Abstract
Biomimetic reconstruction of tooth enamel is a significant topic of study in material science and dentistry as a novel approach for prevention, restoration, and treatment of defective enamel. We developed a new amelogenin-containing chitosan hydrogel for enamel reconstruction that works through amelogenin supramolecular assembly, stabilizing Ca-P clusters and guiding their arrangement into linear chains. These amelogenin Ca-P composite chains further fuse with enamel crystals and eventually evolve into enamel-like co-aligned crystals, anchoring to the natural enamel substrate through a cluster growth process. A dense interface between the newly-grown layer and natural enamel was formed and the enamel-like layer had improved hardness and elastic modulus compared to etched enamel. We anticipate that chitosan hydrogel will provide effective protection against secondary caries because of its pH-responsive and antimicrobial properties. Our studies introduce amelogenin-containing chitosan hydrogel as a promising biomaterial for enamel repair and demonstrate the potential of applying protein-directed assembly to biomimetic reconstruction of complex biomaterials.
Keywords: Enamel, Amelogenin, Chitosan Hydrogel, Apatite, Biomimetic
1. Introduction
Enamel is the exterior layer of the mammalian tooth and a hard biomaterial with significant resilience that protects the tooth from external physical and chemical damage [1]. The remarkable mechanical properties of enamel are associated with its hierarchical levels of structure from the nanoscale to the macroscale [1]. The building blocks of enamel, the enamel rods, are densely packed arrays of elongated apatite crystals organized into an intricate interwoven structure [2]. Cellular activities and protein-controlled process of mineralization are key to achieving such precisely organized structures [1]. The proteins that mediate the mineralization of apatite crystals are gradually degraded and eventually removed during enamel maturation [1, 3, 4]. Mature enamel is non-living and cannot regenerate itself after substantial mineral loss, which often occurs as dental caries or erosion. Currently, the conventional treatments for carious lesions include refilling with amorphous materials like amalgam, ceramics, or composite resin [5]. However, even after those treatments, secondary caries often arise at the interface between the original enamel and the filling materials due to weakening adhesion over time [6]. There is therefore a need for alternative restorative material with improved adhesion to the tooth surface. One such alternative is a synthetic enamel-like material that can be prepared by biomimetic regrowth on the enamel surface.
Various biomimetic systems have been developed by investigators for repair of enamel defects, including liquids and pastes that contain nano-apatite or different organic additives, for the remineralization of early, sub-micrometer-sized enamel lesions. A glycerine-enriched gelatin system has been used to form dense fluorapatite layers on human enamel [7, 8]. Growth in small cavities of enamel-like nanocrystals from a paste containing fluoride-substituted hydroxyapatite has been achieved in vitro [9], and a compacted fluorapatite film with a prism-like structure was synthesized on metal plates using a hydrothermal technique [10]. Formation of enamel-like structures under ambient conditions was also performed in vitro using liquid and pastes with different organic additives [11–15]. Recently, an electrospun hydrogel mat of amorphous calcium phosphate (ACP)/poly(vinylpyrrolidone) nanofibers was developed for the in vitro remineralization of dental enamel. [16] These investigations constitute significant progress in the study of enamel-like structures. Overall, however, biomimetic strategies still face an ongoing challenge in the fields of dentistry and material science.
In natural enamel, the formation of apatite crystals occurs in an amelogenin-rich matrix that plays a critical role in controlling the oriented and elongated growth of apatite crystals [4, 17–20]. Accordingly, we have used several strategies to prepare enamel-like materials that contain nano- and microstructures using amelogenin to control the crystallization of biomimetic calcium and phosphate [20–23]. The results have opened up the promising possibility of remodeling of complex enamel minerals in an amelogenin-containing system.
Here we report development of a new amelogenin-containing chitosan (CS-AMEL) hydrogel to synthesize an organized, enamel-like mineralized layer on an acid-etched enamel surface used as an early caries model. Compared to the previous amelogenin-containing system that was developed, CS-AMEL is easier to handle in the clinic. It is biocompatible, biodegradable, and has unique antimicrobial and adhesion properties that are practical for dental applications [24–26]. Chitosan has been observed to have antimicrobial activity against fungi, viruses, and some bacteria, including streptococci and lactobacilli, which are known as the principal etiological factors of dental caries [27–29]. Therefore, we expect that the “synthetic enamel” formed in CS-AMEL hydrogel will have antimicrobial properties that can prevent bacterial infection and subsequent demineralization. In addition, chitosan is mucoadhesive to both hard and soft surfaces [30]. Importantly, the newly formed crystals in CS-AMEL hydrogel grow directly on the original enamel, achieving a complete adhesion of the repaired layer to the natural enamel with a dense interface. The robust attachment of the newly grown layer demonstrated in the present work can potentially improve the durability of restorations and avoid the formation of new caries at the margin of the restoration.
2. Materials and Methods
2.1. Amelogenin preparation
Recombinant full-length porcine amelogenin rP172 was expressed in E.coli and purified as described previously. The rP172 protein has 172 amino acids and is an analogue of the full-length native porcine P173, but lacking the N-terminal methionine as well as a phosphate group on Ser16 [21].
2.2. Tooth slice preparation
Human third molars (extracted following the standard procedures for extraction at the Ostrow School of Dentistry of the University of Southern California and handled with approval of the Institutional Review Board) without any restored caries were selected. Slices 0.1–0.2 cm in thickness (Fig. 1a), were cut longitudinally using a low speed diamond saw cooled by water. To simulate early caries lesions, tooth slices were acid-etched with 30% phosphoric acid for 30 s and rinsed with deionized water.
