Abstract
Purpose
This study was conducted in order to characterize the prevalence of falls and functional impairments (FIs) and their association with chemotherapy-induced peripheral neuropathy (CIPN) in cancer survivors.
Methods
We analyzed baseline assessments from a phase III RCT in cancer survivors with self-reported CIPN scores of ≥4 out of 10. Patients completed the EORTC QLQ-CIPN-20 for neuropathy and reported falls in the previous 3 months. FIs were defined using the Activities of Daily Living subsection of the Vulnerable Elder’s Scale. Associations of baseline characteristics and CIPN with falls and FIs were examined using logistic regression.
Results
Of 421 patients, 11.9 % experienced recent falls and 26.6 % reported FIs. Motor neuropathy was the only factor associated with falls (OR=1.127, p=0.01). Factors associated with FIs included non-white race (OR=0.335 white relative to non-white, 0.781, p=0.01) and greater motor neuropathy scores (OR=1.262, p<0.0001).
Conclusion
CIPN, primarily motor, is associated with falls and FIs. Future prospective research should investigate the ability of motor neuropathy severity to predict falls.
Keywords: Falls, Functional impairments, Neuropathy, Cancer survivors
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) occurs in as many as 70 % of patients treated with taxanes, vinca alkaloids, or platinum agents [1]. In a poorly defined subset of these patients, CIPN persists even after the completion of chemotherapy. Peripheral neuropathy causes impaired sensory and motor function. Sensory symptoms include tingling, numbness, increased sensitivity to heat and cold, and pain in the hands and feet. Motor dysfunction includes symptoms of muscle weakness and impaired balance [1–5]. Although the incidence, severity, and pattern of CIPN have been investigated during chemotherapy, the relationships between CIPN and falls or functional capacity are less well characterized. One study in a small number of patients receiving chemotherapy found that 20 % of patients with CIPN fell recently [6]. The prevalence of falls in larger samples of patients with CIPN and the association of CIPN with falls are unknown.
Evidence suggests that the prevalence of falls and functional impairment is higher for cancer patients than their age-matched counterparts [7–9]. The few studies that have assessed falls in cancer patients report yearly rates that are up to two times higher than in persons of similar ages without cancer [10–12]. One such study recently published by Stone et al. [13] followed advanced cancer patients prospectively for 6 months. They found that 50.3 % of the patients fell during the follow-up period. Over one third of the falls resulted in soft tissue injuries, and 3.2 % resulted in fractures, further demonstrating the debilitating effect of falls on an at-risk population. The aforementioned studies investigated the prevalence of falls and functional impairment in cancer patients aged 65 and over. Little information regarding these issues, especially falls, is available for a more age diverse group of cancer patients. Almost nothing is known about the association between CIPN that persists beyond the completion of chemotherapy with falls or function in both younger and older cancer survivors with CIPN.
We conducted a secondary analysis of baseline data collected during a clinical trial executed by the University of Rochester Cancer Center Community Clinical Oncology program (CCOP). The study was designed to test the efficacy of a topical analgesic compound on CIPN in patients who had completed chemotherapy. The objectives of this study were to determine the prevalence of falls and functional impairments in cancer survivors with CIPN and to examine whether sensory and/or motor CIPN are independently associated with falls and/or functional impairment.
Patients and methods
Data source and sample
The data for this secondary analysis were obtained from a double-blind, placebo-controlled, randomized clinical trial testing an analgesic cream in patients with CIPN. This study was led by the University of Rochester CCOP and was approved by the University of Rochester IRB and the IRBs of all participating CCOP sites. All patients provided their informed consent prior to their inclusion into the study. Participants included patients who reported persistent chemotherapy-induced peripheral neuropathy. Patients were eligible to enter the trial if their average rating of “pain, numbness, or tingling in your hands and feet over the past 24 hours” for the 7 days prior to randomization was ≥4 on an 11-point rating scale ranging from 0 (not at all) to 10 (as bad as you can imagine). Patients were recruited at 23 community oncology sites of the URCC CCOP research base. All patients had completed chemotherapy at least 1 month prior to enrollment in the study, had been on stable doses of pain medication for at least 2 weeks prior to beginning the study, and had no clinical history of peripheral neuropathy from other conditions. The enrolling physicians obtained an extensive medical history and performed a medical exam to identify neuropathy from sources other than chemotherapy. This included testing for diabetes and asking detailed questions about alcohol use and HIV. The inclusion criteria were designed specifically so that physicians enrolled patients who had chemotherapy-induced neuropathy and who did not have any other clinical reasons for neuropathy.
