Introduction
A diagnosis of lung cancer threatens one’s physical well-being as well as one’s overall quality of life (QOL). Spiritual well-being (SWB), as one aspect of QOL, has received little attention in cancer survivors and even less empirical attention in lung cancer survivors. The research that exists on lung cancer survivors has focused on the spiritual needs identified by patients [1], spiritual coping [2] and end-of-life SWB [3]. A few studies examined the associations between spirituality or prayer and psychological [3–6] and physical responses [3,4,7].
While there is no consensus on the definition of SWB, it is generally identified as multidimensional that includes the concepts of faith, meaning of life and peace of mind. SWB has been identified by lung cancer survivors as an important dimension affecting their QOL [2] and has been found to be a predictor of emotional difficulties including depression [6] and hopelessness [5]. These are important constructs clinically as both depression and hopelessness were found to be predictors of a desire for hastened death [5]. In contrast, high SWB and identifying meaning in life have been shown to be associated with high psychological well-being and lower symptom distress [4]. A component of SWB, faith, has been found to have a positive association with lung cancer survivors’ tumor response rate and an increased level of post chemotherapy lymphocytes [7]. Taken together these findings all demonstrate the significance of SWB in lung cancer survivors, yet little is known about how SWB is affected over the trajectory of this disease process.
The aims of this study were to explore the SWB of individuals with a diagnosis of lung cancer, examine potential changes in SWB over time, and to identify factors associated with SWB.
Methods
Since January 1, 1997, all patients with pathologically confirmed lung cancer at Mayo Clinic Rochester who agreed to participate (over 90%) [8–9] signed a consent form and completed surveys at baseline, 6 and 12 months and then were followed annually by mailed survey. The trained study staff obtained informed consent for research in the clinic setting before or after scheduled appointments. Trained abstractors reviewed the medical records for demographic data, cancer related information, tobacco use and exposure and treatment. This study, part of a larger epidemiology study [8–9] was approved by the Mayo Clinic Institutional Review Board.
Instruments
QOL data were collected using the Functional Assessment in Chronic Illness Therapy – Spiritual (FACITSp) to measure SWB, a QOL Linear Analog Scale Assessment (LASA) scale to measure overall QOL and the Medical Outcome Short Form 8 (SF8) that measures the various dimensions of QOL. The FACIT-Sp, is comprised of 12 items that focus on meaning, peace and faith with demonstrated reliability, discriminate and convergent validity [10–12]. Cronbach’s alpha for the FACIT-Sp was 0.84 to 0.91 across measurement times in our study. The SF8 is comprised of 8 questions that measure physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional and mental health [13–14]. Cronbach’s alpha for the SF-8 was 0.91 to 0.94 across measurement times in our study. The QOL LASA was anchored by 0 = “as bad as it can be” and 100 “as good as it can be”. This scale has been used in clinical trials involving oncology patients and demonstrated to be prognostic for survival [15–17]. Smoking status was defined as “never smoked”, smoking less than 100 cigarettes in their lifetime; “former smoker”, not smoking in the last 30 days, and “current smoker”, those who smoked in the last 30 days.
Statistical Approach
QOL LASA scores ranged from 0–100, SF8 scores were normed with a mean of 50 and SD of 10 and FACIT-Sp scores were converted to a scale of 0–100. All scales were scored so that high scores represented favorable aspects of the measured concept. Associations between demographics and FACIT-Sp scores were compared using Fisher’s exact tests and chi-squared tests for categorical variables and Kruskal-Wallis tests for ordinal variables. Correlations between FACIT-Sp, SF8 and QOL scores were tested using Spearman correlations. Linear regression model were used to explore multivariate relationships between SWB and demographics. Percentages of participants experiencing difficulty with specific FACIT-Sp items were determined by grouping not at all, a little bit and somewhat together and quite a bit, very much together. A minimal important difference (MID) was determine using 0.33 of a standard deviation [18]. With 1578 patients, correlation coefficients could be estimated to within .03 with 95% confidence, and proportions could be estimated within 2.5% with 95% confidence. In this sample, tests for differences in proportions between males and females would have over 90% power to detect differences of 9% using 2-sided tests with a 5% type I error rate.
Results
Patient Characteristics
For this cohort study, data from 1578 unique patients were included at various time points after their cancer diagnosis with study entry being their first visit for lung cancer at Mayo Clinic. A total of 531 patients provided data 1 year from diagnosis with numbers decreasing with increased time from diagnosis. Approximately a third of the patients provided data for only one time point, the remainder provided data for up to 4 time points. Patients ranged in age from 18 to 93 at the time of their lung cancer diagnosis (mean age of 65.6), 52% were male, 93% were Caucasian, 54% were former smokers and 29% current smokers. See Table 1 for details.
Table 1.