Fig. 1.

a) Optical photograph of a tooth slice used in this work. b) SEM image of acid-etched enamel surface.
2.3. Etched enamel repaired by amelogenin-containing chitosan hydrogel
Amelogenin-containing chitosan hydrogel was prepared by mixing chitosan (medium molecular weight, 75–85% deacetylated, Sigma-Aldrich) solution (960 μl, 1% m/v), Na2HPO4 (15 μl, 0.1 M), CaCl2 (25 μl, 0.1 M) and amelogenin rP172 (200 μg), followed by stirring at room temperature overnight, and the pH value was adjusted to 6.5 with 1M NaOH. Twenty μl of chitosan-based hydrogel was carefully applied onto the enamel surface and dried in air at room temperature. The tooth slices were then immersed in 30 ml of artificial saliva solution (MgCl2 0.2 mM, CaCl2·H2O 1 mM, HEPES buffer 20 mM, KH2PO4 4 mM, KCl 16 mM, NH4Cl 4.5 mM, NaF 300 ppm, pH = 7.0, adjusted with 1 M NaOH) [16] at 37 °C for 7 days. After the allotted time, the tooth slice was removed from the solution, rinsed with running deionized water for 50 s and air-dried.
2.4. Characterization
SEM imaging was performed on a field emission scanning electron microscope (JEOL JSM-7001F), operating at an accelerating voltage of 10 kV. X-ray diffraction (XRD) patterns were recorded on a Rigaku Diffractometer with Cu Kr radiation (λ = 1.542 Å) operating at 70 kV and 50 mA with a step size of 0.02°, at a scanning rate of 0.1° s−1 in a 2θ range from 10° to 60°. HRTEM images were obtained on a JEOL JEM-2100 microscope using an accelerating voltage of 200 kV. The hardness and elastic modulus were measured at 20 test points in each sample (n = 3) using a Nano-indenter (Agilent-MTS XP) with a Berkovich tip. Circular dichroism spectropolarimetry (CD) was performed using a JASCO J-815 spectropolarimeter (JASCO, Easton, Md., USA). The spectra were recorded between 190 and 260 nm with a step size of 0.5 nm and a scan rate of 50 nm/min. Fluorescence spectroscopy was performed using a PTI QuantaMaster QM-4SE spectrofluorometer (PTI, Birmingham, N.J., USA). The amelogenin solutions were excited at 290 nm. The emission spectra were monitored between 300 and 400 nm with a step size of 1 nm.
2.5. Antimicrobial evaluation
Human saliva was collected as described in the literature [32] for the antimicrobial experimentation: Healthy adults were chosen as subjects for collecting saliva. Subjects were asked to refrain from eating, drinking, and oral hygiene procedures for at least 1 h prior to the collection. Subjects were given distilled drinking water and asked to rinse their mouths out with it for 1 min. Five minutes after this oral rinse, subjects were asked to spit into a 50 ml sterile tube, which was placed on ice while collecting more saliva. Subjects were instructed to tilt the head forward and let the saliva run naturally to the front of the mouth; Upon the collection of approximately 5 ml volume of saliva from the subject, the saliva sample was taken to the laboratory immediately for processing. 20μl of saliva were added to tubes with 1 ml of lysogeny broth (LB) media containing chitosan-amelogenin hydrogel or amelogenin, and then incubated at 37 °C overnight. The OD600 of the overnight cultures was measured by a Beckman DU-640 Spectrophotometer.
2.6 Statistical analysis
Enamel remineralization experiments were repeated three times. The mechanical tests and antimicrobial experiments were conducted in triplicate and data were expressed as mean ± standard deviations. For mechanical testing the Student’s t-test was applied to identify differences in the hardness and elastic modulus between etched and repaired enamel of samples (n = 3). For the antimicrobial experiments (n = 3) the OD values were compared between control and samples containing chitosan-amelogenin hydrogel or amelogenin by the same test. In all experiments the differences were considered statistically significant at p≤0.05 and highly significant at p < 0.001. All the statistical analyses were carried out using Origin 8.0 (Origin lab, Northampton, MA) and Microsoft Office Excel 2007.
3. Results and Discussion
3.1. Enamel remineralization without CS-AMEL hydrogel
Dental caries is caused by an imbalance in the dynamic process of demineralization–remineralization of enamel [33]. Enamel demineralization occurs at a low pH caused by acids of bacterial origin. To produce artificial caries, a tooth slice was etched with 30% phosphoric acid. When examined, the etched enamel crystals were seen to be discontinuous and broken, resembling crystals from carious enamel (Fig. 1b) [34]. Although the calcium and phosphate ions in the saliva permit the recovery of some lost enamel mineral, the remarkably organized structure of enamel cannot be regained without protein mediation. We prepared an artificial saliva (AS) solution to simulate the oral environment for enamel remineralization. After soaking in AS solution alone for 7 days, a calcium phosphate coating with a thickness of 1 μm was formed on the surface of enamel. As shown in Fig 2a and b, the remineralized crystals had rod-like structure and the coating was porous. A similar layer but with a thickness of 10 μm was formed on the etched enamel soaked in chitosan hydrogel without amelogenin. This remineralized apatite layer also consisted of loosely packed crystals with a porous structure (Figures 2c and d). These porous layers did not resemble natural enamel structure, which has a high packing density of apatite crystals.
Fig. 2.
SEM images of newly grown layer without amelogenin after remineralization in an artificial saliva solution for 7 days. a, c) top view, b, d) side view. a, b) Without, c, d) with chitosan gel.