Study variables
All outcomes used for this analysis were obtained from baseline data collected before patients were randomized to intervention or placebo. Patients were asked to report a history of recent falls (within 3 months of randomization). Patients were grouped dichotomously as those who did and did not experience a fall. Deficits in functional capacity were assessed using the Vulnerable Elders Survey (VES) [14]. Questions utilized in the VES to identify functional impairment in this study were selected from the full panel Activity of Daily Living and Instrumental Activity of Daily Living questions that had the most predictive value for future disability and mortality. The VES was derived from the Medicare Current Beneficiary Survey to create a screening tool to identify patients at highest risk for future adverse outcomes. The VES has also been utilized to identify cancer patients with disability who are at risk of adverse outcomes. The VES was chosen for this study for its brevity and predictive value [14–16]. A subsection of the VES assesses functional impairment using a series of “yes or no” questions that ask whether as a result of their health, patients are unable to perform, or need help with, daily living activities (shopping for personal items, managing money, walking across the room, doing light housework, and bathing). Items of the physical activities subsection of the VES such as ability to crouch or kneel, lift objects, or reach above shoulder level were not considered for this analysis. If a patient reported he/she was unable to perform or needed assistance with any of the listed activities because of underlying health issues, he/she was considered to have a functional impairment.
The severity of sensory and motor neuropathy was assessed with the EORTC QLQ-CIPN20 [17]. Briefly, this measure asks patients to rate the severity of symptoms related to sensory or motor neuropathy. Answers include not at all (1), a little (2), quite a bit (3), or very much (4). The sensory neuropathy subscale includes the cumulative score for nine questions that assess sensory toxicities, with total scores ranging from 0 to 36. The motor neuropathy subscale includes the cumulative score for seven questions, with total scores ranging from 0 to 28. The sensory neuropathy subscale includes questions about tingling, numbness, and burning pain in the hands and toes or feet and problems standing or walking because of an inability to feel the ground beneath one’s feet. The motor subscale asks questions about cramps in the hands and feet, difficulty holding a pen, difficulty manipulating small objects, difficulty opening a jar or bottle because of weakness in one’s hands, difficulty walking because one’s feet dropped downwards, and difficulty climbing stairs or getting out of a chair because of weakness in one’s legs. The EORTC QLQ-CIPN 20 was shown in pretesting to have internal reliability with Cronbach’s alpha scores of 0.82 and 0.73 for the sensory and motor neuropathy subscores, respectively [17]. Cavaletti et al. [18] found the test–retest reliability of the EORTC QLQ-CIPN20 to be adequate in a sample of 272 patients with CIPN (r=0.836 and 0.844 for the sensory and motor subscores, respectively). They also found that similar to the objective, clinical evaluation NCI-CTC CIPN assessment measures, the EORTC QLQ-CIPN20 detected higher incidences of sensory neuropathy than motor neuropathy in the sample. However, they did not report correlations between the objective and self-report measures [18]. The EORTC QLQ-CIPN20 was the primary outcome in a phase III randomized, placebo-controlled trial investigating a topical intervention for CIPN [19].
In a brief interview, study coordinators obtained background information from the patients. Characteristics such as age, race, ethnicity, and cancer and cancer treatment history were obtained. Cancer and cancer treatment history were also verified with the medical record.
Statistical analysis
The goal of our analysis was to determine the prevalence of having a fall or functional impairment in patients with persistent CIPN after chemotherapy. Further, we assessed the association of CIPN (sensory and motor) with these two clinical outcomes. Statistical significance was assessed at the 5 % significance level. No adjustments for multiple comparisons were performed.
Baseline differences in demographics, cancer and chemotherapy history, and neuropathy between groups of patients who had or had not reported any falls were evaluated using χ2 tests for categorical variables and t tests for continuous variables. Separate logistic regression models were used to determine the association of the severity of sensory and motor neuropathy with each clinical outcome. These clinical outcomes included the following dichotomous variables: any fall and any functional impairment. The independent variables were selected either because they are clinically relevant demographics and characteristics or because they are clinical characteristics that are associated with falling. Breast or alimentary cancer history was included due to the high prevalence of these subgroups in the overall sample (40 % breast and 27 % alimentary). Further, these cancers are most often treated with taxane (breast) and platinum (alimentary) therapies, which could help generate hypotheses as to whether certain types of chemotherapy are more frequently associated with falls. Variable selection was not performed to ensure no bias in significance levels.
Each model controlled for age, gender, race, marital status, educational level, previous surgery or radiation therapy, cancer type (breast vs. alimentary vs. other). Adjusted odds ratios and their 95 % confidence intervals (95 % CIs) were reported to indicate the association of each variable with the likelihood of the outcome, controlling for the effects of all other variables in each model. Individual EORTC QLQ-CIPN20 motor neuropathy items were dichotomized into low (subjects reporting “not at all” or “a little”) or high (subjects reporting “quite a bit” or “very much”) levels of each symptom. Each item was compared between fallers and nonfallers and those with and without any functional impairment using χ2 tests. Bonferroni correction for seven motor items and two outcomes was used, setting the p value for significance at p=0.0036 (0.05/14). All analyses were performed in JMP 10.
