Abstract
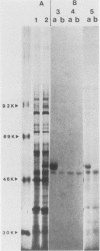
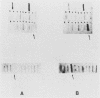
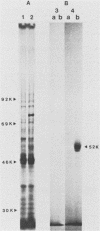
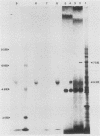
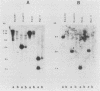
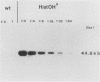
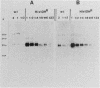
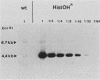
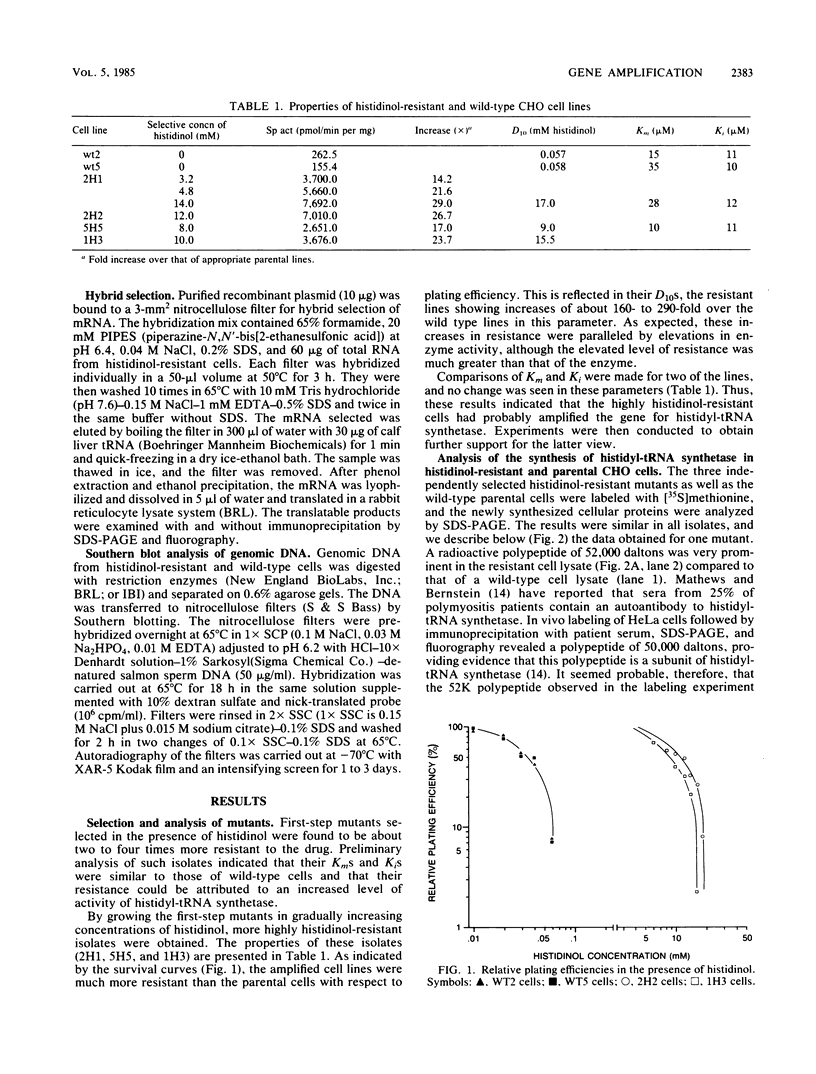
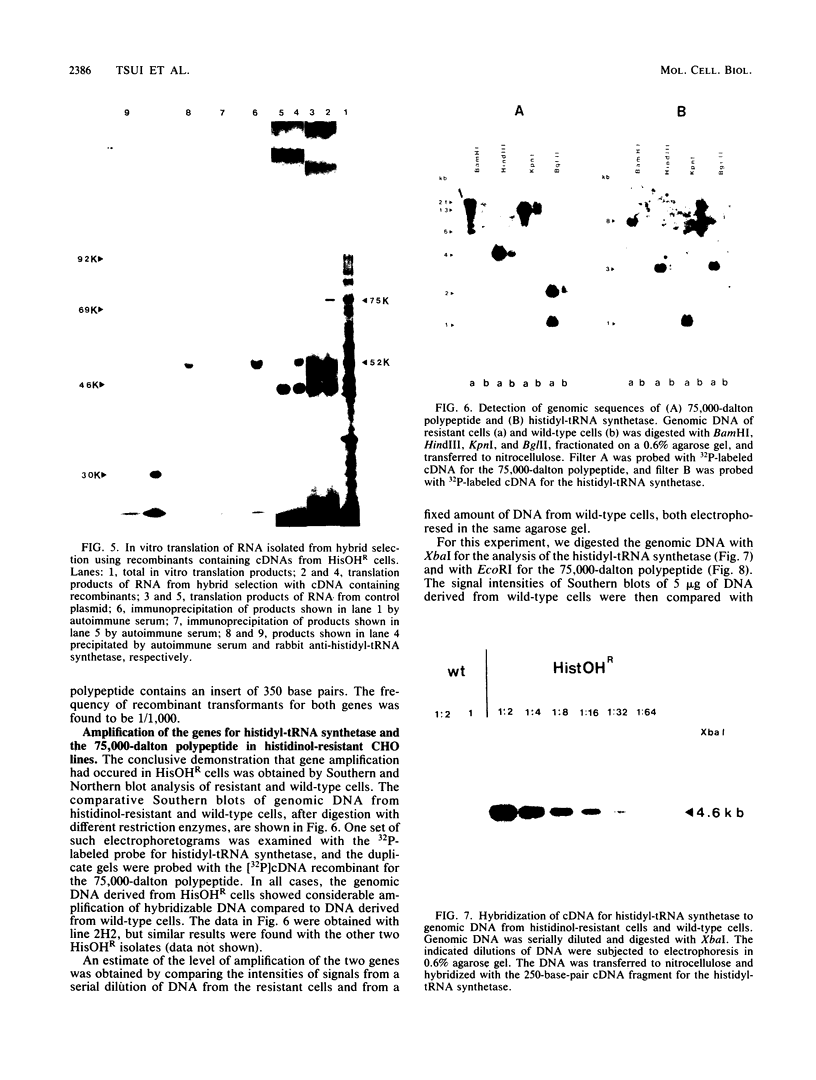
Histidinol-resistant (HisOHR) mutants with up to a 30-fold increase in histidyl-tRNA synthetase activity have been isolated by stepwise adaptation of wild-type Chinese hamster ovary (CHO) cells to increasing amounts of histidinol in the medium. Immunoprecipitation of [35S]methionine-labeled cell lysates with antibodies to histidyl-tRNA synthetase showed increased synthesis of the enzyme in histidinol-resistant cells. The histidinol-resistant cell lines had an increase in translatable polyadenylated mRNA for histidyl-tRNA synthetase. A cDNA for CHO histidyl-tRNA synthetase has been cloned, using these histidyl-tRNA synthetase-overproducing mutants as the source of mRNA. Southern blot analysis of wild-type and histidinol-resistant cells with this cDNA showed that the histidyl-tRNA synthetase DNA bands were amplified in the resistant cells. These HisOHR cells owed their resistance to histidinol to amplification of the gene for histidyl-tRNA synthetase.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adair G. M., Thompson L. H., Lindl P. A. Six complementation classes of conditionally lethal protein synthesis mutants of CHO cells selected by 3H-amino acid. Somatic Cell Genet. 1978 Jan;4(1):27–44. doi: 10.1007/BF01546491. [DOI] [PubMed] [Google Scholar]
- Andrulis I. L., Chiang C. S., Arfin S. M., Miner T. A., Hatfield G. W. Biochemical characterization of a mutant asparaginyl-tRNA synthetase from Chinese hamster ovary cells. J Biol Chem. 1978 Jan 10;253(1):58–62. [PubMed] [Google Scholar]