3.2. Enamel remineralization with CS-AMEL hydrogel
Fig. 3 shows the microstructures of human molar enamel and the newly-grown layer on an etched enamel surface soaked for 7days in CS-AMEL hydrogel. At the nanoscale (Fig. 3a), natural enamel is made of highly organized arrays of apatite crystallites growing preferentially along the c-axis, perpendicular to the surface (black arrows in Fig. 3a). After mineralization for 7 days, similar organized crystals were formed on the etched enamel surface treated by CS-AMEL hydrogel. The crystals grown in CS-AMEL hydrogel were composed of numerous nanorods oriented preferentially along the c-axis with a diameter of ~50 nm, nearly parallel to each other in the longitudinal direction (white arrows in Figs. 3b). The newly-grown layer, with a thickness of 15 μm, was tightly bound to the surface of the natural enamel (Fig. 3b inset). Examination at higher magnification revealed no obvious boundary at the interface (Fig. 3c). The bulk of the newly-grown layer contained needle-like crystals that were bundled to form a fundamental organization unit analogous to that of natural enamel crystallites (Figs. 3d and 3e). The high-resolution transmission electron microscopy (HRTEM) image showed clear lattice fringes perpendicular to the nanorod axis with an interplanar spacing of d = 0.3429 nm, in accordance with the distance between the (002) crystal planes of hydroxyapatite (JCPDS 09-0432), which suggests that the nanorods formed in CS-AMEL hydrogel grow in the [001] direction (white arrow in Fig. 3e). Selected area electron diffraction (SAED) of the newly-grown layer resulted in an arc-shaped pattern along the (002) diffraction plane, indicating a hierarchical alignment of the c-axes of the newly formed crystals (Fig. 3f).
Fig. 3.
SEM and TEM images of natural enamel and newly-grown layer after remineralization in amelogenin-chitosan gel for 7 days. a) Microstructure of native enamel. Black arrows indicate the crystallographic orientations of the apatite crystallites in native enamel. b) After 7 days of remineralization with chitosan-amelogenin hydrogel, an enamel-like layer was formed on the surface of etched enamel; Inset shows thickness of newly-grown layer; Rectangle 1 and 2 represent the selected areas corresponding to b and c. White arrows indicate the apatite orientations in the newly-grown layer. c) The newly-grown layer was bound firmly to the surface of enamel. d) Bundles of organized crystals were found inside the repaired layer. The arrows present a typical bundle of paralleled crystals inside the newly-grown layer. Inset shows the homogeneous surface of the repaired layer. e) HRTEM image of a rod-like crystal taken from the area outlined by the red rectangle on the crystal bundle in the inset. The arrow indicates the crystallographic direction of an apatite crystal along the c-axes. HRTEM image represents a typical bundle of paralleled crystals. f) Selected area electron diffraction (SAED) image of the newly-grown layer. Inset shows TEM image of the repaired layer prepared by focused ion beam (FIB) milling.
The orientation and composition of the newly-grown crystals were further confirmed by x-ray diffraction (XRD) and energy dispersion spectroscopy (EDS) (Fig. 4). All of the diffraction peaks can be readily indexed to hexagonal phase hydroxyapatite (JCPDS 09-0432) crystals. The unsplit diffraction peak around 2θ = 32° indicates the poor crystallinity of newly-formed apatite in CS-AMEL hydrogel (Fig. 4a) [35]. Sharp and intense 002 and 004 peaks indicates that the (001) faces are parallel to the surface (Fig. 4a), i.e., the crystals align in an orderly fashion along the crystallographic c-axis, in accordance with the microstructure obtained by SEM and TEM observations (Fig. 3). EDS revealed the presence of calcium, phosphate and fluorine ions in the newly-grown layer (Fig 4b). The structural and compositional analyses indicated that the newly-formed layer contain fluoridated hydroxyapatite (FAP) with poor crystallinity [17].
Fig. 4.
a) XRD spectra of newly-grown layer after remineralization in a chitosan gel a) with and b) without amelogenin for 7 days. b) EDS spectrum of repaired layer after remineralization in a chitosan gel with amelogenin for 7 days.
3.3. Functions of chitosan and amelogenin in enamel remineralization with CS-AMEL hydrogel
Comparing the morphologies of remineralized layers formed in chitosan hydrogel with and without amelogenin, we observed that disordered structures with porous morphology were formed without the protein (Fig. 2), but ordered enamel-like structures were obtained in the presence of amelogenin (Figs. 3b–f). These results indicate that amelogenin mediation is an essential factor for the formation of the orderly enamel-like structure in the chitosan hydrogel system. Although chitosan hydrogel has also been reported as a mineralization matrix because of its charged surface [36], the chitosan molecules were not found to affect the function of amelogenin during the synthesis of the repaired layer.
The chitosan-amelogenin interaction was studied by using Circular Dichroism (CD) and Fluorescence spectroscopy at pH 3.5, 5.5 and 8.0 (Fig 5). All the CD spectra of pure amelogenin showed negative ellipticities around 203 nm, which were characteristic of unordered polyproline type II structures [37]. At pH 3.5, the intensity of the minima gradually increased and the trough shifted to a higher wavelength with increasing ratios of chitosan to amelogenin, indicating a possible change in the conformation of amelogenin in the presence of chitosan. In the corresponding fluorescence spectra, red shifts of the emission maxima were also observed with increasing amounts of chitosan, indicating the exposure of tryptophan residues belonging to the amelogenin (Fig 5a) [38, 39]. These changes in the CD and fluorescence spectra clearly illustrated that there was a direct interaction between amelogenin and chitosan at pH 3.5. At pH 5.5, both the intensity of the negative dichroic signals and the positions of their minima were changed with the addition of chitosan to the amelogenin; however, there was no shift in the fluorescence spectra (Fig 5b). When the pH reached 8.0, it was difficult to find the dichroic signal or the emission band in CD and fluorescence spectra of amelogenin in association with chitosan (Fig 5c). The results from the CD and fluorescence spectra revealed that the interaction between chitosan and amelogenin is dependent on pH. At pH below the pKa of chitosan (6.5) [40], the amino groups were almost completely ionized, and the charge density of chitosan increased; thus chitosan interacted with amelogenin through electrostatic interaction. In contrast, when pH was higher than 5.5, chitosan’s interaction was weak because of its low solubility and deprotonation.
