii and v). The tumors in HNF4α-KO mice exhibited distinct histological features including expansion of oval cell population (Figure 4D-ii, 4D-iv and 4E-iii) and presence of inflammatory cell foci (Figure 4D-vi).
HNF4α Deletion Results in Increased Pro-Mitogenic Signaling in DEN-induced Hepatocellular Carcinoma
We hypothesized that increased progression of HCC in HNF4α-KO mice treated with DEN may be due to increased pro-mitogenic signaling. Western blot analysis of control, control+DEN (normal tissue), tumor tissues and surrounding normal tissues from HNF4α-KO mice treated with DEN indicated increased Cyclin D1 and c-Myc expression only in normal liver tissue surrounding the tumors and in the tumors observed in HNF4α-KO mice treated with DEN (Fig. 6A). Real time PCR analysis showed that expression of several genes up regulated following HNF4α deletion were further increased in the tumors observed in HNF4α-KO mice (Figure 6B to G).
Figure 6.
Increased pro-mitogenic gene expression in HNF4α-KO tumors. (A) Western blot analysis of HNF4α, Cyclin D1, and c-Myc using either nuclear proteins (HNF4α) or total liver extracts (all other) of Control mice, Control mice treated with DEN, normal liver tissue of HNF4α-KO treated with DEN and tumor tissue of HNF4α-KO treated with DEN. (B) to (G) Real time PCR analysis of putative negative targets of HNF4α identified by combined RNA-seq-ChIP-seq bioinformatics analysis.
Discussion
HNF4α is known as the master regulator of hepatocyte differentiation because it regulates many hepatocyte specific genes involved in bile acid metabolism, drug metabolism, blood coagulation and lipid metabolism. Recent studies suggest a novel function of HNF4α in the regulation of hepatocyte proliferation and indicate that HNF4α may actively inhibit hepatocyte proliferation; however, the mechanisms of HNF4α-mediated inhibition of hepatocyte proliferation are not known. In this study, we explored the mechanisms by which HNF4α inhibits hepatocyte proliferation using a novel mouse model combined with RNA sequencing.
Previous models of HNF4α deletion, produced using constitutively active albumin-cre, result in deletion of HNF4α soon after birth, when the liver is still growing and differentiating, leading to early postnatal lethality (4). This makes it difficult to distinguish the role of HNF4α in the regulation of hepatocyte differentiation from its role in the regulation of cell proliferation. We have independently developed a novel model of HNF4α deletion using a TAM inducible albumin-cre, first described by Bonzo, et al. (17). In this model, HNF4α is deleted in three-month-old male mice by activating albumin-cre using TAM treatment, which allows us to study the role of HNF4α in adult, fully mature livers. Our study clearly indicates that deletion of HNF4α in adult mouse liver results in initiation of hepatocyte proliferation and leads to an increase in liver/body weight ratio as early as one week following deletion of HNF4α. Because we could not detect any liver injury either biochemically or histologically, the increase in cell proliferation is not a compensatory regeneration in response to injury; however, we did observe biochemical changes in liver following HNF4α deletion. Hepatocytes in normal liver store significant amount of glycogen, but hepatocytes in HNF4α-KO mice exhibited a decrease in glycogen and a substantial increase in hepatic fat content. These data are reflective of the metabolic changes induced in the liver due to a lack of HNF4α, which regulates many of the genes involved in glycogen synthesis (Gys2) (26) and lipid transport (Apoa2, Apoa4, Apob, Apoc2, Apoc3, and MTP) (17) and are consistent with the observations made by Bonzo, et al.
To determine the mechanisms by which HNF4α inhibits hepatocyte proliferation, we performed a global gene expression study using Illumina Hiseq2000-based RNA sequencing combined with functional and pathway analysis. A majority of the genes down regulated after HNF4α deletion are previously known HNF4α targets, mainly involved in hepatic differentiation. Interestingly, many of the genes up regulated are involved in cell cycle control and cancer (Table 2). IPA-mediated functional analysis reveals that the major classes of genes changed following HNF4α deletion are in cancer and cell proliferation category. The up-regulation of pro-mitogenic genes explains the significant increase in proliferation within the liver of HNF4α-KO mice. This observation also raises questions regarding the mechanism by which HNF4α is regulating promitogenic genes. Whereas beyond the scope of this study, a closer look at the up-regulated genes in HNF4α-KO mice raises the possibility that HNF4α inhibits hepatocyte proliferation via both direct and indirect inhibition for select sub-populations of genes.