Demographic and Medical Information
| Age at Diagnosis | N(%) |
|---|---|
| N | 1578 |
| Mean (SD) | 65.6 (10.27) |
| Gender | |
| Female | 756 (47.9%) |
| Male | 822 (52.1%) |
| Race | |
| Caucasian | 1459 (92.5%) |
| Hispanic | 19 (1.2%) |
| Alaskan-Indian | 87 (5.5%) |
| Black | 7 (0.4%) |
| Asian-Pacific Islander | 5 (0.3%) |
| Missing | 1 |
| Pack-Years | |
| N | 1302 |
| Mean (SD) | 47.0 (29.89) |
| Cigarette Smoking Status | |
| Never | 267 (16.9%) |
| Former | 845 (53.5%) |
| Current | 462 (29.3%) |
| Some Smoking History | 4 (0.3%) |
| Stage | |
| Limited | 72 (4.6%) |
| Extensive | 25 (1.6%) |
| Stage IA | 483 (30.9%) |
| Stage IB | 293 (18.7%) |
| Stage IIA | 46 (2.9%) |
| Stage IIB | 105 (6.7%) |
| Stage IIIA | 159 (10.2%) |
| Stage IIIB | 165 (10.5%) |
| Stage IV | 217 (13.9%) |
| Missing | 13 |
| Grade | |
| Grade I | 122 (7.7%) |
| Grade II | 334 (21.2%) |
| Grade III | 601 (38.1%) |
| Grade IV | 415 (26.3%) |
| Missing | 106 (6.7%) |
| Recurrent Disease | |
| No | 1418 (89.9%) |
| Yes | 160 (10.1%) |
| Disease Progression | |
| No | 1531 (97.0%) |
| Yes | 47 (3.0%) |
| Surgery | |
| No | 369 (25.1%) |
| Yes | 1104 (74.9%) |
| Missing | 105 |
| Radiation | |
| No | 1070 (72.6%) |
| Yes | 403 (27.4%) |
| Missing | 105 |
| Chemotherapy | |
| No | 822 (55.8%) |
| Yes | 651 (44.2%) |
| Missing | 105 |
| Radiation and Chemotherapy | |
| No | 1134 (77.0%) |
| Yes | 339 (23.0%) |
| Missing | 105 |
| Other Lung Treatment | |
| No | 1462 (99.3%) |
| Yes | 11 (0.7%) |
| Missing | 105 |
| Year of First Spirituality Form | |