- Ashman C. R. Mutations in the structural genes of CHO cell histidyl-, valyl-, and leucyl-tRNA synthetases. Somatic Cell Genet. 1978 May;4(3):299–312. doi: 10.1007/BF01542844. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cirullo R. E., Wasmuth J. J. Isolation of Chinese hamster ovary cells that overproduce asparaginyl-tRNA synthetase. Mol Cell Biol. 1984 Sep;4(9):1939–1941. doi: 10.1128/mcb.4.9.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatisse M., de Saint Vincent B. R., Buttin G. Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J. 1984 Dec 20;3(13):3123–3127. doi: 10.1002/j.1460-2075.1984.tb02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Chinese hamster ovary cells resistant to borrelidin overproduce threonyl-tRNA synthetase. J Biol Chem. 1984 Jul 25;259(14):9202–9206. [PubMed] [Google Scholar]
- Gerken S. C., Arfin S. M. Threonyl-tRNA synthetase from Chinese hamster ovary cells is phosphorylated on serine. J Biol Chem. 1984 Sep 25;259(18):11160–11161. [PubMed] [Google Scholar]
- Kessler S. W. Cell membrane antigen isolation with the staphylococcal protein A-antibody adsorbent. J Immunol. 1976 Nov;117(5 Pt 1):1482–1490. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehrach H., Frischauf A. M., Hanahan D., Wozney J., Fuller F., Boedtker H. Construction and characterization of pro alpha 1 collagen complementary deoxyribonucleic acid clones. Biochemistry. 1979 Jul 10;18(14):3146–3152. doi: 10.2196/47873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B., Bernstein R. M. Myositis autoantibody inhibits histidyl-tRNA synthetase: a model for autoimmunity. Nature. 