Bonzo, et al. first reported the observation that deletion of HNF4α results in an increase in hepatocyte proliferation due to an increase in pro-mitogenic gene expression. The data obtained in this study further confirmed that HNF4α inhibits proliferation through the inhibition of genes involved in cell cycle control. Analysis within the Bonzo et al. study is performed 19 days following initial tamoxifen exposure. Our study strengthens their findings by showing that hepatocyte proliferation and changes in pro-mitogenic gene expression occur as early as 7 days after HNF4α deletion. This suggests that the increased pro-mitogenic gene expression and hepatocyte proliferation may be due directly to the loss of HNF4α as opposed to another factor that HNF4α may regulate. We have recently made similar observation using a adeno-associated virus 8-mediated Cre system (19).
Our analysis revealed that a large number of the genes up-regulated after HNF4α deletion are regulated by c-Myc. The RNA-seq data showed a 3.8-fold increase in c-Myc gene expression corroborating these results. Previous studies have indicated that HNF4α competes with c-Myc for binding on the promoter of cell cycle inhibitor p21/WAF1 (27). Further analysis revealed that several genes up regulated in the c-Myc gene network are involved stimulation of cell proliferation and cancer pathogenesis including the set oncoprotein, fus, ccnb1, and ccnb2. These data indicate that HNF4α may indirectly down regulate these genes via suppressing c-Myc activation in normal adult hepatocytes.
It has been speculated that deletion of HNF4α will result in rapid liver failure making it difficult to directly study its role in the pathogenesis of HCC (17). Whether HNF4α deletion itself can result in hepatocarcinogenesis is not known and may be difficult to study due to limitations of the model system; therefore, we decided to investigate whether HNF4α deletion can promote existing tumors in the liver and can be tested using the two-stage DEN-induced chemical carcinogenesis model. Our studies indicate that HNF4α deletion during the late stage of HCC progression can substantially promote DEN-induced hepatic tumor formation. Our results show that deletion of HNF4α in mice treated with DEN results in a vast expansion in tumor size and tumor number. Furthermore, the tumors, as well as surrounding tissues, in HNF4α-KO mice showed extensive up regulation of c-Myc and Cyclin D1. These data further support the hypothesis that HNF4α inhibits hepatocyte proliferation by inhibiting the c-Myc gene network.
RNA-seq analysis revealed several up regulated genes, which are potentially negatively regulated by HNF4α. A few of these genes have a putative HNF4α binding site on their promoter and may be targets of direct inhibition by HNF4α (Ect2 and Cdc20), one of which we have confirmed in previous studies using ChIP (Ect2)(19); however, a vast number of the up regulated genes do not have an HNF4α binding site including Cyclin D1 and c-Myc, and direct regulation of these genes at the level of transcription is unlikely. It is possible that HNF4α may regulate these genes indirectly via an intermediary pathway, or via miRNAs as shown by Hatziapostolou, et al. (28). They provide evidence of an “HNF4α circuit” involving miR-124, IL6R, STAT3, and miR-24/miR-629 in the regulation of hepatocarcinogenesis. They show a correlation between the downregulation of HNF4α and miR-24 and an upregulation of IL6R and STAT3 associated with the progression of HCC. We cannot comment on the expression of miRs in our model at this time, but we do not observe an increase in IL6R or STAT3. This may be due to a lack of inflammatory responses within our model, which may be a mediating event in the activation of the “HNF4α circuit”. With this said, it is still very much a possibility that HNF4α is regulating many of the gene expression changes that we observe by an indirect mechanism involving miRNAs.
Taken together, our data indicates that HNF4α is not only an important factor in the regulation of hepatocyte differentiation, but also as a critical player in the inhibition of hepatic proliferation. Our study sheds light on the mechanism of HNF4α-mediated inhibition of cell proliferation and indicates that HNF4α inhibits hepatocyte proliferation by down regulation of pro-mitogenic genes such as c-Myc. These data suggest a novel role as a tumor suppressor and highlight HNF4α as a potential therapeutic target, as well as a prognostic marker, for liver cancers.
Supplementary Material
Figure 5.
HNF4α deletion promotes DEN-induced hepatic tumors in mice. (A) Scheme showing protocol of DEN-induced hepatic tumor induction. (B) Representative photographs of livers of Control mice (left) and HNF4α-KO mice (right) treated with DEN. (C) Liver to body weight ratios of Control and HNF4α-KO mice treated with DEN. (D) Representative photomicrographs of paraffin embedded liver sections stained for H&E from Control (i) and HNF4α-KO mice (ii to vi). Large arrowheads show oval cell like cells in (ii) and (iv). Arrows point to biliary duct proliferation in the mixed type tumors in (iii) and (v). Small arrowheads indicate inflammatory cell focus in (vi). (E) Representative photomicrographs of PCNA immunohistochemistry on paraffin section from control (i) and HNF4α-KO mice (ii and iii). Arrowheads point to proliferating biliary duct cells.
