Abstract
Oligodendrogliomas originate from oligodendrocyte progenitor (OPs), whose development is regulated by the Sonic hedgehog and Wnt/beta-catenin pathways. We investigated the contribution of these pathways in the proliferation and differentiation of human oligodendroglioma cells (HOG). Inhibition of Hedgehog signaling with cyclopamine decreased cell survival and increased phosphorylated beta-catenin without altering myelin protein levels. Conversely, treatment of HOG with the Wnt antagonist secreted Frizzled Related Protein (SFRP1), led to increased myelin protein levels and reduced cell proliferation, suggesting cell cycle arrest and differentiation. Unlike normal primary human OPs, beta-catenin in HOG cells is not associated with endogenous Sox17 protein despite high levels of both proteins. Retroviral overexpression of recombinant Sox17 increased HOG cell cycle exit and apoptosis, and raised myelin protein levels and the percentage of O4+ cells, indicating increased differentiation. Recombinant Sox17 also increased beta-catenin-TCF4-Sox17 complex formation and decreased total cellular levels of beta-catenin. These changes were associated with increased SFRP1, and reduced expression of Wnt-1 and Frizzled-1,−3 and −7 RNA, indicating that Sox17 induced a Hedgehog target, and regulated Wnt signaling at multiple levels. Our studies indicate that Wnt signaling regulates HOG cell cycle arrest and differentiation, and that recombinant Sox17 mediates modulation of the Wnt pathway through changes in beta-catenin, SFRP1 and Wnt/Frizzled expression. Our results thus identify Sox17 as a potential molecular target to include in HOG therapeutic strategies.
Keywords: Sonic hedgehog, Wnt, Sox17, oligodendrocyte progenitor, cell cycle
1. Introduction
Oligodendrogliomas (HOGs) are the third most common type of glioma, as they comprise 2% to 5% of primary brain tumors, and 4% to 15% of all gliomas [36]. The updated WHO guidelines classify oligodendrogliomas into grade II and grade III tumors. Grade III oligodendroglioma is synonymous with anaplastic oligodendroglioma (or malignant oligodendroglioma), which grows more quickly. The median survival of patients with grade II oligodendroglioma is 4–10 years, but is only 3–4 years for anaplastic oligodendrogliomas. 80% of HOGs display co-deletion of chromosomal arms 1p and 19q and are generally less aggressive, showing better sensitivity to treatment and prognosis. Since these tumors can affect parts of the brain that control speech, vision or motor functions, surgery may be associated with the risk of disability. Thus, an understanding of their molecular pathogenesis may provide important alternative options for therapy.
Recent studies have shown that the cells which give rise to HOGs in mice resemble oligodendrocyte progenitor cells (OPCs) rather than stem cells [33], indicating a greater level of developmental commitment. HOGs share many antigenic markers of OPCs -including Olig2, NG2 and growth factor receptors - and frequently occur in subcortical white matter, where OPCs are most abundant. HOG cells respond to developmental cues, indicating an innate, albeit limited, ability to differentiate, despite frequent chromosomal aberrations. These features support the notion that identification of signaling pathways controlling cell proliferation and differentiation may offer additional treatment strategies.
The mammalian oligodendroglial lineage is regulated by two well-established signaling pathways in development: Sonic hedgehog (Shh) - which is responsible for the promotion of oligodendroglial cell fate and generation of immature progenitor cells [11] - and Wnt - which inhibits the maturation of these progenitor cells [17]. Dysregulation of one or both of these pathways underlies a wide variety of human malignancies. As HOG cells remain immature in adulthood, it is possible that the Wnt pathway may be involved in HOG pathogenesis, but the relative contribution of both of these pathways in HOG development is unknown.
Sox17, a transcription factor, inhibits the canonical Wnt/β-catenin signaling in malignant tumors [19, 24, 53] and in OPCs [8]. We have previously demonstrated that Sox17 expression is required for oligodendrocyte development lineage progression, and that Sox17 promotes OPC cell cycle exit and differentiation [41]. However, the effect of Wnt modulation by Sox17 in HOGs has not been investigated.
In this study, we demonstrate that Shh signaling regulates cell growth and survival in HOG cells, whereas Wnt signaling plays an important role in HOG cell differentiation. We investigate endogenous Sox17-beta-catenin interaction, and analyzed the consequence of overexpressing Sox17 in HOG cells. Recombinant Sox17 not only upregulated the levels of the Wnt antagonist SFRP1, but also decreased endogenous beta-catenin levels in HOG cells and Wnt/Frizzled expression, leading to cell cycle exit and differentiation. These studies suggest that such approaches involving Wnt modulation at multiple levels by Sox17 may help to improve strategies in cancer therapy.
2. Materials and methods
2.1. Chemicals and antibodies
Cyclopamine and SFRP1 inhibitor were obtained from Millipore (Billerica, MA). Human recombinant proteins SFRP1 and Shh were from R&D systems (Minneapolis, MN). The following antibodies were used: mouse anti-beta catenin, (1:1000; BD Biosciences; San Jose, CA); goat beta-catenin (1:1000; R&D Systems); anti-P-beta-catenin (1:1000; Cell signaling Technology; Danvers, MA ); anti-Sox17 (1:200); anti-actin (1:5000), anti-olig2 (1:500) normal mouse IgG polyclonal (1:300) (all from Millipore); anti-cyclinD1 (1:500) and, p27Kip1 (1:800) from Santa Cruz biotechnology; Santa Cruz, CA. Anti-SFRP1 (1:100) was from Pierce (Rockford, IL) and Gli2 was from Abcam (1:200; Cambridge, MA). Secondary horseradish peroxidase-conjugated antibodies were goat anti-mouse or goat anti-rabbit from Jackson lab (1:2000; West Grove, PA). OPC lineage markers: anti-myelin basic protein (MBP) (1:1000) and anti-2’, 3’ cyclic nucleotide 3’ phosphodiesterase (CNPase) (1:1000) (Covance; Princeton, NJ).
2.2. Cells and human tissue samples
Four established human oligodendroglioma cell lines were used in this study. HOG was kindly provided by Dr. G. Dawson (University of Chicago, Ml). BT54 and BT 88 cells were generated from patients with 1p/19q co-deleted grade III anaplastic oligodendrogliomas, generously donated by Dr. S. Weiss (University of Calgary, Calgary, Canada). These cells were isolated and cultured as described in their respective publications [27, 34]. SJOA1 cells were acquired from Dr. S. Baker (St Jude Children’s Research Hospital, Memphis, TN) and grown in suspension in Neurobasal medium containing 1% penicillin and streptomycin, 2 mM L-glutamine, and supplemented with N2, B27 (all from Invitrogen; Grand Island, NY) in the presence of 20 ng/ml bFGF and EGF (both from R&D Systems). Normal human oligodendrocyte precursor cells (hOPC) were purchased from ScienCells (Carlsbad, CA) and cultured in the growth medium recommended by manufacturer. Rat cortical oligodendrocyte precursor cells were cultured as previously described [41]. U87 is a human glioblastoma cell line obtained from American Type Culture Collection (Manassas, VA) and cultured according to manufacturer’s instructions. Histological diagnoses were confirmed by pathologic review according to WHO criteria [52]. Four adult human oligodendroglioma specimens were provided by Dr. Sontheimer (University of Alabama, Birmingham, AL). These patient specimens were collected in accordance with IRB certification assurance ID FWA00005960, IRB Registration 00000726 (Neurosurgery Brain Bank), snap-frozen at the time of surgery and stored in liquid nitrogen, shipped in dry ice. These 4 tumor specimens were subsequently homogenized in RIPA buffer for Western blotting analysis. The sources of cells and tissues, along with corresponding experimental procedures performed on them are summarized in Table 1.
Table 1.