| 0.5 | 442 (28.0%) |
| 1 | 244 (15.5%) |
| 2 | 165 (10.5%) |
| 3 | 177 (11.2%) |
| 4 | 149 (9.4%) |
| 5 | 139 (8.8%) |
| 6 | 115 (7.3%) |
| 7 | 68 (4.3%) |
| 8 | 63 (4.0%) |
| 9 | 14 (0.9%) |
| 10 | 2 (0.1%) |
| Number of Spirituality Evaluations | |
| 1 | 492 (31.2%) |
| 2 | 444 (28.1%) |
| 3 | 525 (33.3%) |
| 4 | 117(7.4%) |
Grade – classification of how abnormal cells look and how likely they will spread (National Cancer Institute – www.cancer.gov/cancertopics/factsheet/detection/tumor-grade).
SWB
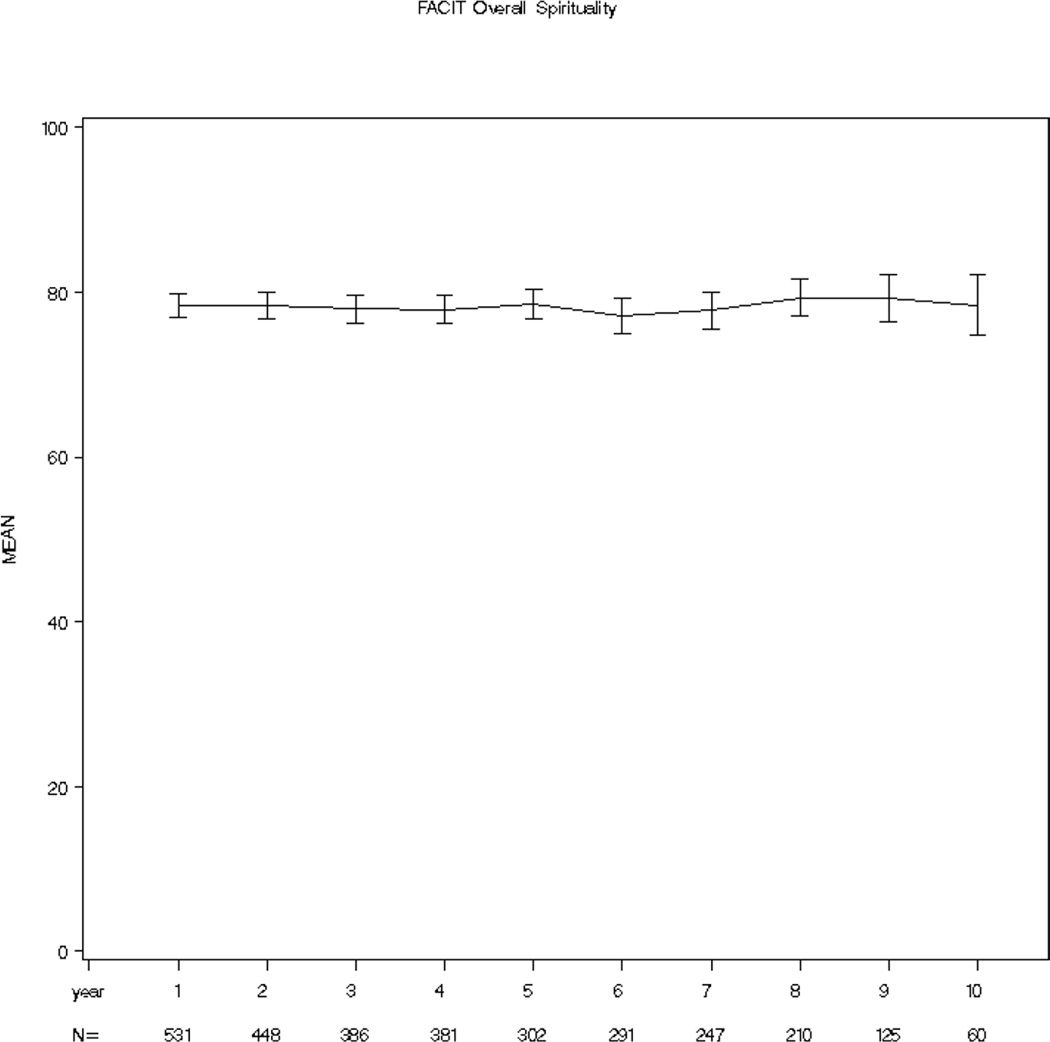
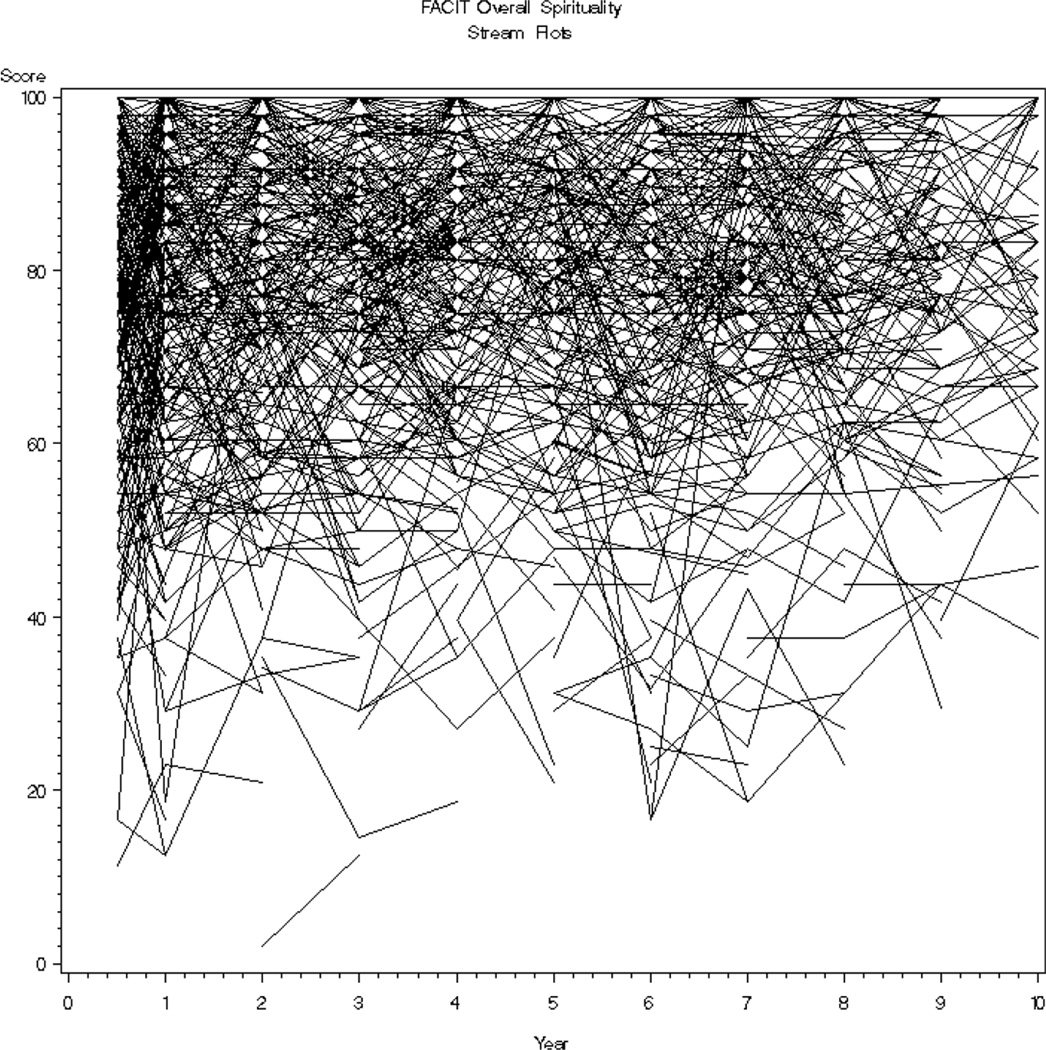
SWB of the entire cohort was high and stable over the 10 year period of time (x̅ = 77.1 – 79.3, SD = 14.47 – 18.46, possible scale 0–100). See Figure 1. However, the range of scores was wide and consumed most of the scale (2.1 – 100) with 63% (N=995) having initial scores greater or equal to 70, 11% (N=173) scores less than or equal to 50 and the remainder with scores between 51 and 69 (N=410). Examining patients’ worst score, the mean score was favorable at 73.3 but also consumed most of the scale (2.1–100) with a standard deviation of 17.7 points. Participants’ mean SWB score change was small, −0.2, however, the standard deviation (11.7) and change score range of −75.0 to 66.7 was large enough to warrant concern. Additionally, in contrast to stable group scores, a stream plot of individual scores from one measurement point to the next revealed a chaotic picture in which individual scores change over time (see Figure 2).