1983 Jul 14;304(5922):177–179. doi: 10.1038/304177a0. [DOI] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Coleman P. F., Stark G. R. N-(Phosphonacetyl)-L-aspartate-resistant hamster cells overaccumulate a single mRNA coding for the multifunctional protein that catalyzes the first steps of UMP synthesis. J Biol Chem. 1979 Feb 10;254(3):974–980. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Scornik O. A., Ledbetter M. L., Malter J. S. Role of aminoacylation of histidyl-tRNA in the regulation of protein degradation in Chinese hamster ovary cells. J Biol Chem. 1980 Jul 10;255(13):6322–6329. [PubMed] [Google Scholar]
- Stanners C. P., Eliceiri G. L., Green H. Two types of ribosome in mouse-hamster hybrid cells. Nat New Biol. 1971 Mar 10;230(10):52–54. doi: 10.1038/newbio230052a0. [DOI] [PubMed] [Google Scholar]
- Stark G. R., Wahl G. M. Gene amplification. Annu Rev Biochem. 1984;53:447–491. doi: 10.1146/annurev.bi.53.070184.002311. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Harkins J. L., Stanners C. P. A mammalian cell mutant with a temperature-sensitive leucyl-transfer RNA synthetase. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3094–3098. doi: 10.1073/pnas.70.11.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson L. H., Lofgren D. J., Adair G. M. Evidence for structural gene alterations affecting aminoacyl-tRNA synthetases in CHO cell mutants and revertants. Somatic Cell Genet. 1978 Jul;4(4):423–435. doi: 10.1007/BF01538864. [DOI] [PubMed] [Google Scholar]
- Thompson L. H., Stanners C. P., Siminovitch L. Selection by [3H] amino acids of CHO-cell mutants with altered leucyl- and asparagyl-transfer RNA synthetases. Somatic Cell Genet. 1975 Apr;1(2):187–208. doi: 10.1007/BF01538547. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Padgett R. A., Stark G. R. Gene amplification causes overproduction of the first three enzymes of UMP synthesis in N-(phosphonacetyl)-L-aspartate-resistant hamster cells. J Biol Chem. 1979 Sep 10;254(17):8679–8689. [PubMed] [Google Scholar]