Summary of cell and tissue specimen analyses
| 1. Cell line | Identification | Source | Experimental procedure |
Figure |
|---|---|---|---|---|
| hOPC | Human Oligodendrocyte Precursor | ScienCell Inc. (Carlsbad, CA) | RNA analysis, Western blotting, Immunoprecipitation | Figures 1 through 3 |
| HOG | Derived from Oligodendroglioma WHO grade II | Dr. Glyn Dawson (University of Chicago) | RNA analysis, Apoptosis and growth assays, Western blotting, Flow cytometry, Inhibitor treatments, Immunoprecipitation, Retroviral transduction, Immunofluorescence | Figures 1 through 5 |
| SJOA1 | Derived from Oligodendroglioma WHO grade II | Dr. Suzanne Baker (St. Jude Children’s Research Hospital) | Western blotting | Figure 3 |
| BT54 | Derived from Anaplastic Oligodendroglioma WHO grade III | Dr. Samuel Weiss (University of Calgary) | Western blotting | Figure 3 |
| BT88 | Derived from Anaplastic Oligodendroglioma WHO grade III | Dr. Samuel Weiss (University of Calgary) | Western blotting | Figure 3 |
| U87 | Derived from Human Glioblastoma | ATCC Inc. (Manassas, VA) | Western blotting, Immunoprecipitation | Figure 3 |
| II. Tissue | Identification | Source |
Experimental procedure |
Figure |
| #1 | Oligodendroglioma WHO grade II | 48 yo white male, donated by Dr. Harald Sontheimer, (University of Alabama). | Western blotting | Figure 3 |
| #2 | Oligodendroglioma WHO grade II | 43 yo white female, donated by Dr. Harald Sontheimer, (University of Alabama). | Western blotting | Figure 3 |
| #3 | Oligodendroglioma WHO grade II | 65 yo white female, donated by Dr. Harald Sontheimer, (University of Alabama). | Western blotting | Figure 3 |
| #4 | Oligodendroglioma WHO grade II | 40 yo white male, donated by Dr. Harald Sontheimer, (University of Alabama). | Western blotting | Figure 3 |
2.3. RNA extraction and RT-PCR
Total RNA was extracted following standard protocol of TRIzol® RNA reagent (Invitrogen). Contaminating genomic DNA was removed using DNA-free™ Kit (Ambion; Grand Island, NY) containing RNase-free DNase I. Double-stranded cDNA was synthesized from 0.5 µg cleaned RNA with an olig-dT primer in a 20 µl Super Script III First-Stand Synthesis SuperMix reaction (Invitrogen). Subsequent PCR reactions were performed with 3 µl cDNA as input DNA using GoTaq® Hot Start Green Master Mix (Promega; Madison, Wl). The primers for RT-PCR assay are listed in Table 2. Temperature cycling was performed in C1000 thermal cycler (BioRad; Hercules, CA). Cycling parameters were as follows: 95°C, 180 s for initial denaturation and activation of Hot start Taq polymerase followed by 40 cycles of denaturing at 95°C 30s, annealing at 60°C, 55°C or 50°C, 30s and extension at 72°C, 60 s. At the conclusion of cycling, 5 min incubation at 72°C was applied. PCR products were resolved on 1% (w/v) agarose gels stained with ethidium bromide and visualized using Quantity one imaging system (BioRad)
Table 2.
Sequence information for primers used in RT-PCR assays
| Gene name | orwar | Reverse |
|---|---|---|
| patched 1 (ptch1) | TCTGCTGGGTGTACTGATGC | AGAGTCCAGGTGGGGCTGTT |
| smoothened (smo) | CCTCCTGGTGGAGAAGATCAA | CTGGGGAGATCTCTGCCTCA |
| sonic hedgehog (shh) | GCCATCATTCAGAGGAGTCTC | CACGAAGAGCAGGTGCGCGG |
| indian hedgehog (ihh) | TTCCGGGCCACATTTGCCAG | GCTGCTGGTTCTGTATGATTGT |
| desert hedgehog (dhh) | TCCACGCAAACTGTTGCTCAC | ATGCATGCCAGTCGGCTGGA |
| wnt1 | GAGTGCAAATGCCACGGGATG | AGCTGACGTGGCAGCACCAG |
| wnt3a | CCATCTGTGGGTGCAGCAGC | GCCTCGTAGTAGACCAGGTC |
| wnt5a | CTTCACGTACGCGGTGAGCG | GGGTCGATGTAGACCAGGTC |
| wnt10b | CCGCTGACGGCCAACACCGT | ATCCCGAGAGAACTTCTCTCC |
| frizzled1(fzd1) | CGGGCAGCAGTACAACGGCGA | GTTCTGGCCCACGCACAGCTC |
| frizzled3(fzd3) | GGAATATGGACGTGTCACACT | GCGAGCAAATGACAGTTCTTC |
| frizzled7(fzd7) | ACACGAACCAAGAGGACGCG | GAGCCGTCGGACGTGTTCTG |
2.4. MTT reduction assay
HOG cells were plated at 3 × 104 cells per well in 12-well plate and treated with cyclopamine, SFRP1, viral CMV or SOX17 next day of plating in 1 ml 10% FBS medium. Cells were harvested after additional 2 or 4 days prior to analysis. On the day of analysis, 300ul MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent (Promega) was added to each well and incubated at 37°C for 2h prior to addition of 300ul detergent reagent to solubilize the crystals. Supernatants (200ul) were transferred to 96 well plates and read on a Molecular Devices ThermoMax 96 well plate reader (Sunnyvale, CA) using a test wavelength of 570 nm. Data are presented as percentage of absorbance in the control group.
2.5. Apoptosis analysis
HOG cells were harvested after 3–5 days in culture in either cyclopamine, SFRP1, retroviral CMV or Sox17 treatments. The annexin V and viability dye 7-Aminoactinomycin D (7-AAD) for flow cytometry were purchased from Invitrogen. Cells were resuspended in 100ul of 1x annexin-binding buffer (Invitrogen) and 5 µl annexin V was added to each 100 µl of cell suspension. Cells were incubated at room temperature for 15 minutes followed by 10µl 7AAD. 250 µl 1X annexin-binding buffer was added, mixed gently and analyzed with a FACSCalibur flow cytometer (BD Biosciences). CellQuest software (BD Biosciences). Was used for flow data acquisition and FlowJo software (Tree Star Inc.; Ashland, OR) for analysis.
2.6. BrdU incorporation and cell-cycle analysis
After 3 to 5 days incubation under various conditions, HOG cells were harvested and processed by flow cytometry using FACSCalibur. Cells were washed twice with PBS and were dissociated with 0.05% Typsin-EDTA (Life technologies; Grand Island, NY) following by three washes with autoMACS running buffer (Miltenyi Biotec; Auburn, CA) before 4% paraformaldehyde fixation for 30 minutes. Samples for BrdU staining were exposed to 10nM BrdU (Sigma; St. Louis, MO) in 7mM NaOH for 3 hours before neutralization with 2N HCI. Fluorophore-conjugated primary antibodies, anti-BrdU-Allophycocyanin (APC), anti-Human Ki-67- Phycoerythrin (PE) (both from eBioscience; San Diego, CA) and anti-O4-APC (Miltenyi Biotec.) were applied according to manufacturer’s instructions. Background fluorescence was measured using unlabeled cells and cells labeled with isotype control alone; these were used to set gating parameters between positive and negative cell populations. Cell doublets and small debris were excluded from analysis or isolation on the basis of side (measuring cell granularity) and forward scatter (measuring cell size). For cell cycle analysis, cells were passaged and cell suspensions fixed by adding ice-cold 70% ethanol and incubation at −20° C for at least 2 hours. Cells were pelleted and washed with PBS twice before incubating with 100 µg/ml RNase A (Sigma) with PI (40 µg/ml, Invitrogen) for 30 minutes at room temperature. Flow cytometric analysis was performed on a FACSCalibur, in order to determine the proportions of cells in G0/G1, S, G2/M and hypodiploid nuclei phases of the cell cycle. CellQuest software (BD Biosciences) was used for flow data acquisition and FlowJo software (Tree Star Inc.; Ashland, OR) for analysis. Fluorescent intensities of stained cells were plotted.
2.7. Protein Extraction and Western blotting
Cells were harvested after treatment with cyclopamine or SFRP1 for 3 days, or after transduction with Sox 17 retrovirus for 24 hours. Whole-cell lysates were prepared using RIPA after additional 5 days in culture. Protein concentrations of samples were determined by the bicinchoninic acid (BCA) protein assay (Pierce). Protein samples (20 µg of each protein) were mixed with 5x reducing Sample buffer (Thermo Scientific; Rockford, IL) and boiled for 7 minutes, separated in 4–15% precast Tris-HCI polyacrylamide gel (BioRad), and transferred onto Immobilon P membranes (Millipore). Membranes were blocked for 1 h at room temperature with 5% skim milk or 5% BSA for phosphorylated-beta-catenin in tris-buffered saline (TBS) and incubated overnight at 4 °C with primary antibodies following manufacturer’s instructions. Immunoblots were washed three times with TBS containing 0.1% Tween 20 (TBST) and incubated with secondary antibodies conjugated with horseradish peroxidase (HRP) against mouse IgG or rabbit IgG in TBS for 1 h at room temperature with agitation. After washing with TBST three times, the membranes were incubated with Super Signal Chemiluminescent Substrate Stable Peroxide Solution (Pierce) for 5 minutes and exposed to Biomax-MR film (Kodak; Rochester, NY).