Figure 1.
Cohort Spiritual Wellbeing Across 10 Year Period of Time
Figure 2.
Stream Plot of Individuals’ SWB Across 10 Year Period of Time
Exploring the individual FACIT-Sp items, the vast majority of individuals felt life had been productive (84%–92% across time points) and that they had reason for living (87%–94% across time points). Approximately 10% of the participants across time points reported feeling that life lacked meaning and purpose, approximately 20% reported difficulty with feeling that things would be okay, feeling a sense of purpose in life, finding comfort in spiritual beliefs, finding peace of mind, or finding strength in spiritual beliefs. About 25% of the participants reported difficulty with their ability to reach down inside for comfort or trouble feeling a sense of harmony with self. About 60% felt that their illness strengthened their faith.
Patient characteristics associated with SWB
Three patient characteristics collected were associated with a patient’s lowest reported level of SWB. These included gender (p<0.0001), smoking status (p=0.0455) and pack-years (p=0.0004). Men experienced lower SWB than women. Forty-two percent of the males and 31% of the women reported SWB scores ≤ 70. 14% of the males and 8% of the women reported SWB scores ≤ 50.
Individuals who never smoked experienced the most favorable SWB, followed by former smokers and those doing the worse, current smokers. 43% of the current smokers reported SWB scores ≤ 70, 14% a score ≤ 50 while 35% of the former smokers reported SWB scores ≤ 70 and 10% a score ≤ 50. In contrast, 33% of the never smokers reported SWB scores ≤ 70 and 8% scores ≤ 50.
Higher pack-years were also associated with lower SWB scores. Individuals with SWB scores greater than or equal to 70 had mean pack-years of 45.0 (SD=30.16), those with SWB scores from 51–69 had mean packyears of 48.5 (SD=28.02) and those with SWB scores of ≤ 50 had a mean of 53.7 (SD = 31.47) pack-years. See Table 2.
Table 2.
Patient Characteristics Associated with Worst Reported SWB
| SWB ≤ 50 N(%) |
SWB 51 to 69 N(%) |
SWB ≥ 70 N(%) |
P | |
|---|---|---|---|---|
| Gender | <0.0001 | |||
| Males (N=822) | 114 (14%) | 234 (28%) | 474 (58%) | |
| Females (N=756) | 59 (8%) | 176 (23%) | 521 (69%) | |
| Smoking Status | 0.0455 | |||
| Current Smoker (N=462) | 63 (14%) | 134 (29%) | 265 (57%) | |
| Former Smoker (N=849) | 90 (11%) | 209 (25%) | 550 (65%) | |
| Never Smoked (N=267) | 20 (7%) | 67 (25%) | 180 (67%) | |
| Pack-years, x̅(SD) | 53.7 (31.47) | 48.5 (28.02) | 45.0 (30.16) | 0.0004 |
| Age at Diagnosis, x̅(SD) | 65.0 (10.20) | 65.1 (11.22) | 65.8 (9.87) | 0.54 |
Individual’s worst reported SWB was not associated with age at diagnosis, educational status, lung cancer type, tumor stage or grade, status of metastasis, or type of treatment. Additionally, SWB was not prognostic with no significant difference between the SWB of those who survived and those who died within the year after completing their surveys.
SWB scores were modelled via linear regression using age at diagnosis, gender, smoking status, and pack years as independent variables. In this model, only smoking status was not related to SWB score. Females were on average 4.3 points higher than males (p<0.0001), older patients had higher SWB scores (mean increase of 0.10 points for every year increase in age, p=0.03), and higher pack years were associated with lower SWB (mean decrease of 0.04 points for every year increase in pack years).
Associations between SWB, Overall QOL, and QOL imensions
Mean (70 – 80) and median (72.7 – 75.8) QOL LASA scores over time showed limited difficulty with overall QOL as a group. However, the range varied from 0 to 100. The areas where patients exhibit the most difficulty was with the physical component (x̅ = 42.1 – 45.3, SD = 9.26 – 10.27 over time). Specific areas under the physical component that were problematic included physical function (x̅ 40.2 – 42.8, SD = 8.39 – 9.20 over time), physical function of one’s role (x̅ = 41.3 – 43.1, SD = 8.83 – 10.95), and vitality (x̅ = 47.5 – 49.9, SD = 7.30 – 8.18).