Fig. 5.
CD and fluorescence spectra were measured with different mass ratio at pH a) 3.5, b) 5.5 and c) 8.0, revealing that the interaction between chitosan and amelogenin is pH dependent.
Therefore, under our experimental conditions (pH > 6.5), amelogenin is the crucial factor in controlling the oriented growth of fluoridated hydroxyapatite crystals. Even so, the role of chitosan is likely more than just as an amelogenin carrier. Chitosan in the CS-AMEL system could provide effective protection from enamel erosion because of its pH-responsiveness. The development of caries is associated with a continuous pH change in plaque biofilm due to the accumulation of acid byproducts from metabolism of fermentable carbohydrates [33, 41, 42]. As the pH decreases (in the general range of 5.5-5.0), positive hydrogen ions from the acid bind to the negative phosphate and hydroxyl ions from enamel mineral leading to mineral loss. The potential advantage of having chitosan present on the enamel surface is that the amino group of chitosan could capture the hydrogen ions from the acid, forming a positive protective layer to prevent the diffusion of hydrogen ions to the mineral surface, as well as interacting with amelogenin to avoiding amelogenin loss into the saliva. When normal pH is restored (to the range of 6.3–7.0) by the saliva [43], the weakly-interacting amelogenin would be released from the chitosan to regulate the remineralization of enamel.
Our HRTEM and SEM analyses provide further insight into the function of amelogenin in remineralization of newly-grown mineral. In the original CS-AMEL hydrogel, we observed linear chains of ~10 nm nanoclusters, as shown in Fig 6a. The calcium phosphate (Ca-P) clusters formed in the Ca-P supersaturated condition are thought to be building blocks of both amorphous calcium phosphate as well as apatitic mineral phase [44, 45]. Generally, without a stabilizing agent, these Ca-P clusters aggregate randomly to form plate-like mineral particles [46, 47]. Indeed, we could not find any oriented aggregation of Ca-P clusters in the original chitosan hydrogel in the absence of amelogenin. We suggest that the presence of amelogenin provides an opportunity for stabilization of the precritical clusters at a minimum free energy since the coassembly of Amel-Ca-P clusters imparts kinetic and thermodynamic stability to the system [47]. As a result and as in previous studies [46, 47], we suggest that amelogenin assemblies stabilized the Ca-P clusters in the CS-AMEL hydrogel and guided their arrangement of clusters into linear chains that eventually evolved into enamel-like co-aligned crystals anchored to the natural enamel substrate.
Fig. 6.
a) TEM image of the original CS-AMEL hydrogel showing the elongated nanochain-like structure (white arrows). b) Cross-section SEM image of repaired layer after remineralization in amelogenin-chitosan gel for 3 days fused to the surface of the natural enamel. The white and black arrows indicate the crystallographic orientations of the crystals in newly-grown layer and natural enamel, respectively. The dot line shows the boundary of the natural enamel and the newly grown layer. c) HRTEM image taken from the interface between the enamel and regrown crystal, showing seamless growth of repaired crystal on the enamel. The black arrows indicate the interface between regrown and enamel crystals. The inset shows the fast Fourier transform (FFT) images corresponding to enamel and regrown crystals. d) Schematic illustration of the enamel repair process.
3.4. Continuous growth of newly-formed crystals on the enamel
SEM images of the side view of a layer grown in CS-AMEL hydrogel for 3 days (Fig. 6b) indicate that the newly-grown crystals are mostly oriented perpendicular to the surface of the substrate and in non-prismatic orientations (white arrows in Fig. 6b). The interface, indicated by the dotted line, between the repaired layer and the enamel substrate reveals no apparent gap. To further explore the interface microstructure, we used a focused ion beam (FIB) technique to prepare TEM samples for higher resolution analysis. Fig. 6c depicts the HRTEM image of the interface where the new crystals nucleate, clearly exhibiting lattice fringes from the (301) and (103) planes of the enamel crystal (d = 0.261 nm and d = 0.236 nm), as well as the (002) plane of the synthetic crystal (d = 0.339). The corresponding fast Fourier transform (FFT) images (inset in Fig. 6c) show two different patterns, indicating that the crystals in the enamel and in the fused repaired layer grew with different orientation, which is consistent with the SEM observations in Fig 6b. Remarkably, the enamel and the newly-grown crystals fused together to form a seamless interface (black arrows in Fig 6c).