2.8. Immunoprecipitation assays
HOG, hOPC or U87 cells cultured for 3–5 days under various conditions were lysed in RIPA lysis buffer containing protease inhibitors. Following BCA protein analysis, about 200–600 ug of protein per sample was first pre-cleared with1/10 volume of protein G sepharose beads (Millipore) at 4°C for an hour with rotation. After pelleting the beads by centrifugation, the supernatants from these reactions were incubated with 2 ug primary antibody (goat anti-beta-catenin) or control polyclonal goat IgG at 4°C on an end-over-end rotator. After overnight incubation, protein G sepharose beads were added at 1/10 total volume and incubated for an additional 2 hrs at 4°C. Beads were then centrifuged, washed twice with RIPA buffer and resuspended in Laemmli sample buffer (BioRad). After boiling for 5 minutes, samples were resolved on 4–15% precast Tris-HCI polyacrylamide gel (BioRad) and analyzed by Western blotting.
2.9. Retroviral overexpression of Sox17
A full-length mouse Sox17 cDNA sequence used to generate the retroviral expression construct for HOG cells transduction was previously described [8]. In brief, the CMV promoter sequence was added to pMXs-IRES-GFP (Cell Biolabs; San Diego, CA) to generated pMXs-CMV-IRES-GFP. Sox17 was then inserted as a direct fusion with an N-terminal HA epitope tag into the Xhol site between the CMV promoter and IRES of the pMXs-CMV-IRES-GFP. The plasmid lacking the Sox17 insert, but carrying the CMV promoter was used to generate control GFP-expression retrovirus. The viral titers assayed with NIH3T3 fibroblasts cells gave estimates of ~ 1 × 106 colony forming units/ ml. Retroviral stocks were prepared with reagents A and B (ViraDuctin retrovirus transduction kit, Cell Biolabs). Equivalent titers were used for different viral stocks. Following overnight incubation with diluted retroviral stock, cells were rinsed twice with DME/10% FBS medium before and after incubation with diluted Reagent C (Cell Biolabs). Cells were analyzed 3–5 days post transduction after recovering in growth medium.
2.10. Immunofluorescence
Cells (1 × 104 cells per well in 12- well plate) were transduced by retroviral Sox17 or control vector for 24 hours, and then washed and rinsed as described above. 5 days later, transduced cells were fixed with 4% paraformaldehyde, permeabilized with Triton X-100 and stained with primary antibodies against anti-mouse BrdU (1:100; Roche; Indianapolis, IN) overnight at 4 °C followed by fluorescent secondary antibodies (Alexa dyes, Invitrogen). Cell nuclei were counterstained with 4′, 6-diamidino-2-phenylindole (DAPI; Sigma) to measure total cell number. Staining was visualized by epifluorescence (BX61 upright microscope, Olympus; Center Valley, PA) and images captured using Olympus DP70 camera and software.
2.11. Statistical analysis
Statistical analysis was performed using Prism 5.00 for Windows (GraphPad, CA). Student’s T-test was used to compare data between two groups. One-way ANOVA and Dunnett’s test were used to compare data between three or more groups. P< 0.05 was considered statistically significant. All histograms shown in figure panels were means ± SEM of at least three independent experiments. The asterisks denote significant levels and were shown as *P<0.05, **P<0.01, **P<0.001 vs. respective controls.
3. Results
3.1. Hedgehog signaling supports HOG growth and survival, rather than differentiation
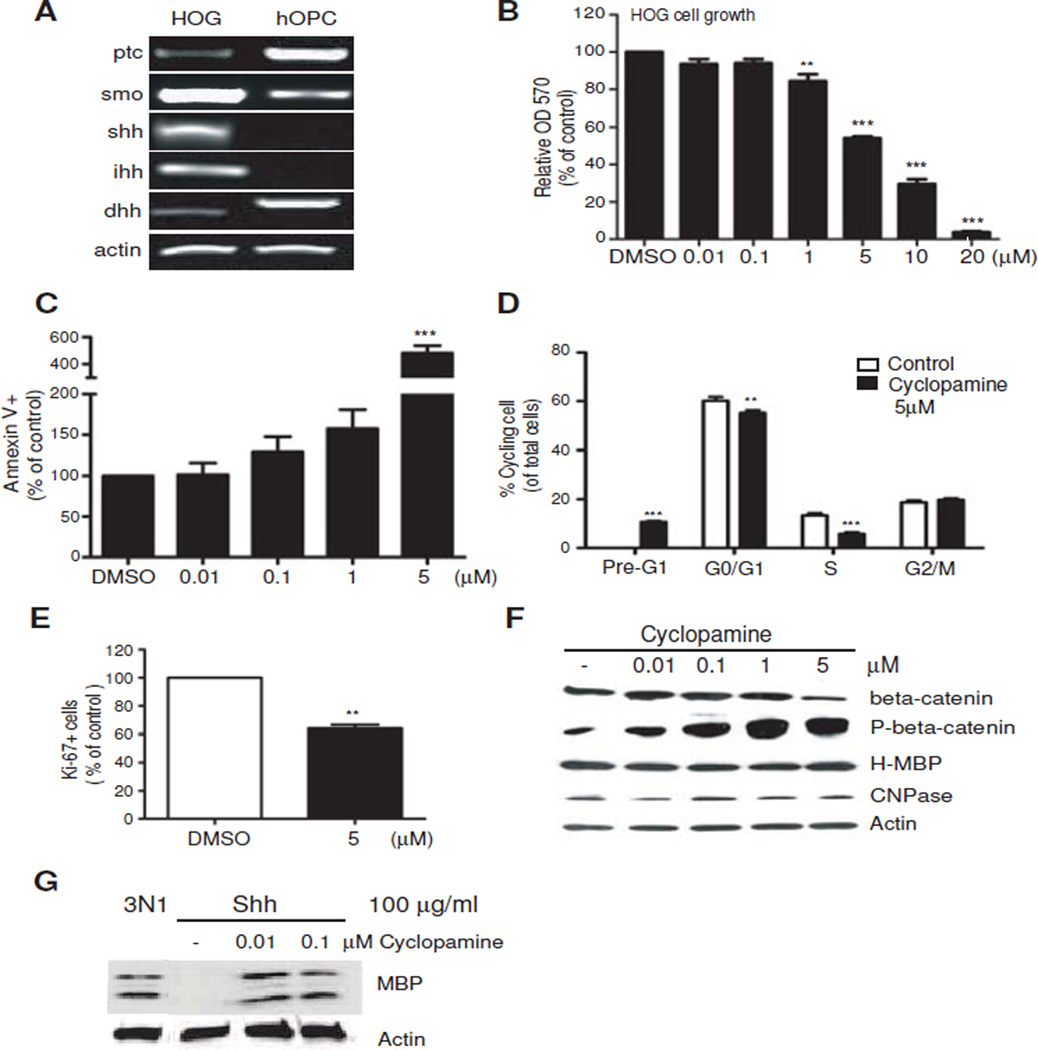
To understand the contribution of the Hedgehog pathway in HOG developmental properties, we wanted to determine whether cyclopamine, a widely used small molecule Smoothened antagonist would modulate HOG cell growth. Figure 1A shows that HOG cells express RNA for the receptor complex and ligands for Hedgehog signaling, suggesting the capacity to respond to cyclopamine and the likelihood that HOG cell growth could be sustained by autocrine signaling. The Smoothened repressor and Hedgehog receptor, Patched (Ptc), is expressed at relatively low levels in HOG cells. By comparison, primary human oligodendrocyte precursor cells (hOPC) cultured in growth medium express higher levels of Ptc and lower levels of Smoothened (Figure 1A). There is also preferential expression of desert hedgehog in hOPCs rather than Sonic and Indian hedgehog which predominate in HOG cells (Figure 1A). Taken together, the enhanced expression of Smoothened and two of its ligands in HOG cells support an autocrine mode of stimulation. Indeed, application of cyclopamine inhibited HOG cell growth in a dose-dependent manner in MTT reduction assays (Figure 1B). To determine whether this was due to an effect on cell survival, an annexin V assay was performed. The results in Figure 1C indicate that cell apoptosis is increased by cyclopamine. Cyclopamine not only induced apoptosis of HOG cells, which is also detected as a Pre-G1 peak in DNA analysis (Figure 1D) but also reduced the percentage of cells in G1 and S phase (Figure 1D). This decrease in cell proliferation is also observed in a Ki-67 assay, where application of cyclopamine significantly reduced the percentage of actively dividing HOG cells (Figure 1E).
Figure 1.