We found SWB after 5 years of post-lung cancer survival to be strongly associated with overall QOL (Spearman correlation, r=0.52) and was moderately associated with the QOL components mental health (r=0.44), role function (r=0.42), vitality (r=0.34), general health (0.33), and social function (r=0.30). Low correlations were found between SWB and the two QOL dimensions physical function (r=0.29) and bodily pain (r=0.24). Changes in QOL between years were positively correlated with changes in SWB. These correlations ranged from 0.17 to 0.42 and were higher in the years farther removed from initial diagnosis and treatment.
Discussion
While the SWB of the cohort is relatively and consistently high as a group over time, there was remarkable variance in some individuals’ scores over time. Males, current smokers and those with higher pack-years experienced lower SWB compared to females, non-smokers and those with lower pack-years. SWB was strongly associated with overall QOL with mental health and role function being the QOL components most highly associated with SWB.
High SWB scores have been found in lung and other cancer populations [19–20]. The stream plot in this study shows a vivid picture of the chaotic trajectory that SWB takes with this population. While the majority of these scores are 70 or above on a scale of 0 – 100, there are obvious ups and downs in individuals’ SWB. These findings provide impetus for the exploration of factors associated with changes in SWB and underscore the necessity of conducting ongoing assessments of SWB in the clinical setting. Additionally, with a significant portion (20–25%) of patients experiencing difficulty with items that reflect purpose in life, peacefulness and comfort/strength from spiritual beliefs there is a need for spiritual intervention. Patients themselves have expressed interest in receiving spiritual information [21]. Various spiritual interventions have been identified as being helpful including prayer, centering prayer [22], meditation, journaling, life review, chaplain/pastoral visits, and building spiritual awareness [23–24].
Like others, we have found SWB to be different in males and females [25–28] with males experiencing lower levels of SWB. Understanding these differences can affect the success of interventions. It has been suggested that women are higher than men on the spiritual constructs of belonging, meaning, hope, spiritual connection, and praying for one’s health [25–28]. We also found more men’s worst score to reflect difficulty with meaning and spiritual connection as evidenced by significantly lower scores than women on the SWB items life lacks meaning (30.4%, 24.2% respectively), comfort in spiritual beliefs (33.7, 20.9% respectively) and strength in spiritual beliefs (34%, 21.2% respectively). Potential interventions that address SWB difficulties in males may include spiritual support through a religious community or the medical system. Assisting the individual to identify ways to enhance relationships with family and friends may be a means to reinforce meaning in life.
We did not find other research that explored the relationship between SWB and smoking in individuals with lung cancer. In related research, well individuals with a high level of SWB were less likely to use tobacco [33–35]. Research focused on persons with lung cancer revealed that non-smokers had a higher level of QOL than smokers [36–37] and a negative association between religious activity and smoking [38]. Others have identified an association between spirituality and healthy lifestyles [39]. These associations may suggest an overall approach to life in which individuals undertake activities, such as spiritual and healthy lifestyle approaches, that enhance their wellbeing. Thus, focusing on interventions that incorporate a holistic approach to enhancing well-being may be important.
Our finding that current smokers have the lowest SWB reveals an important population in need of intervention. While the mechanisms behind this finding were not explored, it is possible that smoking may be related to self-blame or guilt about smoking and its relationship to lung cancer. Additionally, smoking may be the main coping mechanism used by a number of these individuals. Interventions aimed at building other coping mechanisms while undergoing smoking cessation interventions may be useful for both smoking cessation and increased SWB. Support from others may provide the needed nurturance to adapt new coping mechanisms and enhance well-being. Spiritual support has been shown to have a positive association with patient QOL [29–31]. Good relationships with family and others have been identified as essential for finding meaning and hope [32]. Attributional theory may provide guidance for interventions that assist with smoking cessation and lessening blame. Interventions aimed at cognitive appraisal of the situation as controllable, changeable and a result of the person’s behavior has been identified as promoting behavior change. Interventions focused on seeking forgiveness have been identified as lessening blame [40].
Like others, we found SWB to be associated with QOL [19,41–43]. In this lung population, SWB was associated more strongly with mental health, ability to perform roles, vitality, perceived general health and social function. The association between SWB and QOL is likely multifaceted, including a systems theory perspective in which each aspect of an individual, such as SWB, influences other aspects, such as overall perceived QOL. Spirituality is also seen as a coping mechanism [43]. Increased use of spirituality as a coping mechanism contributes to the enhancement of SWB, favorable adjustment and consequently favorable QOL.
We found that SWB in the last year of life was not prognostic of those who died in the subsequent year. With the significant variations in SWB over time, exploration of the prognostic ability of SWB in the last month of life may reveal different findings. We also found that changes in SWB were more strongly associated with changes in QOL in years farther removed from initial diagnosis. These findings may suggest that SWB and QOL change in concert with each other only once the individual has adapted to their diagnosis. Weisman and Worden [44] referred to the period of time after a diagnosis of cancer as existential plight in which there is a predominance of life and death concerns and worries about health. These overriding concerns may prevent the person from adjusting to a new normal which is needed for stabilizing the various aspects of their being. Further research focusing on the relationship between QOL and SWB is warranted.