Results
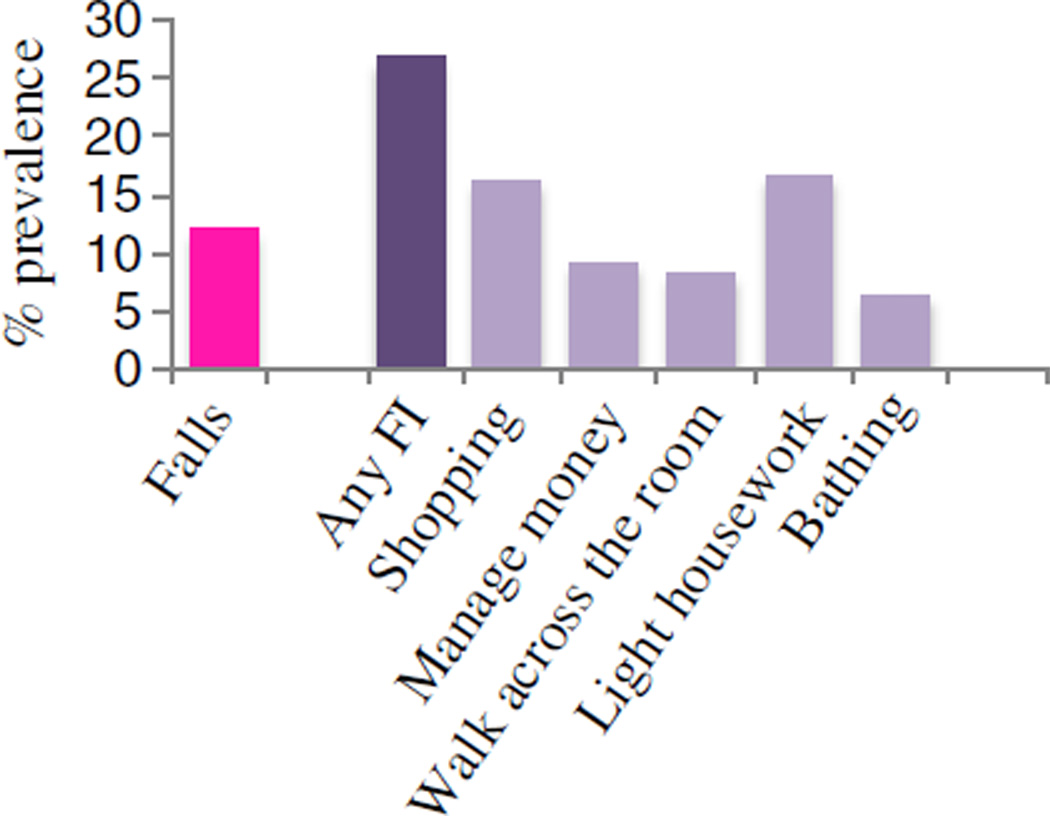
Four hundred seventy-one subjects with average CIPN scores ≥4 were included in the study. Patients were predominantly female (71 %) and most had a history of breast (40 %) or alimentary (27 %) cancers. They were on average 60 years of age (range 28–86 years). Figure 1 depicts the prevalence of falls and impairments in functional capacity items. Twelve percent (n=51) of subjects reported having had a fall in the 3-month period prior to enrolling in the study. Twenty-seven percent (n=113) of participants reported any impairment in functional capacity. Difficulty shopping (17 %, n=69) and doing light household tasks required to live in the home independently (17 %, n=71) were the most prevalent issues reported in terms of functional capacity. Table 1 lists the demographic and clinical characteristics of the groups who did and did not report a fall prior to randomization. The elapsed time between completion of chemotherapy and assessment was similar for fallers and nonfallers (21.7 vs. 14.7 months, p=0.127). Subjects who reported a fall were less likely to have had breast cancer (27.5 % of fallers vs. 41.5 % of nonfallers, p=0.049). Those reporting a fall had higher (worse) sensory (24.3 vs. 21.5 out of 36, p=0.0008) and motor neuropathy (18.4 vs. 15.2 out of 28, p=0.0003) scores.
Fig. 1.
Prevalence of falls and impairments in functional capacity items
Table 1.
Characteristics of participants who have and have not reported a recent fall
| Total (N=471) |
Any fall (N=51) |
No fall (N=378) |
p | |
|---|---|---|---|---|
| Gender, N (%) | 0.310 | |||
| Male | 125 (29.0) | 18 (35.3) | 107 (28.3) | |
| Female | 306 (71.0) | 33 (64.7) | 271 (71.7) | |
| Race, N (%) | 0.100 | |||
| White | 386 (82.0) | 42 (82.4) | 342 (90.5) | |
| Non-white | 45 (9.6) | 9 (17.7) | 36 (9.5) | |
| Hispanic, N (%) | 0.710 | |||
| Yes | 13 (3.0) | 2 (3.9) | 11 (2.9) | |
| No | 415 (97.0) | 49 (96.0) | 364 (97.1) | |
| Marital status, N (%) | 0.325 | |||
| Married | 305 (70.8) | 33 (64.7) | 270 (71.4) | |
| Widowed | 41 (9.5) | 8 (15.7) | 33 (8.7) | |
| Other | 85 (19.7) | 10 (19.6) | 75 (19.8) | |
| Education, N (%) | 0.668 | |||
| ≤HS | 142 (33) | 18 (35.3) | 122 (32.3) | |
| ≥Partial college | 289 (67) | 33 (64.7) | 256 (67.7) | |
| Previous surgery, N (%) | 0.174 | |||
| Yes | 367 (85.2) | 40 (78.4) | 325 (86.0) | |
| No | 64 (14.9) | 11 (21.6) | 53 (14.0) | |
| Previous RT, N (%) | 0.478 | |||
| Yes | 203 (47.1) | 25 (50.0) | 176 (46.6) | |
| No | 228 (52.9) | 25 (50.0) | 202 (53.4) | |
| Breast cancer, N (%) | 0.049* | |||
| Yes | 173 (40.1) | 14 (27.5) | 171 (41.5) | |
| No | 258 (59.9) | 37 (72.5) | 258 (58.4) | |
| Previous taxane treatment, N (%) | 0.768 | |||
| Yes | 253 (53.7) | 26 (51.0) | 201 (46.8) | |
| No | 198 (46.3) | 25 (49.0) | 177 (53.2) | |
| Age, mean (SD) | 60.0 (10.3) | 62.0 (9.6) | 59.9 (10.2) | 0.141 |
| Months from end of chemotherapy, mean (SD) | 15.5 (21.4) | 21.7 (31.1) | 14.7 (19.7) | 0.127 |
| Sensory, mean (SD) | 21.8 (4.9) | 24.3 (5.2) | 21.5 (4.7) | 0.0008* |
| Motor, mean (SD) | 15.56 (4.7) | 18.4 (5.6) | 15.2 (4.5) | 0.0003* |
P value is based on χ2 test (categorical variables) or t test (continuous variables) between groups of participants who reported any falls or no fall HS high school, RT radiation therapy
p=0.05