Inhibition of autocrine Hedgehog signaling reduces HOG cell survival and growth without altering myelin protein levels. A. Semiquantitative RT-PCR analysis showing that HOG cells express signaling proteins of the Hedgehog pathway, including the ligands Shh, Indian hedgehog (ihh) and desert hedgehog (dhh). HOG cells were maintained in 10% FBS for 3 days. B. Application of cyclopamine significantly inhibits HOG cell growth. MTT assay OD570 absorbance readings showing the effect of the indicated doses of cyclopamine after 3 days of incubation. Control wells received an equal volume of DMSO, and readings are expressed as a percentage of control wells treated with DMSO. All values shown are mean ± SEM of at least 3 independent experiments. ** P<0.01, ***P<0.001, one way ANOVA, Dunn’s posthoc test. C. Annexin V assay showing cyclopamine causes HOG apoptosis at higher cyclopamine doses (5uM). Cell number values are expressed as percentage of control which received DMSO. ***P<0.001 one way ANOVA, Dunn’s posthoc test. D. Cell cycle profile revealed by propidium iodide staining for HOG cells treated with 5 µM cyclopamine for 3 days show reduction of cells in G0/G1 and S phases with exclusive pre G1 peak representing induction of apoptotic cells. Values are expressed as a percentage of total cells analyzed. ** P<0.01, ***P<0.001 vs control, Student’s T-test E. Significant change in the percentage of Ki-67+ cells is observed after 3 days of treatment with 5 µM cyclopamine. Values are expressed as percent Ki-67 positive cells of a normalized total of sorted events. ** P<0.01 vs DMSO, Student’s T-test F. Western blot analysis showing the same doses of cyclopamine do not alter H-MBP or CNPase levels after 3 days incubation, but eventually reduce total beta-catenin and produce dose-dependent changes in beta-catenin phosphorylated at S33/S37/T41 (P-beta-catenin). G. Western blot of MBP changes in primary rat OPCs cultured for 3 days in basal conditions (N1) showing inhibition of MBP expression by 100 ug/ml Shh that is relieved by the inclusion of cyclopamine.
We then performed Western blotting to determine whether attenuation of cell proliferation with cyclopamine affected the expression of myelin-specific protein levels, such as myelin basic protein (MBP), as an indicator of lineage progression and OPC differentiation. HOG cells under these growth conditions express a single 45 kDa protein band detected by monoclonal MBP antibodies that also react with the classic membrane-associated 18.5, 21.5, 27 kDa isoforms of primary rat OPCs (Figure 1F and G). HOG cells have previously been reported to display MBP immunoreactivity in the cell soma and nucleus [6]. Because golli RNA is expressed in intermediate-stage, immature oligodendrocytes before MBP, and immunocytochemistry has localized golli proteins to the soma and nucleus [20], it was proposed that golli products might be included among the HOG MBP-reactive peptides [6]. The identity of this 45 kDa peptide in HOG remains unknown, and may represent an uncharacterized pre-processed form, therefore we have designated this high molecular weight species H-MBP. After treatment of HOG cells with cyclopamine, surprisingly little effect on CNPase or H-MBP levels is observed (Figure 1F), indicating lack of an effect on cell differentiation. The phosphorylation levels of S33/37/T41-beta-catenin were noticeably increased and total beta-catenin levels were found to be decreased by 5 uM cyclopamine (Figure 1F), indicating cross-talk between Hedgehog and Wnt pathways.
In contrast to HOG cells, normally differentiating rat oligodendrocyte progenitor cells (OPC) in culture are prevented from expressing MBP by high exogenous levels of Sonic hedgehog (Figure 1G); this was reversed by the inclusion of low doses of cyclopamine (Figure 1G). This indicates that, in normal progenitor cells, high levels of Sonic hedgehog repress myelin gene expression via Smoothened (Smo) activity. These experiments thus indicate that HOG cells rely on autocrine activation of the Hedgehog pathway primarily for survival and self-renewal, and that this is associated with the maintenance of beta-catenin stability through Smo activity in HOG cells. However, unlike primary OPCs, Smo activity in HOG cells could not be modulated to effectively alter differentiation and myelin gene expression.
3.2. Wnt signaling modulates HOG cell proliferation and differentiation
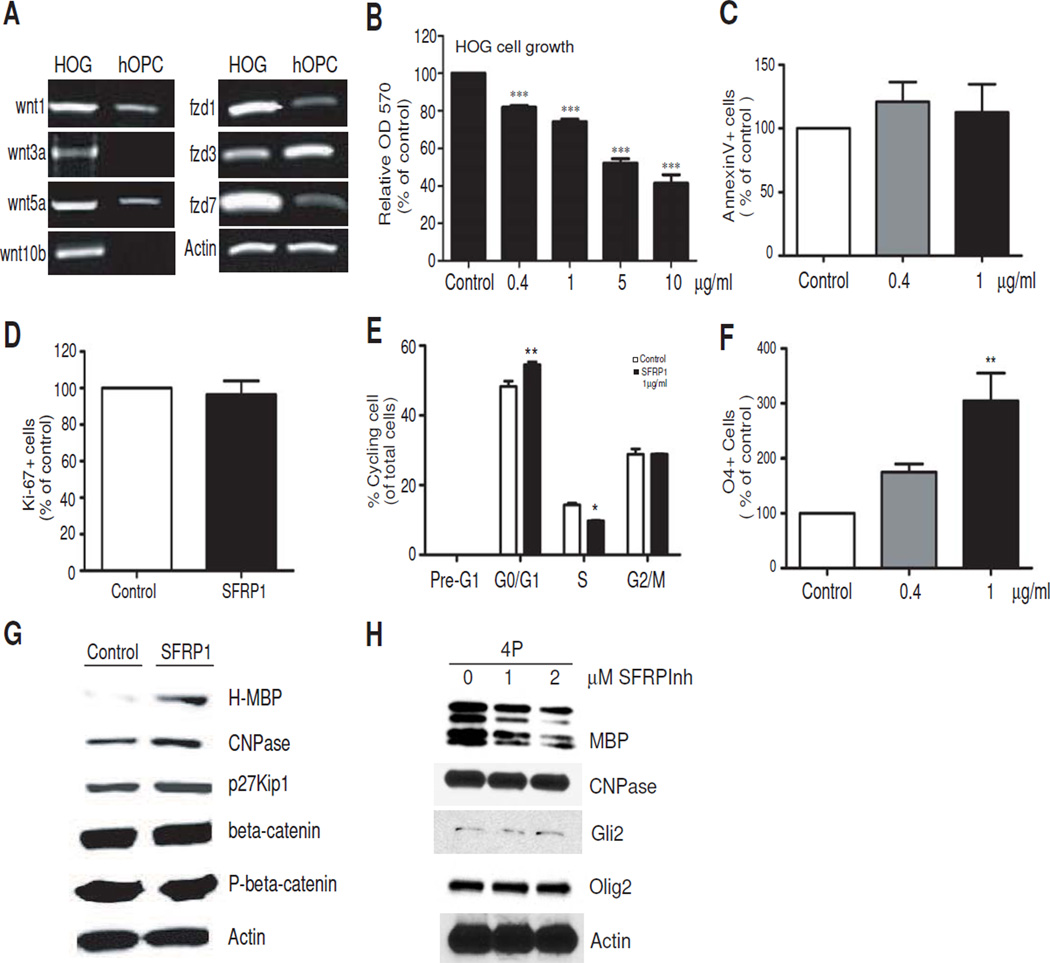
Our lab has previously shown that the Wnt antagonist, secreted Frizzled-related protein-1 (SFRP1) is upregulated in cultured OPCs under differentiating conditions [8], suggesting an autonomous suppression of Wnt signaling during cell maturation. Figure 2A shows that the RNA for Wnt ligands and frizzled receptors are expressed in HOG, suggesting the capacity for modulation by exogenous Wnt antagonists. A comparison with hOPC however shows that HOG cells clearly express higher levels of Wnt1, Wnt3a, Wnt5a and Wnt 10b, as well as frizzled receptors-1 (fzd1) and −7 (fzd7) (Figure 2A). hOPC express these ligands and receptors weakly, if at all, and marginally higher levels of frizzled 3 receptor. This suggests that both canonical and non-canonical Wnt signaling may be abnormally activated in HOG cells, and a Wnt antagonist like SFRP1 would be a more effective inhibitor of Wnt activity than Dickkopf (DKK), which selectively targets LRP5/6-dependent canonical signaling.
Figure 2.