Strengths of this study include the large sample size and the longitudinal assessment of individuals over a 10 year period of time. Limitations to this study includes that this represents a Caucasian population and only 20–25% of the overall group responded at any given time point. Additionally, as identified in other follow-up studies of lung cancer patients, it is likely that individuals with more severe disease and higher mortality are underrepresented [45–49].
Conclusions
While group averaged SWB may reflect that SWB is static, individual patient SWB data reveal that is not uniformly the case, underscoring the need for ongoing assessment of SWB. Males, current smokers, those with a high number of pack-years as well as those with impaired mental health or role function may be particularly vulnerable for poor SWB.
Acknowledgements
This research was funded by 2 NIH grants, R01-84354 and R01-115857, from the National Cancer Institute, Ping Yang, Principal Investigator.
Footnotes
Conflict of Interest
No conflicts of interests. We have full control of the primary data and agree to allow the Journal of Supportive Care in Care to review data if requested.
Contributor Information
Marlene H. Frost, Mayo Clinic Rochester, 200 First Street SW, Charlton Building, 6-127, Rochester, MN 55905, fax: 507-266-2478, telephone: 507-266-6945, frost.marlene@mayo.edu.
Mary E. Johnson, Mayo Clinic Rochester, 200 First Street SW, Stabile 1, Rochester, Minnesota 55905, telephone: 507-923-0503, johnson.mary3@mayo.edu.
Jeff A. Sloan, Mayo Clinic Rochester, 200 First Street SW, Harwick Building, 8, Rochester, MN 55905, telephone: 507-284-9985, fax: 507-284-4186, jsloan@mayo.edu.
Paul J. Novotny, Mayo Clinic Rochester, 200 First Street SW, Harwick Building, 8-35, Rochester, MN 55905, fax: 507-266-2477, telephone: 507-284-4186, novotny@mayo.edu.
Matthew M. Clark, Mayo Clinic Rochester, 200 First Street SW, Mayo Building W11-B, Rochester, MN 55905, telephone: 507-284-2933, fax: 507-284-4158, clark.matthew@mayo.edu.
Ping Yang, Mayo Clinic Rochester, 200 First Street SW, Charlton Building, 6, Rochester, MN 55905, telephone: 266-5369, fax: 507-266-2478, yang.ping@mayo.edu.
References
- 1.Jacobs-Lawson JM, Schumacher MM, Huges T, et al. Gender differences in psychosocial responses to lung cancer. Gender Med. 2010;7:137–148. doi: 10.1016/j.genm.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 2.John LD. Self-care strategies used by patients with lung cancer to promote quality of life. Oncol Nurs Forum. 2010;37:339–347. doi: 10.1188/10.ONF.339-347. [DOI] [PubMed] [Google Scholar]
- 3.Murray SA, Kendall M, Grant E, et al. Patterns of social, psychological, and spiritual decline toward the end of life in lung cancer and heart failure. J Pain Symptom Manage. 2011;34:393–402. doi: 10.1016/j.jpainsymman.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Meraviglia MG. The effects of spirituality on well-being of people with lung cancer. Oncol Nurs Forum. 2004;31:89–94. doi: 10.1188/04.ONF.89-94. [DOI] [PubMed] [Google Scholar]
- 5.Rodin G, Lo C, Mikulincer M, et al. Pathways to distress: the multiple determinants of depression, hopelessness, and the desire for hastened death in metatstatic cancer patients. Soc Sci Med. 2009;68:562–569. doi: 10.1016/j.socscimed.2008.10.037. [DOI] [PubMed] [Google Scholar]
- 6.Zimmermann LC, Rydall C, Walsh A, et al. Longitudinal study of depressive symptoms in patients with metastatic gastrointestinal and lung cancer. J Clin Oncol. 2010;28:3084–3089. doi: 10.1200/JCO.2009.26.9712. [DOI] [PubMed] [Google Scholar]
- 7.Lissoni P, Messina G, Parolini D, et al. A spritual approach in the treatment of cancer : relation between faith score nad respons to chemotherapy in advanced non-smal cell lung cancer patients. In Vivo. 2008;22:577–581. [PubMed] [Google Scholar]
- 8.Yang P, Allen MS, Aubry MC, et al. Clinical features of 5,628 primary lung cancer patients: experience at Mayo Clinic from 1997 to 2003. CHEST. 2005;128(1):452–462. doi: 10.1378/chest.128.1.452. [DOI] [PubMed] [Google Scholar]
- 9.Svobodnik a, Yang P, Novotny PJ, et al. Quality of life in 650 lung cancer survivors 6 months to 4 years after diagnosis. Mayo Clin Proc. 2004;79(8):1024–1030. doi: 10.4065/79.8.1024. [DOI] [PubMed] [Google Scholar]