Although the exact growth mechanism remains unresolved, it is clear that amelogenin-stabilized clusters with orientated aggregation are crucial to the continuous growth of new crystals on the enamel. Recent research has shown that calcium-based biominerals can be formed at a templating surface via stable pre-nucleation clusters, with aggregation into an amorphous precursor phase and transformation of this phase into a crystal [48, 49]. Similar to these cluster-growth models [49, 50], the newly-grown crystals formed in CS-AMEL hydrogel in our experiments started with the aggregation of prenucleation clusters leading to the nucleation of ACP and then the development of oriented apatite crystals. The possible repair processes are schematically presented in Fig 6d. Initially, the pre-nucleation Ca-P clusters, stabilized by amelogenin, aggregate to form linear chains in the CS-AMEL hydrogel. Subsequently, parts of the cluster aggregates in contact with the enamel surface start to become dense by adopting a closer packing of the clusters. The continuation of this process leads to the formation of an amorphous precursor phase that further fuses with enamel crystals and ultimately transforms into crystalline apatite, which is oriented along the c-axis as directed by amelogenin. As a result, the newly-formed crystals are continuously grown on the enamel crystals and oriented by amelogenin so that their long axes run perpendicular to the enamel surface like natural enamel prisms [2].
3.5. Bonding strength between newly-grown layer and enamel surface
The dense interface between synthetic and natural enamel crystals promoted strong bonding between the newly-grown layer and the tooth surface. Fig. 7 shows the backscattered electron and secondary electron images of the newly-grown layer after ultrasonic treatment (42 kHz, 100 W) for 10 min. The results revealed that the newly-grown layer formed in the CS-AMEL hydrogel was tightly bound to the enamel surface (Fig. 7a), and the organized structure was unaffected by the ultrasonic treatment (Fig. 7b). In contrast, following the same treatment we observed a large gap between the enamel and the repaired layer formed in the chitosan hydrogel without amelogen in (Fig 7c). In clinical dentistry bonding strength is one of the most important attributes for enamel restorative materials. Due to poor adhesion that leads to gaps at the enamel-restoration interface the currently available materials often have limitation in their durability. These gaps increase the possibility for bacterial leakage and secondary caries, which are the main causes of restoration failure [50]. In the present study, the robust attachment of the newly-grown layer formed in the CS-AMEL hydrogel can potentially improve the durability of restorations and avoid the formation of new caries at the margin of the restoration.
Fig. 7.
SEM images of reconstructed enamel-like layers after ultrasonic treatment. a) backscattered electron image of the cross section, and b) second electron image of the surface of an ultrasonic-treated newly-grown layer obtained in a chitosan-amelogenin hydrogel. Inset shows the typical morphology of the surface at a higher magnification. c) backscattered electron image of the cross section of an ultrasonic-treated newly-grown layer obtained in a chitosan hydrogel without amelogenin.
3.6. Mechanical properties of reconstructed enamel-like layer repaired by CS-AMEL hydrogel
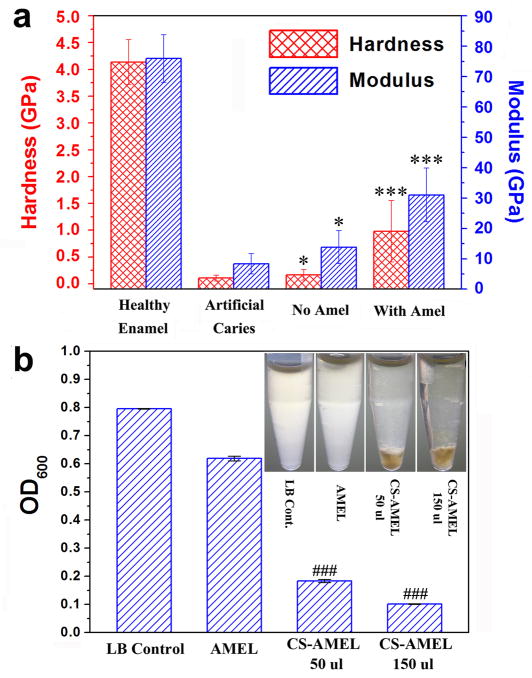
Fig. 8a shows the hardness and elastic modulus of healthy enamel, etched enamel, and the reconstructed enamel-like layer repaired by chitosan hydrogel with and without amelogenin. The hardness and modulus of caries-free enamel slice were estimated to be 4.0 GPa and 70 GPa respectively [51], and both the hardness and modulus were severely compromised by acid etching (nearly 88% decrease in modulus and 98% decrease in hardness). After mineralization in chitosan hydrogel without amelogenin, we only observed slight increase in the hardness and modulus of etched enamel surface (Fig. 8a). Clearly, the porous structure (Fig. 2c and d) caused by conventional remineralization could not provide a satisfied mechanical function. However, after treatment with amelogenin-chitosan hydrogel for 7 days, the hardness and modulus of the etched enamel surface increased significantly (p < 0.001). The modulus increased by nearly 4 times and the hardness was increased by nearly 9 times (Fig. 8a). Although the mechanical properties were not the same as those of native enamel, the repaired enamel treated with CS-AMEL hydrogel showed superior properties compared to the control (without amelogenin) due to the well-organized crystal orientation [52]. The amelogenin and chitosan residues in the repaired layer may limit its mechanical performance, which could potentially be improved by removal of the organic material with proteolytic enzymes [53–55]. Moreover, in clinical practice, the mechanical properties of repaired layer would be further improved by repetitive application of CS-AMEL hydrogel in order to achieve thicker repaired layer. Further work is needed in order to assess the stability of CS-AMEL hydrogel in the oral cavity.
Fig. 8.
a) Hardness and elastic modulus of healthy enamel, etched enamel, and reconstructed enamel repaired by chitosan hydrogel with and without amelogenin. * p ≤0.05 when compared with artificial caries; *** p < 0.001 when compared with artificial caries. b) OD values of the overnight cultures of saliva bacteria in different LB media. ### p < 0.001 when compared with LB control. The inset image shows the turbidity of LB medium with and without chitosan hydrogel.