Inhibition of Wnt signaling with recombinant SFRP1 causes HOG cell growth arrest and differentiation. A. Semi-quantitative PCR analysis showing HOG cells after 3 days in culture express transcripts for Wnt ligands and Frizzled receptor forms. B. SFRP1 retards HOG cell growth. HOG cells were incubated with the indicated concentrations of recombinant SFRP1 for 3 days and MTT assays were performed. All values shown are mean ± SEM of at least 3 independent experiments. ***P<0.001, one way ANOVA, Dunn’s posthoc test. C. Annexin V assay reveals no significant difference in cell survival after treatment with SFRP1 for 3 days. Values are expressed as percentage over control samples. D. No significant change in the percentage of Ki-67+ cells is observed after 3 days of treatment with 1ug/ml SFRP1. Values are expressed as percent Ki-67 positive cells of a normalized total of sorted events. E. Cell cycle analysis of HOG cells treated with 1ug/ml SFRP1 for 3 days. Propidium iodide staining reveals reduction of cells in S phase accompanied by accumulation in G0/G1. Histograms from a representative experiment is shown. Values are expressed as a percentage of total cells analyzed. * P<0.05, ** P<0.01, vs control, Student’s T-test. F. SFRP1 treatment increases HOG differentiation to the O4 stage. FACS-assisted quantitative analysis of O4+ cells following treatment with increasing doses of SFRP1 for 3 days. Values are percentage O4+ cells of total sorted events further expressed as percentage of control. ** P<0.01, one way ANOVA, Dunn’s posthoc test. Values shown in histogram panels are mean ± SEM of at least 3 independent experiments. G. Western blot analysis of proteins in HOG cells shows increased expression of H-MBP, CNPase and p27 after treatment with 1 ug/ml SFRP1 for 3 days. H. Western blot showing myelin protein response of primary rat OPs to SFRP1 inhibitor (SFRPInh). Rat OPs were cultured in the presence of 10 ng/ml human recombinant PDGF-AB for 4 days (4P) in the absence or presence of SFRP1 inhibitor at the indicated concentrations. Control samples received an equal volume of DMSO.
We wanted to determine whether Wnt modulation by SFRP1 application was sufficient to regulate cell proliferation and/or myelin gene expression and cell differentiation. Recombinant SFRP1 decreased HOG cell growth in a dose-dependent manner (Figure 2B), while not significantly affecting cell survival based on annexin V apoptosis assay (Figure 2C). Further analysis of cell proliferation revealed no significant change in the population of Ki-67+ cells, indicating no effect on cell cycle exit or cells in Go (Figure 2D), however SFRP1 led to cell cycle arrest in G1, accompanied by corresponding decreases in S phase (Figure 2E).
The lack of apoptosis was also confirmed through the absence of a pre-G1 peak in DNA content analysis (Figure 2E). SFRP1 also induced a dose-dependent increase in the percentage of cells expressing the O4 antigen, a marker of premyelinating oligodendrocytes (Figure 2F), indicating increased differentiation. SFRP1 at 1ug/ml was found to significantly increase the levels of H-MBP, CNPase and p27Kip1 (Figure 2G), indicating proliferative arrest and differentiation, despite the absence of morphological maturation that is characteristic of developing OPCs (not shown). Interestingly, no change in beta-catenin levels or phosphorylation was found (Figure 2G), suggesting that these effects of SFRP1 on HOG differentiation may be mediated primarily through non-canonical Wnt signaling.
Figure 2H shows that, in primary rat OPCs, application of the small molecule SFRP1 inhibitor attenuates MBP and CNP expression by 67% and 10% respectively at 2uM. The relative changes in MBP and CNP are in agreement with the changes following SFRP1 application in HOG cells (Figure 2G), supporting our observation that SFRP1 activity is normally required in OPC development. These results suggest that SFRP may be important in the transition between cell cycle arrest and differentiation, and that increasing the levels and activity of SFRP1 may help promote differentiation of HOG cells.
3.3. Molecular interaction between endogenous Sox17 and beta-catenin is not observed in human glioma
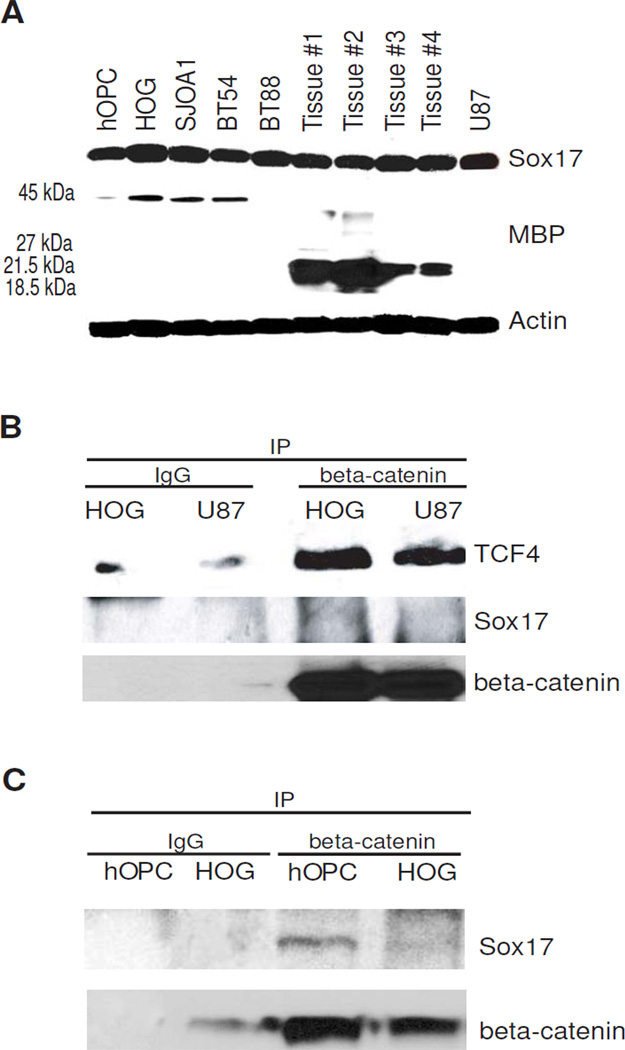
Our lab has previously demonstrated that modulation of canonical Wnt signaling by altering Sox17 levels in primary rodent OPCs regulates progenitor cell proliferation and differentiation [8]. To determine whether glioma cells expressed Sox17 protein, we performed Western blot assay using glioma cell lines and oligodendroglioma tumor biopsy samples. Similar to proliferating primary rat OPCs [8], Sox17 protein was found to be expressed in these glioma lines and tissue. The levels of Sox17 protein in normal human OPCs (hOPC) and in glioma samples were found to be comparable (Figure 3A). This is in contrast to reports of malignant breast and gastric tumors where Sox17 expression is epigenetically silenced [19, 53]. Interestingly, Western analysis also indicates that the expression of MBP in our samples reflects distinct processing of the MBP protein in human oligodendroglial lineage cells and tissue. Figure 3A shows that hOPCs maintained in growth medium also express low levels of H-MBP, whereas oligodendroglioma tissue show the smaller MBP forms that are believed to be associated with myelin membranes.
Figure 3.
Sox17 protein is expressed in human oligodendrocyte progenitor cells, glioma cells and oligodendroglioma tumor tissue. A. Western blot showing robust Sox17 and MBP expression in human glioma cells and tissue. The 45 kDa band (or H-MBP) is detected in cultured hOPC and cell lines, while the 27, 21.5, 18.5 kDa forms are found in tumor tissue. Human oligodendrocyte precursor cells (hOPC) were maintained in growth medium. HOG cells were maintained in growth medium for 3 days before analysis. SJ0A1, BT54 and BT88 are cell lines that were derived from human oligodendroglioma tissue (see Materials and Methods). Cell cultures were harvested for Western analysis when cells reached 80% confluence.Tissue #1–4 are individual biopsy or resected tissue samples from adult patients diagnosed with oligodendroglioma, WHO Grade II. U87 is a human astrocytoma cell line derived from malignant glioblastoma. B. Beta-catenin/TCF complexes do not contain Sox17 in HOG and U87. The HOG and U87 cell lysates were precleared with protein G-beads, and incubated with mouse IgG (IgG) as negative control or mouse anti-beta-catenin. The resulting immunoprecipitates (IP) were analyzed by Western blotting for TCF4, Sox17 and beta-catenin. C. Sox17 binds beta-catenin in cultured hOPC and not HOGs. The precipitated beta-catenin in both samples is also shown. Immunoprecipitation was performed as in B.
We had previously described a correlation between MBP expression, cell cycle activity and beta-catenin/Sox17 complex formation in primary rat OPCs [8]. However, unlike in developing white matter and differentiating oligodendroglial cells [8], Sox17 was not found in beta-catenin complexes that also contained the transcription factor TCF4 in HOG cell or U87 astrocytoma lysates (Figure 3B). In a separate experiment, however, we immunoprecipitated beta-catenin from normal hOPCs and clearly detected Sox17 in the complex (Figure 3C). These results indicate that endogenous Sox17 in HOG cells are incapable of, or inhibited from, binding beta-catenin despite strong expression levels, and suggest that the absence of Sox17 from these beta-catenin complexes may contribute to aberrant cell cycle exit and differentiation in HOG cells.