Acknowledgments
Financial Support: These studies were supported by NIH - P20 RR021940 (Udayan Apte), and AASLD/ALF Liver Scholar Award (Udayan Apte). The RNA sequencing studies were performed by the Kansas University Medical Center-Microarray Facility (KUMC-MF), which is supported by the Kansas University-School of Medicine, KUMC Biotechnology Support Facility, the Smith Intellectual and Developmental Disabilities Research Center (HD02528), and the Kansas IDeA Network of Biomedical Research Excellence (RR016475).
List of Abbreviations
- HNF4α
hepatocyte nuclear factor 4 alpha
- PCNA
proliferating cell nuclear antigen
- HCC
hepatocellular carcinoma
- IPA
Ingenuity Pathway Analysis
- RNA-seq
RNA sequencing
- DEN
diethylnitrosamine
- TAM
tamoxifen
References
- 1.Sladek FM. Orphan receptor HNF-4 and liver-specific gene expression. Receptor. 1993;3:223–232. [PubMed] [Google Scholar]
- 2.Sladek FM, Zhong WM, Lai E, Darnell JE. Liver-Enriched Transcription Factor Hnf-4 Is a Novel Member of the Steroid-Hormone Receptor Superfamily. Genes & Development. 1990;4:2353–2365. doi: 10.1101/gad.4.12b.2353. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez FJ. Regulation of hepatocyte nuclear factor 4 alpha-mediated transcription. Drug Metab Pharmacokinet. 2008;23:2–7. doi: 10.2133/dmpk.23.2. [DOI] [PubMed] [Google Scholar]
- 4.Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inoue Y, Peters LL, Yim SH, Inoue J, Gonzalez FJ. Role of hepatocyte nuclear factor 4 alpha in control of blood coagulation factor gene expression. Journal of Molecular Medicine-Jmm. 2006;84:334–344. doi: 10.1007/s00109-005-0013-5. [DOI] [PubMed] [Google Scholar]
- 6.Inoue Y, Yu AM, Inoue J, Gonzalez FJ. Hepatocyte nuclear factor 4 alpha is a central regulator of bile acid conjugation. Journal of Biological Chemistry. 2004;279:2480–2489. doi: 10.1074/jbc.M311015200. [DOI] [PubMed] [Google Scholar]
- 7.Inoue Y, Yu AM, Yim SH, Ma X, Krausz KW, Inoue J, Xiang CC, et al. Regulation of bile acid biosynthesis by hepatocyte nuclear factor 4alpha. J Lipid Res. 2006;47:215–227. doi: 10.1194/jlr.M500430-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weissglas-Volkov D, Huertas-Vazquez A, Suviolahti E, Lee J, Plaisier C, Canizales-Quinteros S, Tusie-Luna T, et al. Common hepatic nuclear factor-4alpha variants are associated with high serum lipid levels and the metabolic syndrome. Diabetes. 2006;55:1970–1977. doi: 10.2337/db06-0035. [DOI] [PubMed] [Google Scholar]
- 9.Yamagata K, Furuta H, Oda N, Kaisaki PJ, Menzel S, Cox NJ, Fajans SS, et al. Mutations in the hepatocyte nuclear factor-4alpha gene in maturity-onset diabetes of the young (MODY1) Nature. 1996;384:458–460. doi: 10.1038/384458a0. [DOI] [PubMed] [Google Scholar]
- 10.Lazarevich NL, Cheremnova OA, Varga EV, Ovchinnikov DA, Kudrjavtseva EI, Morozova OV, Fleishman DI, et al. Progression of HCC in mice is associated with a downregulation in the expression of hepatocyte nuclear factors. Hepatology. 2004;39:1038–1047. doi: 10.1002/hep.20155. [DOI] [PubMed] [Google Scholar]
- 11.Ning BF, Ding J, Yin C, Zhong W, Wu K, Zeng X, Yang W, et al. Hepatocyte nuclear factor 4 alpha suppresses the development of hepatocellular carcinoma. Cancer Res. 2010;70:7640–7651. doi: 10.1158/0008-5472.CAN-10-0824. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Hui L, Wang S, Gong J, Jin Y, Wang Y, Ji Y, et al. Expression profiling suggested a regulatory role of liver-enriched transcription factors in human hepatocellular carcinoma. Cancer Res. 2001;61:3176–3181. [PubMed] [Google Scholar]
- 13.Erdmann S, Senkel S, Arndt T, Lucas B, Lausen J, Klein-Hitpass L, Ryffel GU, et al. Tissue-specific transcription factor HNF4alpha inhibits cell proliferation and induces apoptosis in the pancreatic INS-1 beta-cell line. Biol Chem. 2007;388:91–106. doi: 10.1515/BC.2007.011. [DOI] [PubMed] [Google Scholar]