- 10.Brady MJ, Peterman AH, Fitchett G, et al. A case for spirituality in quality of life measurement in oncology. Psychooncology. 1999;8:417–428. doi: 10.1002/(sici)1099-1611(199909/10)8:5<417::aid-pon398>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Bredle JM, Salsman JM, Debb SM, et al. Spiritual well-being as a component of healthrelated quailty of life: the Functional Assessment of Chronic Illness Therapy – Spiritual Well-Being Scale (FACIT-Sp) Religions 2011. 2011;2:77–94. [Google Scholar]
- 12.Peterman A, Fitchett G, Brady M, et al. Measuring spiritual well-being in people with cancer: The Functional Assessment of Chronic Illness Therapy-Spiritual Well-being Scale (FACIT-Sp) Ann Behav Med. 2002;24:49–58. doi: 10.1207/S15324796ABM2401_06. [DOI] [PubMed] [Google Scholar]
- 13.Lefante JJ, Harmon GN, Ashby KM, et al. Use of the SF-8 to assess health-related quality of life for a chronically ill, low-income population participating in the Central Louisiana Medication Access Program (CMAP) Qual Life Res. 2005;14(3):665–673. doi: 10.1007/s11136-004-0784-0. [DOI] [PubMed] [Google Scholar]
- 14.Ware JE, Kosinski M, Dewey JE, et al. How to score and interpret single-item health status measures: a manual for users of the SF-8 TM Health Survey. Lincoln, RI: QualityMetric Inc; 1999. [Google Scholar]
- 15.Bretscher M, Rummans T, Sloan J, et al. Quality of life in hospice patients. A pilot study. Psychosomatics. 1999;40:309–313. doi: 10.1016/S0033-3182(99)71224-7. [DOI] [PubMed] [Google Scholar]
- 16.Sloan J, Symonds T, Vargas-Chanes D, et al. Practical guidelines for assessing the clinical significance of health-related quality of life changes within clinical trials. Drug Inf J. 2003;37:23–31. [Google Scholar]
- 17.Rummans TA, Clark MM, Sloan JA, et al. Impacting quality of life for patients with advanced cancer with a structured multidisciplinary intervention : a randomized controlled trial. J Clin Oncol. 2006;224:635–642. doi: 10.1200/JCO.2006.06.209. [DOI] [PubMed] [Google Scholar]
- 18.Sloan JA, Aaronson N, Cappelleri JC, et al. Assessing the clinical significance of single items relative to summated scores. Mayo Clinic Proceedings. 2002;77:479–487. [PubMed] [Google Scholar]
- 19.Frost MH, Johnson ME, Atherton TJ, et al. Spiritual Well-being and Quality of Life of Women with Ovarian Cancer and Their Spouses. J Support Oncol. 2012;10(2):72–80. doi: 10.1016/j.suponc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 20.Highfield MF. Spiritual health of oncology patients. Nurse and patient perspectives. Cancer Nurs. 1992;15:1–8. [PubMed] [Google Scholar]
- 21.Manii D, Ammerman D. Men and cancer: a study of the needs of male cancer patients in treatment. J Psychosoc Oncol. 2008;26:87–102. doi: 10.1300/j077v26n02_06. [DOI] [PubMed] [Google Scholar]
- 22.Johnson ME, Dose AM, Pipe TB, et al. Centering prayer for women receiving chemotherapy for recurrent ovarian cancer: a pilot study. Oncol Nurs Forum. 2009;36:421–428. doi: 10.1188/09.ONF.421-428. [DOI] [PubMed] [Google Scholar]
- 23.Cole B, Pargament K. Re-creating your life: a spiritual/psychotherapeutic intervention for people diagnosed with cancer. Psychooncology. 1999;8:395–407. doi: 10.1002/(sici)1099-1611(199909/10)8:5<395::aid-pon408>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 24.Mytko JJ, Knight SJ. Body, mind and spirit: towards the integration of religiosity and spirituality in cancer quality of life research. Psychooncology. 1999;8:439–450. doi: 10.1002/(sici)1099-1611(199909/10)8:5<439::aid-pon421>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 25.Flannelly KJ, Galek K. Discipline and sex differences in religiosity and spirituality among health care professionals. Psychol Rep. 2006;99:803–804. doi: 10.2466/PR0.99.3.803-804. [DOI] [PubMed] [Google Scholar]
- 26.Galek K, Flannelly KJ, Jacobs MR, et al. Spiritual needs: Gender differences among professional spiritual care providers. J Pastoral Care Counsel. 2008;62:29–35. doi: 10.1177/154230500806200104. [DOI] [PubMed] [Google Scholar]
- 27.Ross LE, Hall IJ, Fairley TL, et al. Prayer and self-reported health among cancer survivors in the United States, National Health Interview Survey 2002. J Altern Complement Med. 2008;14:931–938. doi: 10.1089/acm.2007.0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHOQOL, SRPB, Group. A cross-cultural study of spirituality, religion, and personal beliefs as components of quality of life. Soc Sci Med. 2006;62:1486–1497. doi: 10.1016/j.socscimed.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 29.Balboni TA, Vanderwerker LC, Block SD, et al. Religiousness and spiritual support among advanced cancer patients and associations with end-of-life treatment preferences and quality of life. J Clin Oncol. 2007;25:555–560. doi: 10.1200/JCO.2006.07.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tarakeshwar N, Vanderwerker LC, Paulk E, et al. Religious coping is associated with the quality of life of patients with advanced cancer. J Palliat Med. 2006;9:646–657. doi: 10.1089/jpm.2006.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weaver AJ, Flannelly KJ. The role of religion/spirituality for cancer patients and their caregivers. South Med J. 2004;97:1210–1214. doi: 10.1097/01.SMJ.0000146492.27650.1C. [DOI] [PubMed] [Google Scholar]