3.7. Antimicrobial properties of CS-AMEL hydrogel
Human saliva used as a source for bacteria was cultured in LB medium to examine the antimicrobial properties of CS-AMEL by observing the optical density (OD) values and turbidity (Fig 8b). After overnight culture, the medium without chitosan gel was opaque due to presence of bacteria, while the medium with chitosan gel was clear (insert in Fig. 8b). The OD value was significantly reduced when the CS-AMEL hydrogel was added to the LB medium (p < 0.001, Fig. 8b). These results demonstrate that the CS-AMEL hydrogel can effectively inhibit bacteria growth. We believed that the antimicrobial effect of CS-AMEL hydrogel was attributed to the chitosan. Chitosan has been observed to have antimicrobial activity against a wide variety of bacteria, including streptococci and lactobacilli, which are known as the principal etiological factors of dental caries. Moreover, chitosan has several advantages over other types of antiseptic agents, including a higher antibacterial activity, a broader spectra of activity, a higher killing rate, and lower toxicity toward mammalian cells [56]. Therefore, we expect that the clinical application of the CS-AMEL hydrogel can not only fulfill the superficial enamel reconstruction, but also effectively suppress the bacterial infection and subsequent demineralization.
4. Conclusions
In summary, taking advantage of the potential of amelogenin to control organized growth of apatite crystals and the potential antimicrobial activity of chitosan, we have developed a new amelogenin-containing chitosan hydrogel for superficial enamel reconstruction. Amelogenin assemblies stabilized Ca-P clusters in CS-AMEL hydrogel and guided their arrangement into linear chains. These amelogenin Ca-P composite chains further fused with enamel crystal and eventually evolved into enamel-like co-aligned crystals, anchored to the natural enamel substrate through a cluster growth process [48, 49]. The continuous growth of crystals formed an excellent bond between the newly-grown layer and the enamel. Furthermore, the hardness and elastic modulus of etched enamel were increased by 9 and 4 times after treatment with amelogenin-chitosan hydrogel. We anticipate that chitosan hydrogel will provide effective protection against secondary caries because of its pH-responsive and antimicrobial properties. Our studies introduce a promising amelogenin-chitosan hydrogel method for superficial enamel repair and demonstrate the potential of applying protein-directed assembly to the biomimetic reconstruction of complex biomaterials.
Acknowledgments
Research was supported by NIH-NIDCR grants; DE-13414 and DE-020099 to J.M.O. The authors would like to thank Dr. Sisi Liu for assistant with the transmission electron microscopy, and the Center for Electron Microscopy and Microanalysis (CEMMA) at USC for electron microscopy.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moradian-Oldak J. Protein - mediated enamel mineralization. Front Biosci. 2012;17:1996–2023. doi: 10.2741/4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui FZ, Ge J. New observations of the hierarchical structure of human enamel, from nanoscale to microscale. J Tissue Eng Regen Med. 2007;1:185–91. doi: 10.1002/term.21. [DOI] [PubMed] [Google Scholar]
- 3.Fincham AG, Moradian-Oldak J, Simmer JP. The structural biology of the developing dental enamel matrix. J Struct Biol. 1999;126:270–99. doi: 10.1006/jsbi.1999.4130. [DOI] [PubMed] [Google Scholar]
- 4.Bartlett JD, Simmer JP. Proteinases in developing dental enamel. Crit Rev Oral Biol Med. 1999;10:425–41. doi: 10.1177/10454411990100040101. [DOI] [PubMed] [Google Scholar]
- 5.Ruttermann S, Trellenkamp T, Bergmann N, Raab WHM, Ritter H, Janda R. A new approach to influence contact angle and surface free energy of resin-based dental restorative materials. Acta Biomater. 2011;7:1160–5. doi: 10.1016/j.actbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Onuma K, Yamagishi K, Oyane A. Nucleation and growth of hydroxyapatite nanocrystals for nondestructive repair of early caries lesions. J Cryst Growth. 2005;282:199–207. [Google Scholar]
- 7.Busch S. Regeneration of human tooth enamel. Angew Chem Int Ed. 2004;43:1428–31. doi: 10.1002/anie.200352183. [DOI] [PubMed] [Google Scholar]
- 8.Guentsch A, Busch S, Seidler K, Kraft U, Nietzsche S, Preshaw PM, et al. Biomimetic mineralization: effects on human enamel in vivo. Adv Eng Mater. 2010;12:B571–B6. [Google Scholar]
- 9.Yamagishi K, Onuma K, Suzuki T, Okada F, Tagami J, Otsuki M, et al. A synthetic enamel for rapid tooth repair. Nature. 2005;433:819. doi: 10.1038/433819a. [DOI] [PubMed] [Google Scholar]
- 10.Chen H, Tang Z, Liu J, Sun K, Chang S-R, Peters MC, et al. Acellular synthesis of a human enamel-like microstructure. Adv Mater. 2006;18:1846–51. [Google Scholar]