3.4. Overexpression of recombinant Sox17 promotes cell cycle exit and differentiation in HOG cells
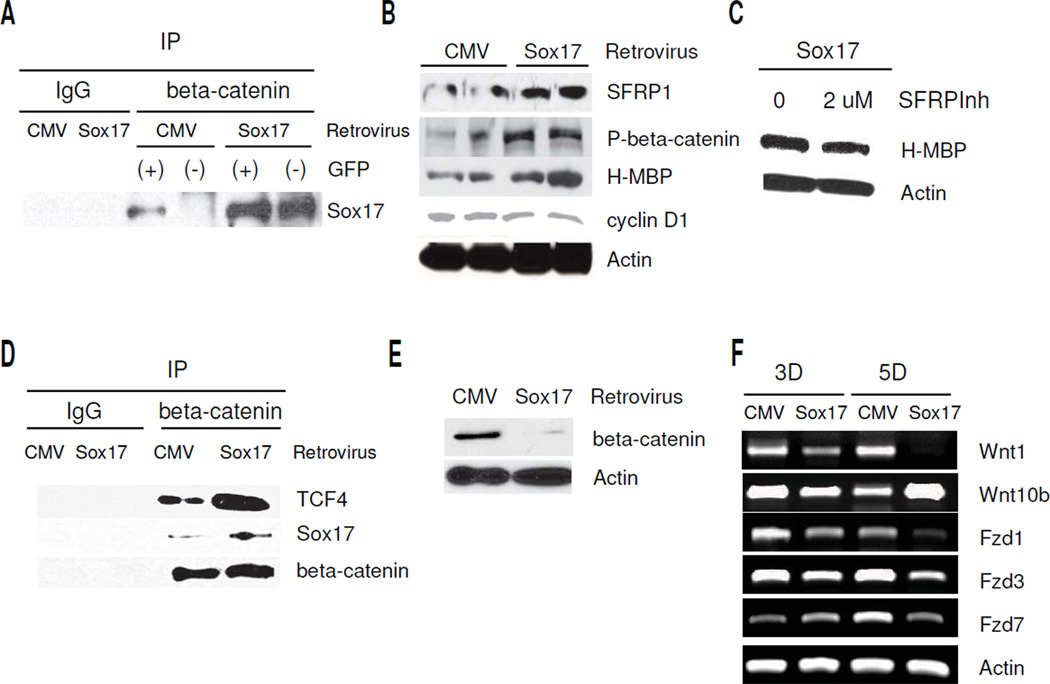
Since HOG cells are capable of further differentiation, we wanted to determine whether restoring Sox17 to the HOG beta-catenin complex could modify the growth characteristics of HOG cells. To study the effects Sox17 overexpression in HOG cells, we chose a previously generated retroviral expression vector that was used on rodent OPCs [8]. Recombinant mouse Sox17 from this construct has been shown to be localized in the nucleus and is expressed with the GFP reporter through an IRES element [8]. After incubating HOG cells with Sox17 retrovirus expression vector, we analyzed changes in cell proliferation, myelin protein levels and differentiation to the O4 stage. In agreement with our previous findings in proliferating rat OPCs in culture [8], retroviral overexpression of Sox17 results in growth inhibition of HOG cells (Figure 4A). This is not only due to decreased S-phase DNA synthesis in GFP+ cells, as detected by BrdU assay (Figure 4B–C), but also due to increased cell cycle exit, as indicated by reduced Ki-67 immunostaining (Figure 4D). DNA content analysis confirms changes in both S-and G0/G1 -phase cells induced by Sox17 overexpression (Figure 4E). However, unlike SFRP1 application, the decrease in cell proliferation by Sox17 was also accompanied by apoptosis and possibly necrosis, as measured by Annexin V/7AAD staining (Figure 4F). Despite the promotion of cell death, we also observed increased O4+ cells (Figure 4G), indicating enhanced HOG cell differentiation promoted by recombinant Sox17.
Figure 4.
Recombinant Sox17 promotes growth arrest and differentiation in HOG cells. A. Relative absorbance readings of MTT assays performed 5 days post-transduction with CMV control vector or Sox17 retroviruses and recovery in DMEM/10%FBS. Values are expressed as percentage absorbance relative to CMV vector control for each experiment. ***P<0.001 vs CMV, Student’s T-test. B. Immunocytochemical detection of BrdU+ cells following HOG cell transduction with retroviral vectors. HOG cells were allowed to recover for 3 days in DME/10% FBS following transduction. The top row of images shows cells with control CMV vector virus while bottom row shows Sox17 retrovirus. Arrowheads indicate GFP+ transduced cells and the corresponding levels of BrdU in those cells. With Sox17 retrovirus, fewer GFP+ cells appear to be BrdU+. Merged panels show BrdU cells and Dapi nuclear counterstain. C. FACS-assisted quantitative analysis of BrdU proliferation assay performed at 5 days post-transduction. Values are percent BrdU+ cells of total GFP+ population in each group. **P<0.01 vs CMV, Student’s T-test. D. FACS-assisted quantitative analysis of Ki-67+ cells 5 days after retrovirus transduction. Values are percent Ki-67+ cells of the analyzed GFP+ in each group. **P<0.01 vs CMV, Student’s T-test. In C. and D, values are mean ± SEM of 3 experiments performed in triplicate. E. Cell cycle analysis of HOG cells 3days after transduction with retrovirus. Propidium iodide staining reveals reduction of cells in S phase accompanied by accumulation in G0/G1. Histograms from a representative experiment are shown. Values are expressed as a percentage of total cells analyzed. ***P<0.001, **P< 0.01 vs CMV, Student’s T-test. F. Annexin V apoptosis assay showing early as well as late- stage apoptosis based on 7AAD staining. ***P<0.001 vs CMV, Student’s T-test. G. FACS-assisted quantitative analysis of O4+ cells showing Sox17-mediated promotion of differentiation. Values are calculated as a percentage of O4+ GFP+ double positive cells of total GFP+ cells, and finally expressed as a percentage over CMV controls. Data shown are mean ± SEM of 3 separate experiments. *** P < 0.001 vs CMV, Student’s T-test.
3.5 Sox17 overexpression decreases beta-catenin levels and promotes myelin gene expression through SFRP1
To determine the molecular changes underlying Sox17-induced cell differentiation, we performed a series of Western blotting experiments following retroviral transduction in HOG cells. In order to demonstrate increased Sox17 binding activity in the transduced cells, we isolated GFP+ and GFP− HOG cells by FACS in a first set of experiments, and analyzed immunoprecipitated beta-catenin-Sox17 protein complexes in these lysates. The immunoprecipitation experiment shown in Figure 5A indicates that beta-catenin in the Sox17 retrovirus -transduced HOG cells clearly binds a greater amount of Sox17 than the CMV vector-transduced cells (comparing GFP+ lanes). Furthermore, analysis of the GFP− negative populations also revealed an increase in Sox17-beta-catenin binding that was specific to the Sox17 sample (Figure 5A). This result indicates an additional non cell-autonomous effect of recombinant Sox17, suggesting a secreted mediator(s) acting via a paracrine mechanism with an effect on Sox17-beta-catenin interaction. To determine the collective effect of Sox17 overexpression in HOGs, all of our subsequent experiments therefore utilized lysates from the Sox17-retrovirus-transduced cells without prior FACS sorting. At 3 days post-transduction, the protein levels of SFRP1, P-S33/37/T41 - beta-catenin and H-MBP were increased by recombinant Sox17 (Figure 5B). In the Sox17-transduced cells, cyclin D1 protein levels fell 15% after 3 days. Since exogenous application of SFRP1 led to increased myelin protein levels (Figure 2G), we wanted to determine whether the Sox17-induced increase in H-MBP was mediated by Sox17-induced fsFRPL To block the effect of SFRP1, 2uM SFRP inhibitor (SFRPInh) was added during recovery after Sox17 retrovirus transduction. Figure 5C shows that SFRPInh reduced the levels of H-MBP by approximately 25% in the presence of Sox17 retrovirus, indicating that at least part of the effect of Sox17 on H-MBP levels could be attributed to Sox17-induced SFRP1.
Figure 5.