- 32.Benzein E, Norberg A, Saveman BI. The meaning of the lived experience of hope in patients with cancer in palliative home care. Palliat Med. 2001;15:117–126. doi: 10.1191/026921601675617254. [DOI] [PubMed] [Google Scholar]
- 33.Leigh J, Bowen S, Marlatt GA. Spirituality, mindfulness and substance abuse. Addict Behav. 2005;30:1335–1341. doi: 10.1016/j.addbeh.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 34.Mann JR, McKeown RE, Bacon J, et al. Religiosity, spirituality, and tobacco use by pregnant women. South Med J. 2007;100:867–872. doi: 10.1097/SMJ.0b013e318137a422. [DOI] [PubMed] [Google Scholar]
- 35.Turner-Musa J, Lipscomb L. Spirituality and social support on health behaviors of African American undergraduates. Am J Health Behav. 2007;31:495–501. doi: 10.5555/ajhb.2007.31.5.495. [DOI] [PubMed] [Google Scholar]
- 36.Garces YI, Yang P, Parkinson J, et al. The relationship between cigarette smoking and quality of life after lung cancer diagnosis. CHEST. 2004;126 doi: 10.1378/chest.126.6.1733. 1733-174. [DOI] [PubMed] [Google Scholar]
- 37.Croghan IT, Schroeder DR, Hays JT, et al. Nicotine dependence treatment: perceived health status improvement with 1-year continuous smoking abstinence. Eur J Public Health. 2005;15:251–255. doi: 10.1093/eurpub/cki076. [DOI] [PubMed] [Google Scholar]
- 38.McFadden D, Croghan IT, Piderman KM, et al. Spirituality in tobacco dependence: a Mayo Clinic survey. EXPLORE. 2011;7:162–167. doi: 10.1016/j.explore.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Koenig H, McCullough M, Larson D. Handbook of Religion and Health. Oxford, NY: Oxford University Press; 2001. [Google Scholar]
- 40.Weiner B. Judgments of responsibility: a foundation for a theory of social conduct. New York: Guilford Press; 1995. [Google Scholar]
- 41.Johnson ME, Piderman KM, Sloan JA, et al. Measuring spiritual quality of life in patients with cancer. J Support Oncol. 2007;5:437–442. [PubMed] [Google Scholar]
- 42.Lin HR, Bauer-Wu SM. Psycho-spiritual well-being in patients with advanced cancer: an integrative review of the literature. J Adv Nurs. 2003;44:69–80. doi: 10.1046/j.1365-2648.2003.02768.x. [DOI] [PubMed] [Google Scholar]
- 43.McClain CS, Rosenfeld B, Breitbart W. Effect of spiritual well-being on end-of-life despair in terminally-ill cancer patients. Lancet. 2003;361:1603–1607. doi: 10.1016/S0140-6736(03)13310-7. [DOI] [PubMed] [Google Scholar]
- 44.Weisman AD, Worden JW. The existential plight in cancer: significance of the first 100 days. Int J Psychiatry Med. 1977;7:1–15. doi: 10.2190/uq2g-ugv1-3ppc-6387. [DOI] [PubMed] [Google Scholar]
- 45.Fairclough DL, Peterson HF, Chang V. Why are missing quality of life data a problem in clinical trials of cancer therapy? Stat Med. 1998;17:667–677. doi: 10.1002/(sici)1097-0258(19980315/15)17:5/7<667::aid-sim813>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 46.Fairclough DL, Gagon DD, Zagari MJ, et al. Evaluation of quality of life in a clinical trial with nonrandom dropout: the effect of epoetin alfa in anemic cancer patients. Qual Lif Res. 2003;12:1013–1027. doi: 10.1023/a:1026116426494. [DOI] [PubMed] [Google Scholar]
- 47.Hollen PJ, Gralla RJ, Cox C, et al. A dilemma in analysis: issues in the serial measurment of quality of life in patients with advanced lung cancer. Lung Cancer. 1997;18:119–136. doi: 10.1016/s0169-5002(97)00059-7. [DOI] [PubMed] [Google Scholar]
- 48.Cheville AL, Novotny PJ, Sloan JA, et al. Fatigue, dyspnea, and cough comprise a persistent symptom cluster up to 5 years after diagnosis with lung cacner. J Pain Symptom Manage. 2011;42:201–212. doi: 10.1016/j.jpainsymman.2010.10.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheville AL, Novotny PJ, Sloan JA, et al. The value of a symptom cluster of fatigue, dyspnea, and cough in predicting clinical outcomes in lung cancer survivors. J Pain Symptom Manage. 2011;42:213–221. doi: 10.1016/j.jpainsymman.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]