- 11.Li L, Mao C, Wang J, Xu X, Pan H, Deng Y, et al. Bio-inspired enamel repair via glu-directed assembly of apatite nanoparticles: an approach to biomaterials with optimal characteristics. Adv Mater. 2011;23:4695–701. doi: 10.1002/adma.201102773. [DOI] [PubMed] [Google Scholar]
- 12.Yin YJ, Yun S, Fang JS, Chen HF. Chemical regeneration of human tooth enamel under near-physiological conditions. Chem Commun. 2009:5892–4. doi: 10.1039/b911407f. [DOI] [PubMed] [Google Scholar]
- 13.Li L, Pan HH, Tao JH, Xu XR, Mao CY, Gu XH, et al. Repair of enamel by using hydroxyapatite nanoparticles as the building blocks. J Mater Chem. 2008;18:4079–84. [Google Scholar]
- 14.Xie RQ, Feng ZD, Li SW, Xu BB. EDTA-assisted self-assembly of fluoride-substituted hydroxyapatite coating on enamel substrate. Cryst Growth Des. 2011;11:5206–14. [Google Scholar]
- 15.Qi YP, Li N, Niu LN, Primus GM, Liang JQ, Pashley DH, et al. Remineralization of artificial dentinal caries lesions by biomimetically modified mineral trioxide aggregate. Acta Biomater. 2012;8:836–42. doi: 10.1016/j.actbio.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher J, Walsh D, Fowler CE, Mann S. Electrospun mats of PVP/ACP nanofibres for remineralization of enamel tooth surfaces. Crystengcomm. 2011;13:3692–7. [Google Scholar]
- 17.Petta V, Moradian-Oldak J, Yannopoulos SN, Bouropoulos N. Dynamic light scattering study of an amelogenin gel-like matrix in vitro. Eur J Oral Sci. 2006;114:308–14. doi: 10.1111/j.1600-0722.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 18.Iijima M, Moradian-Oldak J. Control of apatite crystal growth in a fluoride containing amelogenin-rich matrix. Biomaterials. 2005;26:1595–603. doi: 10.1016/j.biomaterials.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 19.Iijima M, Du C, Abbott C, Doi Y, Moradian-Oldak J. Control of apatite crystal growth by the co-operative effect of a recombinant porcine amelogenin and fluoride. Eur J Oral Sci. 2006;114:304–7. doi: 10.1111/j.1600-0722.2006.00324.x. [DOI] [PubMed] [Google Scholar]
- 20.Du C, Falini G, Fermani S, Abbott C, Moradian-Oldak J. Supramolecular assembly of amelogenin nanospheres into birefringent microribbons. Science. 2005;307:1450–4. doi: 10.1126/science.1105675. [Erratum in Science 2005;309:2166] [DOI] [PubMed] [Google Scholar]
- 21.Fan Y, Sun Z, Moradian-Oldak J. Controlled remineralization of enamel in the presence of amelogenin and fluoride. Biomaterials. 2009;30:478–83. doi: 10.1016/j.biomaterials.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fan Y, Sun Z, Wang R, Abbott C, Moradian-Oldak J. Enamel inspired nanocomposite fabrication through amelogenin supramolecular assembly. Biomaterials. 2007;28:3034–42. doi: 10.1016/j.biomaterials.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang L, Guan X, Yin H, Moradian-Oldak J, Nancollas GH. Mimicking the self-organized microstructure of tooth enamel. J Phys Chem C. 2008;112:5892–9. doi: 10.1021/jp077105+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee H-S, Tsai S, Kuo C-C, Bassani AW, Pepe-Mooney B, Miksa D, et al. Chitosan adsorption on hydroxyapatite and its role in preventing acid erosion. J Colloid Interface Sci. 2012;385:235–43. doi: 10.1016/j.jcis.2012.06.074. [DOI] [PubMed] [Google Scholar]
- 25.Stamford Arnaud TM, de Barros Neto B, Diniz FB. Chitosan effect on dental enamel de-remineralization: An in vitro evaluation. J Dent. 2010;38:848–52. doi: 10.1016/j.jdent.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 26.Decker EM, von Ohle C, Weiger R, Wiech I, Brecx M. A synergistic chlorhexidine/chitosan combination for improved antiplaque strategies. J Periodontal Res. 2005;40:373–7. doi: 10.1111/j.1600-0765.2005.00817.x. [DOI] [PubMed] [Google Scholar]
- 27.Rabea EI, Badawy MET, Stevens CV, Smagghe G, Steurbaut W. Chitosan as antimicrobial agent: applications and mode of action. Biomacromolecules. 2003;4:1457–65. doi: 10.1021/bm034130m. [DOI] [PubMed] [Google Scholar]
- 28.Neilands J, Sutherland D, Resin A, Wejse PL, de Paz LEC. Chitosan nanoparticles affect the acid tolerance response in adhered cells of streptococcus mutans. Caries Res. 2011;45:501–5. doi: 10.1159/000331206. [DOI] [PubMed] [Google Scholar]
- 29.de Paz LEC, Resin A, Howard KA, Sutherland DS, Wejse PL. Antimicrobial effect of chitosan nanoparticles on streptococcus mutans biofilms. Appl Environ Microbiol. 2011;77:3892–5. doi: 10.1128/AEM.02941-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dash M, Chiellini F, Ottenbrite RM, Chiellini E. Chitosan - a versatile semi-synthetic polymer in biomedical applications. Prog Polym Sci. 36:981–1014. [Google Scholar]
- 32.Henson BS, Wong DT. Collection, storage, and processing of saliva samples for downstream molecular applications. In: Seymour GJ, Cullinan MP, Heng NCK, editors. Oral Biology: Molecular Techniques and Applications. Humana Press Inc; 999 Riverview Dr, Ste 208, Totowa, Nj 07512–1165 USA: 2010. pp. 21–30. [DOI] [PubMed] [Google Scholar]
- 33.Selwitz RH, Ismail AI, Pitts NB. Dental caries. Lancet. 2007;369:51–9. doi: 10.1016/S0140-6736(07)60031-2. [DOI] [PubMed] [Google Scholar]