Logistic regressions were used to evaluate the associations between sensory and motor neuropathy with the two outcomes (falls and any functional impairment). After adjustment for demographic and cancer characteristics, more severe motor neuropathy was the only variable significantly associated with reported falls (OR=1.127, 95 % CI=1.029–1.238, p=0.01) (AUC for ROC=0.725) (Table 2). Variables significantly associated with any functional impairment included non-white race (OR=0.335 white relative to non-white, 95 % CI=0.143–0.781, p=0.01) and greater motor neuropathy scores (OR=1.262, 95 % CI=1.166–1.375, p<0.0001) (AUC for ROC=0.813) (Table 3). Hence, motor neuropathy was associated with both clinical outcomes. Sensory neuropathy was no longer significantly associated with falls or functional losses after adjustment for clinical and demographic factors.
Table 2.
Multivariable associations of neuropathy and demographic and cancer-related factors with falls in cancer survivors with CIPN
| Predictor | Adjusted odds ratio estimate | |||
|---|---|---|---|---|
| Odds ratio | 95 % confidence interval | p | ||
| Age | 1.019 | 0.983 | 1.057 | 0.32 |
| Gender (female vs. male) | 1.019 | 0.466 | 2.267 | 0.96 |
| Race (white vs. non-white) | 0.501 | 0.204 | 1.304 | 0.15 |
| Marital status | ||||
| Married vs. widowed | 0.672 | 0.233 | 2.165 | 0.49 |
| Married vs. other | 1.094 | 0.488 | 2.646 | 0.83 |
| Education (≥partial college vs. HS or less) | 1.449 | 0.714 | 3.072 | 0.310 |
| Previous surgery (yes vs. no) | 0.602 | 0.241 | 1.571 | 0.29 |
| Previous RT (yes vs. no) | 1.379 | 0.680 | 2.828 | 0.37 |
| Cancer type | ||||
| Breast vs. other | 0.506 | 0.197 | 1.298 | 0.16 |
| Alimentary vs. other | 1.089 | 0.446 | 2.673 | 0.85 |
| Sensory neuropathy | 1.036 | 0.946 | 1.34 | 0.45 |
| Motor neuropathy | 1.127 | 1.029 | 1.238 | 0.01* |
| Any functional impairment (yes vs. no) | 1.110 | 0.498 | 2.396 | 0.80 |
AUC for the ROC curve=0.725 HS high school, RT radiation therapy
p=0.05
Table 3.
Multivariable associations of neuropathy and demographic and cancer-related factors with any functional impairment in cancer survivors with CIPN
| Predictor | Adjusted odds ratio estimate | |||
|---|---|---|---|---|
| Odds ratio | 95 % confidence interval | p | ||
| Age | 1.005 | 0.978 | 1.034 | 0.71 |
| Gender (female vs. male) | 1.552 | 0.800 | 3.064 | 0.19 |
| Race (white vs. non-white) | 0.335 | 0.143 | 0.781 | 0.01 |
| Marital status | ||||
| Married vs. widowed | 1.266 | 0.467 | 3.762 | 0.652 |
| Married vs. other | 1.061 | 0.541 | 2.135 | 0.866 |
| Education (≥partial college vs. HS or less) | 0.898 | 0.513 | 1.593 | 0.71 |
| Previous surgery (yes vs. no) | 0.684 | 0.301 | 1.574 | 0.37 |
| Previous RT (yes vs. no) | 0.809 | 0.445 | 1.463 | 0.48 |
| Cancer type | ||||
| Breast vs. other | 0.375 | 0.172 | 0.806 | 0.01 |
| Alimentary vs. other | 0.731 | 0.417 | 1.558 | 0.42 |
| Sensory neuropathy | 1.064 | 0.987 | 1.148 | 0.11 |
| Motor neuropathy | 1.262 | 1.166 | 1.375 | <0.0001 |
AUC for the ROC curve=0.813 HS high school, RT radiation therapy
The functional impairment subsection of the VES asks if patients have difficulty walking across the room. The motor subscale of the EORTC QLQ-CIPN20 asks, “did you have difficulty walking because your feet dropped downwards?” To ensure that the association between reporting any functional impairment and motor neuropathy score was not enhanced because both surveys ask about walking ability, a sensitivity analysis was performed in which the logistic regressions presented in Table 3 were repeated with a new functional impairment outcome that required patients to report having difficulty with at least one of the functions assessed other than walking across the room. Motor neuropathy scores were still significantly associated with functional impairment when the analysis was modified (p<0.0001).