- 14.Flodby P, Liao DZ, Blanck A, Xanthopoulos KG, Hallstrom IP. Expression of the liver-enriched transcription factors C/EBP alpha, C/EBP beta, HNF-1, and HNF-4 in preneoplastic nodules and hepatocellular carcinoma in rat liver. Mol Carcinog. 1995;12:103–109. doi: 10.1002/mc.2940120207. [DOI] [PubMed] [Google Scholar]
- 15.Grigo K, Wirsing A, Lucas B, Klein-Hitpass L, Ryffel GU. HNF4 alpha orchestrates a set of 14 genes to down-regulate cell proliferation in kidney cells. Biol Chem. 2008;389:179–187. doi: 10.1515/BC.2008.011. [DOI] [PubMed] [Google Scholar]
- 16.Kalkuhl A, Kaestner K, Buchmann A, Schwarz M. Expression of hepatocyte-enriched nuclear transcription factors in mouse liver tumours. Carcinogenesis. 1996;17:609–612. doi: 10.1093/carcin/17.3.609. [DOI] [PubMed] [Google Scholar]
- 17.Bonzo JA, Ferry CH, Matsubara T, Kim JH, Gonzalez FJ. Suppression of hepatocyte proliferation by hepatocyte nuclear factor 4alpha in adult mice. J Biol Chem. 2012;287:7345–7356. doi: 10.1074/jbc.M111.334599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WS, Manova K, Weinstein DC, Duncan SA, Plump AS, Prezioso VR, Bachvarova RF, et al. Disruption of the HNF-4 gene, expressed in visceral endoderm, leads to cell death in embryonic ectoderm and impaired gastrulation of mouse embryos. Genes Dev. 1994;8:2466–2477. doi: 10.1101/gad.8.20.2466. [DOI] [PubMed] [Google Scholar]
- 19.Walesky C, Gunewardena S, Terwilliger EF, Edwards G, Borude P, Apte U. Hepatocyte-Specific Deletion of Hepatocyte Nuclear Factor 4 alpha in Adult Mice Results in Increased Hepatocyte Proliferation. Am J Physiol Gastrointest Liver Physiol. 2012 doi: 10.1152/ajpgi.00064.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Borude P, Edwards G, Walesky C, Li F, Ma X, Kong B, Guo GL, et al. Hepatocyte specific deletion of farnesoid X receptor delays, but does not inhibit liver regeneration after partial hepatectomy in mice. Hepatology. 2012 doi: 10.1002/hep.25918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, Salzberg SL, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoffman BG, Robertson G, Zavaglia B, Beach M, Cullum R, Lee S, Soukhatcheva G, et al. Locus co-occupancy, nucleosome positioning, and H3K4me1 regulate the functionality of FOXA2-, HNF4A-, and PDX1-bound loci in islets and liver. Genome Res. 2010;20:1037–1051. doi: 10.1101/gr.104356.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Langmead B, Salzberg SL. Fast gapped-read alignment with Bowtie 2. Nat Methods. 2012;9:357–359. doi: 10.1038/nmeth.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Liu T, Meyer CA, Eeckhoute J, Johnson DS, Bernstein BE, Nusbaum C, et al. Model-based analysis of ChIP-Seq (MACS) Genome Biol. 2008;9:R137. doi: 10.1186/gb-2008-9-9-r137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sandelin A, Alkema W, Engstrom P, Wasserman WW, Lenhard B. JASPAR: an open-access database for eukaryotic transcription factor binding profiles. Nucleic Acids Res. 2004;32:D91–94. doi: 10.1093/nar/gkh012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mandard S, Stienstra R, Escher P, Tan NS, Kim I, Gonzalez FJ, Wahli W, et al. Glycogen synthase 2 is a novel target gene of peroxisome proliferator-activated receptors. Cell Mol Life Sci. 2007;64:1145–1157. doi: 10.1007/s00018-007-7006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang-Verslues WW, Sladek FM. Nuclear receptor hepatocyte nuclear factor 4alpha1 competes with oncoprotein c-Myc for control of the p21/WAF1 promoter. Mol Endocrinol. 2008;22:78–90. doi: 10.1210/me.2007-0298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hatziapostolou M, Polytarchou C, Aggelidou E, Drakaki A, Poultsides GA, Jaeger SA, Ogata H, et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell. 2011;147:1233–1247. doi: 10.1016/j.cell.2011.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.