Recombinant Sox17 induces beta-catenin-TCF complex formation in HOG cells, and decreases Wnt/beta-catenin signaling by cell autonomous and non cell-autonomous mechanisms. A. Sox17 retrovirus transduction increases Sox17 levels in beta-catenin immunoprecipitates of both GFP+ and GFP− cell populations. GFP+ and GFP− cells were isolated from CMV vector or Sox17-transduced HOG cells by FACS sorting 5 days after transduction. Lysates were prepared from the purified cells and immunoprecipitated with mouse IgG (CMV lysate only) or beta-catenin antibody. Western blotting was performed for Sox17. Sox17 levels are approximately 2.5 fold higher in Sox17 GFP(+) compared with CMV GFP(+). Note increased Sox17-beta-catenin complex formation in GFP- cells after transduction with Sox17 retrovirus, indicating non-cell autonomous activity. B. Western blot showing changes in SFRP1, H-MBP and phospho-S33/37/T41-beta-catenin (P-beta-catenin) at 3 days post-transduction with control (CMV) or Sox17 retrovirus. Two samples for each group are shown. C. Western blot showing inclusion of 2 uM SFRP1 inhibitor (SFRPInh) decreases the Sox17-induced H-MBP 5 days following retroviral transduction. D. Western blot showing increased Sox17 and TCF4 are detected in beta-catenin immunoprecipitates (IP) 5 days after HOG cell transduction with Sox17 retrovirus. Negative controls consisted of CMV-transduced cell lysate precipitated with IgG. E. Western blot showing the levels of total beta-catenin is clearly reduced at 5 days after retroviral transduction. F. Semi-quantitative RT- PCR results showing decreased Wnt1, Fzd1, −3, and −7 expression in HOG cells after transduction with the Sox17 retrovirus. Samples were analyzed after 3 days (3D) and 5 days (5D) recovery from virus addition.
Based on our previous observation of beta-catenin antagonism by Sox17 in primary rodent OPCs, we wanted to determine how recombinant Sox17 affected beta-catenin in HOG cells. We analyzed beta-catenin protein complexes and found increased Sox17 protein in immunoprecipitates after retrovirus transduction (Figure 5D). These beta-catenin-containing complexes also contained a higher level of TCF4 than in the vector-transduced controls. After quantitation and normalization against beta-catenin, the increase in TCF4 was found to be in close agreement with the increase in Sox17 after retroviral transduction (Figure 5D) i.e. 2.3 to 2.4 -fold, which suggests a stoichiometric effect of recombinant Sox17 in the recruitment of TCF4 into the beta-catenin complexes. As predicted from the elevated P-S33/37/T41-beta-catenin levels in Figure 5B, total cellular beta-catenin levels are found to be significantly attenuated following Sox17 overexpression (Figure 5E), indicating increased beta-catenin destabilization [8], and hence reduced signaling potential. This destabilization and reduction in TCF4-mediated beta-catenin function would be consistent with our previous result of reduced TOPFLASH/FOPFLASH and cyclinD1 promoter activity in HOG cells co-transfected with a Sox17 expression plasmid [8].
Since retroviral transduction revealed non cell-autonomous mechanisms, we further explored the possibility that recombinant Sox17 might show other effects on the Wnt signaling pathway in HOG cells. Interestingly, we found that the RNA expression of endogenous Wnt ligands and Frizzled receptors was also regulated by Sox17 overexpression (Figure 5F). The canonical ligand Wnt1 was consistently downregulated over a 5 day time course, while Wnt 10b was only transiently reduced after 3 days. In contrast, the levels of Frizzled receptors-1,−3, and −7 were clearly reduced by Sox17 5 days after transduction (Figure 5F). This result suggests a role for Sox17 in regulating the sensitivity and response of HOG cells | to autocrine/paracrine Wnt signals.
Taken together, these observations indicate that recombinant Sox17 abolishes canonical Wnt signaling by interacting with beta-catenin, forming Sox17-TCF4-beta-catenin complexes and eventually reducing the cellular levels of beta-catenin. In addition, Sox17 also increases the levels of endogenous SFRP1 protein, which is a bona fide Hedgehog target gene [26]. These multiple effects of Sox17 serve to promote proliferative arrest and differentiation of HOG cells.
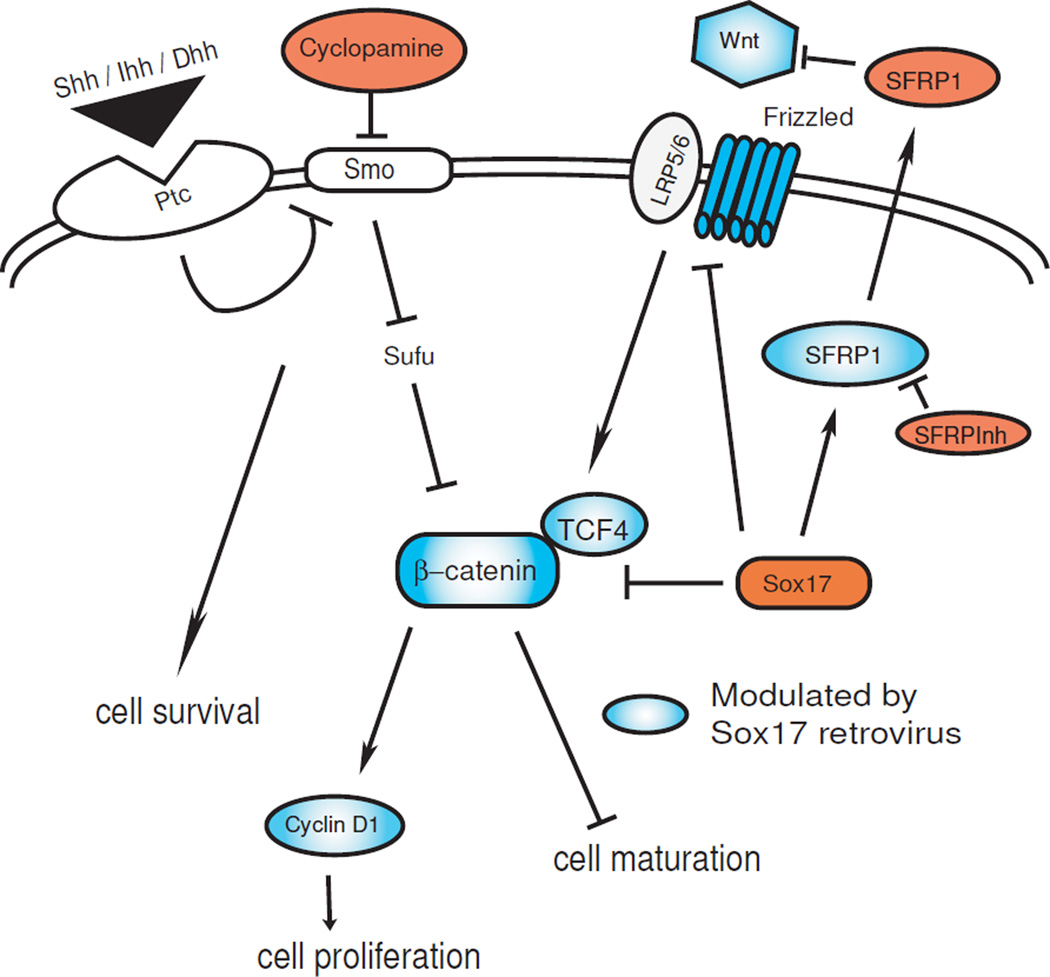
The effects of cyclopamine, SFRP1 and Sox17 retrovirus in HOG cells as found in this study are summarized as a hypothetical model in Figure 6.
Figure 6.
A model for the observed effects of Hedgehog and Wnt inhibition, and Sox17 overexpression in HOG cells. Hedgehog signaling mediates cell survival, while maturation is associated with regulation of Wnt/beta-catenin. Red ovals denote cell treatments used, blue ovals denote proteins regulated by Sox17 retrovirus in this study.
4. Discussion
Recent studies of the origin and molecular tumorigenesis of oligodendrogliomas have revealed that, rather than stem cells, the NG2+ oligodendrocyte progenitor cell population serves as tumor- initiating cells [33] in which asymmetric cell division has gone awry [42]. Since oligodendroglioma cells have been found to resemble immature oligodendrocyte cells [6] - and as oligodendrocyte progenitor development involves the cessation of proliferation at the onset of differentiation, which is at least in part regulated by the Wnt pathway [8, 16, 17] - we have thus investigated the contribution of Hedgehog and Wnt signaling in the growth and development of the oligodendroglioma cell line, HOG. This cell line was previously developed and used for studies of neurotransmitter signaling mechanisms [34, 35]. To our knowledge, our experiments constitute the first studies to have compared the cell differentiation effects of Smoothened inhibition with that of Frizzled inhibition, and to have identified the Wnt pathway as a regulator of differentiation in oligodendroglioma cells.