In order to identify items of the EORTC QLQ-CIPN20 that are most closely associated with falling, differences in the individual EORTC QLQ-CIPN20 items from the motor subscale between fallers and nonfallers were assessed (Supplemental Table 1). Fallers more often reported high “difficulty holding a pen, which made writing difficult” (p=0.0005) and “difficulty walking because your feet dropped downwards” (p=0.0017) than nonfallers. These analyses were repeated to compare those with and without any functional impairment. Those with any functional impairment more often reported high “cramps in your hands” (p=0.0017), “difficulty holding a pen, which made writing difficult” (p<0.0001), “difficulty manipulating small objects with your fingers (for example buttoning small buttons)” (p=0.0006), “difficulty opening a jar or bottle because of weakness in your hands” (p<0.0001), “difficulty walking because your feet dropped downwards” (p<0.0001), and “difficulty walking up stairs or getting out of a chair because of weakness in your legs” (p<0.0001).
Discussion
Approximately 12 % of cancer survivors enrolled in a multicenter clinical trial for persistent CIPN reported falling during the 3 months prior to enrollment in the trial. More than a quarter had a functional impairment. Our analyses demonstrate that motor neuropathy scores (i.e., patient-reported perceived muscle weakness) had the strongest association with falls and functional impairment. To our knowledge, this is the first study that examines prevalence of falls in cancer survivors with CIPN and the associations of neuropathy with falls and functional impairment. Our results also identify an association between non-white race and functional impairments in this cohort of cancer survivors. Although non-white race is known to be associated with functional impairments in older adults [20, 21], our study highlights this disparity in a cohort of younger adults as well. Further research should investigate the disparities in functional impairments based on race and ethnicity among cancer patients and evaluate whether these disparities are enhanced by a cancer diagnosis.
Between 2004 and 2008, 54 % of newly diagnosed cancer patients in the USA were over the age of 65, and this number is projected to increase to 70 % by 2030 [22, 23]. Increased age is associated with decreased ability to perform daily activities and increased risk of falling [7–9]. In the elderly population, a fall can have devastating consequences. Between 28 and 35 % of adults over age 65 are estimated to fall each year. Forty percent to 60 % of falls experienced by elderly persons cause injury, of which 5 % are serious nonfractures and 5 % are fractures [11, 24]. In addition to physical injury, people who experience a fall often become afraid of falling again, which can be psychologically detrimental and lead to an increased risk of falling due to physical decline from lack of activity [25]. In 2009, 2.2 million nonfatal fall injuries were treated in emergency departments, and approximately 26 % of these patients were hospitalized. In 2000, direct medical costs for treating falls totaled over $19 billion [26].
Although falls are common in older adults, very little research has investigated fall prevalence or predictors in cancer patients. Bylow et al. [10] reported that 34 % of older prostate cancer patients (mean age=78) undergoing androgen deprivation therapy experienced a fall over a 6-month observation period. These patients also had significant physical performance problems as measured objectively by gait speed, balance, and strength. Stone et al. [13] reported that 50.3 % of advanced cancer patients reported a fall during a 6-month prospective study. A primary diagnosis of brain cancer or brain metastases, the number of falls in the 3 months prior to enrollment in the study, severity of depression, and daily benzodiazepine dose were identified as predictors of time to fall in a multivariate model. These cancer cohorts exhibited approximately twice the annual rate of fallers (51 % [10] and 75 % [13]) as the average rate reported for the general population over age 65 (28–35 % annually) [11]. A nationally representative population-based study of older Medicare beneficiaries found that over one out of five cancer survivors reported recent falls; the prevalence of falls in cancer survivors was higher than in an age-matched cohort without cancer [7].
In a recent small study, 19 % of cancer patients receiving either taxanes or platinum agents experienced at least one fall during chemotherapy [6]. Falls were associated with increased chemotherapy cycle number, neuropathic symptoms, and muscle weakness, as well as loss of balance. These results are consistent with a study that reported diminished physical function, including muscle weakness and loss of balance, was predictive of falls in osteoporotic elderly men [27].
Ours is the first study to investigate the prevalence of falls in cancer survivors with persistent chemotherapy-induced peripheral neuropathy. Assuming that the rate of falls is consistent over a 1-year period, the extrapolated annual prevalence of falls is 40 %, accounting for the percent of patients who remain at risk in each quarter. Although the mean age of these cancer survivors was only 60 years old, this falls prevalence is between 14 and 40 % greater than the annual falls prevalence reported for healthy adults above 65 years of age [11]. Our study shows that falls are an issue not only for older patients, but also for younger patients who develop neuropathy due to chemotherapy. Further, this is the first study to investigate the relationship between neuropathy and falls. We demonstrate that motor neuropathy is the neuropathy symptom most closely associated with falls and functional limitations, which were common in this sample.