- 34.Cheng ZJ, Wang XM, Cui FZ, Ge J, Yan JX. The enamel softening and loss during early erosion studied by AFM, SEM and nanoindentation. Biomed Mater. 2009:4. doi: 10.1088/1748-6041/4/1/015020. [DOI] [PubMed] [Google Scholar]
- 35.Murugan R, Ramakrishna S. Aqueous mediated synthesis of bioresorbable nanocrystalline hydroxyapatite. J Crys Growth. 2005;274:209–13. [Google Scholar]
- 36.Li B, Wang Y, Jia D, Zhou Y. Gradient structural bone-like apatite induced by chitosan hydrogel via ion assembly. J Biomater Sci Polym Ed. 22:505–17. doi: 10.1163/092050610X487800. [DOI] [PubMed] [Google Scholar]
- 37.Lakshminarayanan R, Fan D, Du C, Moradian-Oldak J. The role of secondary structure in the entropically driven amelogenin self-assembly. Biophys J. 2007;93:3664–74. doi: 10.1529/biophysj.107.113936. [Erratum in Biophys J 2008;94:715] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fan DM, Iijima M, Bromley KM, Yang XD, Mathew S, Moradian-Oldak J. The cooperation of enamelin and amelogenin in controlling octacalcium phosphate crystal morphology. Cells Tissues Organs. 2011;194:194–8. doi: 10.1159/000324208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fan D, Du C, Sun Z, Lakshminarayanan R, Moradian-Oldak J. In vitro study on the interaction between the 32 kDa enamelin and amelogenin. J Struct Biol. 2009;166:88–94. doi: 10.1016/j.jsb.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu WG, Sun SJ, Cao ZQ, Xin Z, Yao KD, Lu WW, et al. An investigation on the physicochemical properties of chitosan/DNA polyelectrolyte complexes. Biomaterials. 2005;26:2705–11. doi: 10.1016/j.biomaterials.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 41.Edgar WM. Role of saliva in control of pH changes in human dental plaque. Caries Res. 1976;10:241–54. doi: 10.1159/000260206. [DOI] [PubMed] [Google Scholar]
- 42.Stephan RM. pH and dental caries. J Dent Res. 1947;26:340. [PubMed] [Google Scholar]
- 43.Lingstrom P, van Ruyven FOJ, van Houte J, Kent R. The pH of dental plaque in its relation to early enamel caries and dental plaque flora in humans. J Dent Res. 2000;79:770–7. doi: 10.1177/00220345000790021101. [DOI] [PubMed] [Google Scholar]
- 44.Onuma K, Ito A. Cluster growth model for hydroxyapatite. Chem Mat. 1998;10:3346–51. [Google Scholar]
- 45.Posner AS, Betts F. Synthetic amorphous calcium-phosphate and its relation to bone-mineral structure. Acc Chem Res. 1975;8:273–81. [Google Scholar]
- 46.Fang PA, Conway JF, Margolis HC, Simmer JP, Beniash E. Hierarchical self-assembly of amelogenin and the regulation of biomineralization at the nanoscale. Proc National Acad Sci U S A. 2011;108:14097–102. doi: 10.1073/pnas.1106228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang X, Wang L, Qin Y, Sun Z, Henneman ZJ, Moradian-Oldak J, et al. How amelogenin orchestrates the organization of hierarchical elongated microstructures of apatite. J Phys Chem B. 2010;114:2293–300. doi: 10.1021/jp910219s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pouget EM, Bomans PHH, Goos J, Frederik PM, de With G, Sommerdijk N. The initial stages of template-controlled CaCO3 formation revealed by Cryo-TEM. Science. 2009;323:1455–8. doi: 10.1126/science.1169434. [DOI] [PubMed] [Google Scholar]
- 49.Gebauer D, Volkel A, Colfen H. Stable prenucleation calcium carbonate clusters. Science. 2008;322:1819–22. doi: 10.1126/science.1164271. [DOI] [PubMed] [Google Scholar]
- 50.Mehdawi I, Neel EA, Valappil SP, Palmer G, Salih V, Pratten J, et al. Development of remineralizing, antibacterial dental materials. Acta Biomater. 2009;5:2525–39. doi: 10.1016/j.actbio.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 51.Cuy JL, Mann AB, Livi KJ, Teaford MF, Weihs TP. Nanoindentation mapping of the mechanical properties of human molar tooth enamel. Arch Oral Biol. 2002;47:281–91. doi: 10.1016/s0003-9969(02)00006-7. [DOI] [PubMed] [Google Scholar]
- 52.Eimar H, Ghadimi E, Marelli B, Vali H, Nazhat SN, Amin WM, et al. Regulation of enamel hardness by its crystallographic dimensions. Acta Biomater. 2012;8:3400–10. doi: 10.1016/j.actbio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Yang XD, Sun Z, Ma RW, Fan DM, Moradian-Oldak J. Amelogenin “nanorods” formation during proteolysis by Mmp-20. J Struct Biol. 2011;176:220–8. doi: 10.1016/j.jsb.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun Z, Carpiaux W, Fan D, Fan Y, Lakshminarayanan R, Moradian-Oldak J. Apatite Reduces Amelogenin Proteolysis by MMP-20 and KLK4 in vitro. J Dent Res. 2010;89:344–8. doi: 10.1177/0022034509360660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gorgieva S, Kokol V. Preparation, characterization, and in vitro enzymatic degradation of chitosan-gelatine hydrogel scaffolds as potential biomaterials. J Biomed Mater Res A. 2012;100A:1655–67. doi: 10.1002/jbm.a.34106. [DOI] [PubMed] [Google Scholar]
- 57.Liu XF, Guan YL, Yang DZ, Li Z, De Yao K. Antibacterial action of chitosan and carboxymethylated chitosan. J Appl Polym Sci. 2001;79:1324–35. [Google Scholar]