Previous studies have identified associations between peripheral neuropathy symptoms and falls or functional impairment in HIV [28], diabetic neuropathy [29], axonal peripheral polyneuropathy [30], and CIPN patients on active treatment [6]. All of these studies defined peripheral neuropathy with general measures, and none of them specifically compared associations between falls and functional impairment with sensory vs. motor neuropathy symptoms. To our knowledge, only one study has investigated associations between falls and sensory and motor neuropathy specifically. Oka et al. [31] found that only deep sensory loss was associated with falls and not muscle weakness. However, the body of this article is only available in Japanese and the abstract does not provide enough information about patient population or methods to compare it to our study. Our study is the first to provide evidence that motor neuropathy symptoms like weakness in the legs and feet dropping downwards are more closely associated with falling than sensory symptoms. Our data suggest that motor neuropathy symptoms may contribute to falls more than sensory neuropathy symptoms. Alternatively, motor neuropathy could be more closely associated with falls because individuals with motor neuropathy often have more severe neuropathy in general. If this hypothesis holds true in future prospective research, severity of motor neuropathy could become an important clinically relevant variable that could help providers identify those cancer survivors who were at greatest risk for falls and help maximize the utility of falls prevention resources.
In an attempt to identify specific factors associated with falls and functional impairment, a more detailed exploration of associations between individual EORTC QLQ-CIPN20 motor neuropathy items and falling or functional impairment was performed. Six out of seven motor neuropathy items were significantly associated with function. Thus, use of the composite EORTC QLQ-CIPN20 motor score is likely the most informative option when investigating associations with functional impairment. On the contrary only two items (“difficulty holding a pen, which made writing difficult” and “difficulty walking because your feet dropped downwards”) were significantly different between fallers and nonfallers. This suggests that these two items may be particularly predictive of falling. The associations between the individual motor items and fall status should be investigated in a larger sample of fallers.
The cross-sectional design of our study limits the interpretation of the results, and causality cannot be determined. For example, subjects who have fallen or have physical limitations may perceive higher levels of motor neuropathy symptoms such as weakness in the legs. Another limitation of this study is that the neuropathy symptoms were assessed using a self-report measure [17]. However, Cavaletti et al. [18] reported consistency between the NCI-CTC sensory and motor neuropathy clinical assessment tools, the EORTC QLQ-CIPN20 in a cohort of CIPN patients. Further, the VES does not capture all aspects of function or activities of daily living and is a limitation. However, it was shown to be highly correlated with functional capacity in older individuals [32] and, thus, is still useful for this hypothesis generating research. The population evaluated in this study was a self-selected group of clinical trial participants who had fairly severe neuropathy symptoms. This limits the generalizability of the results to the general cancer survivor population. Future studies should evaluate the ability of motor neuropathy symptoms to predict falls in a general population of cancer survivors.
Identifying possible relationships between chemotherapy type and dose with falls or functional impairment would be clinically beneficial for risk assessment. Chemotherapy-induced sensory neuropathy is more common than chemotherapy-induced motor neuropathy. Platinum agents cause primarily sensory neuropathy, with the exception of muscle contractions that are triggered or enhanced by extreme cold and occur mostly during active treatment [33]. Taxanes and vinca alkaloids cause sensory and motor neuropathy symptoms, most commonly muscle weakness [4]. Our data suggest that cancer survivors who were most likely to have received taxanes (i.e., breast cancer survivors) were more likely to experience a fall or have functional impairments. Alimentary cancer patients with neuropathy, who likely received oxaliplatin, were no more likely to report falls or functional impairment. Unfortunately, one limitation of our study is that we do not have detailed chemotherapy histories and thus cannot test this hypothesis directly. Future studies should collect specific chemotherapy dosing information and investigate relationships between regimens and falls or functional impairment in cancer survivors.
Despite these limitations, this work indicates that persistent motor neuropathy may have detrimental functional consequences, including increased risk of falls. Although this work must be verified in future prospective studies, clinicians should consider screening for motor neuropathy as part of overall assessments used to devise an intervention plan to prevent falls. Identifying the risk factors that increase falls in high-risk cancer populations could lead to better preventive care and, in turn, decreased injury, hospital stays, and costs associated with falls in the growing population of cancer survivors.
Supplementary Material
Acknowledgments
This work was funded by the National Cancer Institute (R25CA10618 and U10CA37420). The work was also funded by a generous gift from Sandy Lloyd to the Geriatric Oncology Program at the James Wilmot Cancer Center. The study sponsors had no role in the study design, data collection, or data interpretation. We thank the patients, clinicians, and researchers of the University of Rochester Community Clinical Oncology Program who contributed to this study.
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00520-013-1766-y) contains supplementary material, which is available to authorized users.
Conflict of interest The authors have no conflicts of interest to disclose. We have full control of all primary data and agree to allow the journal to review the data if requested.
Contributor Information
J. S. Gewandter, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
L. Fan, Department of Public Health, University of Rochester Medical Center, 265 Crittenden Blvd, Rochester, NY 14642, USA
A. Magnuson, Department of Medicine, Hematology/Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
K. Mustian, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
L. Peppone, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
C. Heckler, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
J. Hopkins, Southeast Cancer Control Consortium, Piedmont HEM/ONC Assocs, 445 Pineview Dr. Suite 200, Kernersville, NC 27284, USA
M. Tejani, Department of Medicine, Hematology/Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
G. R. Morrow, Department of Radiation Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA
S. G. Mohile, Email: supriya_mohile@urmc.rochester.edu, Department of Medicine, Hematology/Oncology, University of Rochester Medical Center, 601 Elmwood Ave, Rochester, NY 14642, USA; Wilmot Cancer Center, 601 Elmwood Avenue, Box 704, Rochester, NY 14642, USA.
References
- 1.Ocean AJ, Vahdat LT. Chemotherapy-induced peripheral neuropathy: pathogenesis and emerging therapies. Support Care Cancer. 2004;12(9):619–625. doi: 10.1007/s00520-004-0657-7. [DOI] [PubMed] [Google Scholar]
- 2.Argyriou AA, Koltzenburg M, Polychronopoulos P, Papapetropoulos S, Kalofonos HP. Peripheral nerve damage associated with administration of taxanes in patients with cancer. Crit Rev Oncol Hematol. 2008;66(3):218–228. doi: 10.1016/j.critrevonc.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Dougherty PM, Cata JP, Cordella JV, Burton A, Weng HR. Taxol-induced sensory disturbance is characterized by preferential impairment of myelinated fiber function in cancer patients. Pain. 2004;109(1–2):132–142. doi: 10.1016/j.pain.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 4.Quasthoff S, Hartung HP. Chemotherapy-induced peripheral neuropathy. J Neurol. 2002;249(1):9–17. doi: 10.1007/pl00007853. [DOI] [PubMed] [Google Scholar]
- 5.Windebank AJ, Grisold W. Chemotherapy-induced neuropathy. J Peripher Nerv Syst. 2008;13(1):27–46. doi: 10.1111/j.1529-8027.2008.00156.x. [DOI] [PubMed] [Google Scholar]
- 6.Tofthagen C, Overcash J, Kip K. Falls in persons with chemotherapy-induced peripheral neuropathy. Support Care Canc. 2012;20(3):583–589. doi: 10.1007/s00520-011-1127-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mohile SG, Fan L, Reeve E, Jean-Pierre P, Mustian K, Peppone L, Janelsins M, Morrow G, Hall W, Dale W. Association of cancer with geriatric syndromes in older Medicare beneficiaries. J Clin Oncol. 2011;29(11):1458–1464. doi: 10.1200/JCO.2010.31.6695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mohile SG, Xian Y, Dale W, Fisher SG, Rodin M, Morrow GR, Neugut A, Hall W. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Canc Inst. 2009;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lisa Sprod SGM, Fan Lin, Janelsins Michelle Christine, Peppone Luke Joseph, Chandwani Kavita Dayal, Morrow Gary R, Mustian Karen Michelle. Physical activity participation and functional limitations in geriatric cancer survivors. J Clin Oncol. 2012;(suppl) abstr 9009. [Google Scholar]
- 10.Bylow K, Dale W, Mustian K, Stadler WM, Rodin M, Hall W, Lachs M, Mohile SG. Falls and physical performance deficits in older patients with prostate cancer undergoing androgen deprivation therapy. Urology. 2008;72(2):422–427. doi: 10.1016/j.urology.2008.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Masud T, Morris RO. Epidemiology of falls. Age Ageing. 2001;30(Suppl 4):3–7. doi: 10.1093/ageing/30.suppl_4.3. [DOI] [PubMed] [Google Scholar]
- 12.Stone C, Lawlor PG, Nolan B, Kenny RA. A prospective study of the incidence of falls in patients with advanced cancer. J Pain Symptom Manag. 2011;42(4):535–540. doi: 10.1016/j.jpainsymman.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 13.Stone CA, Lawlor PG, Savva GM, Bennett K, Kenny RA. Prospective study of falls and risk factors for falls in adults with advanced cancer. J Clin Oncol. 2012;30(17):2128–2133. doi: 10.1200/JCO.2011.40.7791. [DOI] [PubMed] [Google Scholar]
- 14.Saliba D, Elliott M, Rubenstein LZ, Solomon DH, Young RT, Kamberg CJ, Roth C, MacLean CH, Shekelle PG, Sloss EM, Wenger NS. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49(12):1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 15.Mohile SG, Bylow K, Dale W, Dignam J, Martin K, Petrylak DP, Stadler WM, Rodin M. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109(4):802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 16.Luciani A, Ascione G, Bertuzzi C, Marussi D, Codeca C, Di Maria G, Caldiera SE, Floriani I, Zonato S, Ferrari D, Foa P. Detecting disabilities in older patients with cancer: comparison between comprehensive geriatric assessment and vulnerable elders survey-13. J Clin Oncol. 2010;28(12):2046–2050. doi: 10.1200/JCO.2009.25.9978. [DOI] [PubMed] [Google Scholar]
- 17.Postma TJ, Aaronson NK, Heimans JJ, Muller MJ, Hildebrand JG, Delattre JY, Hoang-Xuan K, Lanteri-Minet M, Grant R, Huddart R, Moynihan C, Maher J, Lucey R. The development of an EORTC quality of life questionnaire to assess chemotherapy-induced peripheral neuropathy: the QLQ-CIPN20. Eur J Cancer. 2005;41(8):1135–1139. doi: 10.1016/j.ejca.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 18.Cavaletti G, Cornblath DR, Merkies IS, Postma TJ, Rossi E, Frigeni B, Alberti P, Bruna J, Velasco R, Argyriou AA, Kalofonos HP, Psimaras D, Ricard D, Pace A, Galie E, Briani C, Dalla Torre C, Faber CG, Lalisang RI, Boogerd W, Brandsma D, Koeppen S, Hense J, Storey D, Kerrigan S, Schenone A, Fabbri S, Valsecchi MG. The chemotherapy-induced peripheral neuropathy outcome measures standardization study: from consensus to the first validity and reliability findings. Ann Oncol. 2013;24(2):454–462. doi: 10.1093/annonc/mds329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton DL, Wos EJ, Qin R, Mattar BI, Green NB, Lanier KS, Bearden JD, 3rd, Kugler JW, Hoff KL, Reddy PS, Rowland KM, Jr, Riepl M, Christensen B, Loprinzi CL. A double-blind, placebo-controlled trial of a topical treatment for chemotherapy-induced peripheral neuropathy: NCCTG trial N06CA. Support Care Canc. 2011;19(6):833–841. doi: 10.1007/s00520-010-0911-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrison T. Health disparities among Latinas aging with disabilities. Fam Community Health. 2009;32(1 Suppl):S36–S45. doi: 10.1097/01.FCH.0000342838.05607.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zsembik BA, Peek MK, Peek CW. Race and ethnic variation in the disablement process. J Aging Health. 2000;12(2):229–249. doi: 10.1177/089826430001200205. [DOI] [PubMed] [Google Scholar]
- 22.SEER Cancer Statistics. National Cancer Institute. [Accessed Jan 2012];2011 http://seer.cancer.gov/statfacts/html/all.html.
- 23.Balducci L. Epidemiology of cancer and aging. J Oncol Manag. 2005;14(2):47–50. [PubMed] [Google Scholar]
- 24.Wehren LE. The epidemiology of osteoporosis and fractures in geriatric medicine. Clin Geriatr Med. 2003;19(2):245–258. doi: 10.1016/s0749-0690(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 25.Vellas BJ, Wayne SJ, Romero LJ, Baumgartner RN, Garry PJ. Fear of falling and restriction of mobility in elderly fallers. Age Ageing. 1997;26(3):189–193. doi: 10.1093/ageing/26.3.189. [DOI] [PubMed] [Google Scholar]
- 26.Prevention CfDCa. Falls among older adults: an overview. [Accessed Jan 2012];2010 http://www.cdc.gov/HomeandRecreationalSafety/Falls/adultfalls.html.
- 27.Chan BK, Marshall LM, Winters KM, Faulkner KA, Schwartz AV, Orwoll ES. Incident fall risk and physical activity and physical performance among older men: the Osteoporotic Fractures in Men Study. Am J Epidemiol. 2007;165(6):696–703. doi: 10.1093/aje/kwk050. [DOI] [PubMed] [Google Scholar]
- 28.Erlandson KM, Allshouse AA, Jankowski CM, Duong S, MaWhinney S, Kohrt WM, Campbell TB. Risk factors for falls in HIV-infected persons. J Acquir Immune Defic Syndr. 2012;61(4):484–489. doi: 10.1097/QAI.0b013e3182716e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel S, Hyer S, Tweed K, Kerry S, Allan K, Rodin A, Barron J. Risk factors for fractures and falls in older women with type 2 diabetes mellitus. Calcif Tissue Int. 2008;82(2):87–91. doi: 10.1007/s00223-007-9082-5. [DOI] [PubMed] [Google Scholar]
- 30.Richardson JK, Ching C, Hurvitz EA. The relationship between electromyographically documented peripheral neuropathy and falls. J Am Geriatr Soc. 1992;40(10):1008–1012. doi: 10.1111/j.1532-5415.1992.tb04477.x. [DOI] [PubMed] [Google Scholar]
- 31.Oka N, Sugiyama H, Kawasaki T, Mizutani K, Matsui M. Falls in peripheral neuropathy. Rinsho Shinkeigaku. 2005;45(3):207–210. [PubMed] [Google Scholar]
- 32.Stauder R, Moser K, Holzner B, Sperner-Unterweger B, Kemmler G. Six independent domains are defined by geriatric assessment in elderly cancer patients. Crit Rev Oncol Hematol. 2010;74(2):97–105. doi: 10.1016/j.critrevonc.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 33.Pasetto LM, D'Andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: how and why? Crit Rev Oncol Hematol. 2006;59(2):159–168. doi: 10.1016/j.critrevonc.2006.01.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.