An understanding of molecular targets has provided advantages to the management of medulloblastomas, which are relatively better characterized with regard to molecular pathogenesis and prognosis. Hedgehog and Wnt-subgroup categorization of medulloblastomas [44] provides prognostic value [15, 38]. While glial tumors are far less understood, there is evidence that Hedgehog signaling is an important feature of malignant gliomas [3, 10, 14, 50]. In agreement with recent studies showing that malignancy of a cell line derived from anaplastic oligodendroglioma is dependent upon Hedgehog pathway signaling, and neither Wnt nor Notch [43], our results show that the growth and survival of HOG cells are modulated via Smoothened activity. Autocrine Smo activation thus prevents the apoptotic death of HOG cells grown under proliferative conditions, involving integration of beta-catenin regulation (Figure 1). While some overlap in function, i.e. HOG cell proliferation, appears to be shared between Hedgehog and Wnt pathways, the changes in H-MBP and CNPase protein levels, and O4 expression distinguish the Wnt pathway as an important signal in the differentiation of HOG cells. Indeed, constitutive Wnt signaling arising from epigenetic inactivation of Wnt antagonists such as SFRP, DKK and Wnt inhibitory factor has been implicated in oral carcinogenesis [32], so that the restoration of such inhibitors should be expected to attenuate tumor growth.
Our analysis shows that HOGs, in contrast to human OPCs in culture, express higher levels of Wnt ligands and receptors, indicating an autocrine mechanism that enhances signaling activity, which is known to oppose cell cycle arrest and exit. Oligodendrogliomas with 1p/19q co-deletions [1, 4] frequently show mutations in CIC, the mammalian homolog of the Drosophila gene Capicua, an HMG box-containing transcription factor downstream of receptor tyrosine kinase-RAS-MAP signaling pathway [12], as well as the Far Upstream element Binding Protein-1 (FUBP1), a transcriptional modulator of c-myc oncogene and p27Kip1 cyclin dependent kinase inhibitor [54]. Our previous studies of rodent OPCs showed that Sox17 loss modulated phosphorylated ERK levels as well as p27Kip1, as did Wnt3a application [8], suggesting that these mutations in 1p/19q oligodendrogliomas altered downstream effectors of the Wnt signaling pathway that could potentially be restored by Sox17.
High mobility group (HMG) proteins, such as the Sox transcription factors, regulate stem cell function in multiple tissues [21, 37] and have important roles in oligodendrocyte lineage specification and maturation [48, 49]. Sox17 is expressed in multiple tissue types, and is required for the formation of endoderm [23, 25, 40, 51], vascular endothelium [29], and maintenance of hematopoietic stem cells [28]. Our laboratory has previously identified roles for Sox17 in oligodendrocyte progenitor proliferation and lineage progression [41], in which the peak of Sox17 expression coincides with the developmental transition between proliferative arrest and differentiation onset. In the oligodendrocyte lineage, the expression of Sox17 protein in OPCs is predominantly associated with proliferating progenitors, but its molecular association with the canonical Wnt mediator, beta-catenin, was found to distinguish between conditions of proliferation which led to eventual arrest and differentiation, and those which inhibited cell cycle exit [8]. Based on these findings, and on reports of cell cycle control by Sox17 in other cell types [39], it would not be unreasonable to predict that Sox17 might function as a tumor suppressor of the oligodendrocyte lineage.
In our present studies, we found beta-catenin in HOG cells not to be associated with endogenous Sox17, despite apparently high levels of expression. It remains unclear why endogenous Sox17 is prevented from binding beta-catenin in HOG cells despite H-MBP expression, and how recombinant mouse Sox17 overcomes the inhibition. Possible explanations include: i) recombinant mouse Sox17 possesses an inherently higher affinity for beta-catenin than HOG Sox17, ii) recombinant Sox17 is exclusively nuclear [8], and may be localized more appropriately than HOG Sox17 to bind nuclear beta-catenin, iii) recombinant Sox17 is unresponsive to inhibitory signals which prevent endogenous Sox17 from binding in HOG cells. Identifying the signals regulating Sox17 binding activity could potentially provide an additional level of control in cellular development and aid in a better understanding of the relationship between cell cycle arrest and the onset of differentiation.
Along with studies of epigenetic silencing of WNT antagonists [9], promoter analysis of Sox17 has revealed its methylation and repression in numerous non-CNS cancers [13, 19, 24, 47]. In hepatocellular carcinoma, Wnt pathway activation and nuclear accumulation of beta-catenin has been described, and restoring Sox17 expression effectively inhibited colony formation [24]. Wnt1, Fzd1 and Fzd7 have been implicated in breast and colon cancer [2, 30, 45, 46]; our finding of Wnt1 and Fzd downregulation in HOG by Sox17 suggests that targeting beta-catenin/TCF4 not only disrupts downstream signaling but also perturbs the reinforcement of positive feedback Wnt signaling at the cell surface. Furthermore, such an effect of Sox17 on Wnt ligands and receptors likely constitutes another part of its non cell-autonomous activity in HOG cells in addition to its induction of SFRP1. This aspect of Sox17 action could potentially prove beneficial in therapy since Fzd1 overexpression has been shown to confer clinical chemoresistance in aggressive neuroblastoma [18].
In agreement with our findings in primary OPCs [8], recombinant Sox17 showed multiple effects which promoted HOG cell differentiation i.e. cell cycle exit, O4, CNP and H-MBP expression. It was recently found in embryonic stem cells that Sox17 promoted differentiation through modulation of genes responsible for self-renewal [31]. In radial glial progenitor cells, asymmetric cell division generates neurons while allowing self-renewal, and proteins of the Notch pathway were found to regulate both cell division and daughter cell fate specification [5]. Mechanisms for gliogenesis are still poorly understood, but it is possible that Sox17 is involved in similar developmental fate decisions in proliferating oligodendrocyte progenitor cells. Through its interaction with beta-catenin, stimulation of SFRP1 expression and downregulation of Wnt ligands and receptors in HOG cells, Sox17 performs dual functions of growth control and differentiation. Given that the effects of Sox17 are highly cell-type-, stage- and context-dependent [22], further studies will reveal the specific functions of Sox17 in Hedgehog-Wnt crosstalk in the pathogenesis of oligodendroglioma formation and progression. Given the infiltrating nature of gliomas, targeted chemotherapeutic agents with improved delivery devices for the CNS [7] offer strong options in the management of surgically intractable malignancies.
Acknowledgements
This work was supported by R01 NS056427 (VG), P30 HD040677 (VG), National Multiple Sclerosis Society RG 3954A1/2 (L-J C), Children’s Board of Visitors Grant (L-J C, H-L C), the Daniel and Jennifer Gilbert Neurofibromatosis Institute and the Zickler Family Fund (RJP, H-L C). We thank Dr. Lina Chakrabarti, Children’s Research Institute (CRI) Flow Cytometry Core Facility, for excellent service and technical advice. We thank Dr. Glyn Dawson, University of Chicago, for the gift of human oligodendroglioma (HOG) cells; Dr. Samuel Weiss, University of Calgary, Canada for BT54 and BT88 cells; Dr. Suzanne Baker, St Jude Children’s Research Hospital, for SJ0A1 cells, and Dr. Harald Sontheimer, University of Alabama, Birmingham, for human oligodendroglioma tumor specimens. Epifluorescent microscopy was performed at the CRI Light Microscopy and Image Analysis Core supported by Children’s Research Institute and NIH grant 5P30HD040677-11.
Abbreviations
- HOG
Human oligodendroglioma
- OP
oligodendrocyte progenitor
- Shh
sonic hedgehog
- SFRP1
secreted Frizzled Related protein
- Sox17
SRY-Box containing gene 17
- CNPase
2’,3’-cyclic-nucleotide 3’-phosphodiesterase
- MBP
myelin basic protein
- CMV
cytomegalovirus
- BrdU
5-Bromo-2’-deoxyuridine 5’-triphosphate
- RT-PCR
reverse transcription-polymerase chain reaction
- TCF4 orTCF7L2
T-cell specific transcription Factor
- ihh
Indian hedgehog
- dhh
Desert hedgehog
- fzd
Frizzled
- Smo
Smoothened
- Ptc
Patched
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement
None
REFERENCES
- 1.Alentorn A, Sanson M, Adbaih A. Oligodendrogliomas: new insights from the genetics and perspectives. Curr Opin Oncol. 2012 doi: 10.1097/CCO.0b013e328357f4ea. [DOI] [PubMed] [Google Scholar]
- 2.Ayyanan A, Civenni G, Ciarloni L, Morel C, Mueller N, Lefort K, Mandinova A, Raffoul W, Fiche M, Dotto GP, Brisken C. Increased Wnt signaling triggers oncogenic conversion of human breast epithelial cells by a Notch-dependent mechanism. Proc Natl Acad Sci (USA) 2006;103:3799–3804. doi: 10.1073/pnas.0600065103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar EE, Chaudhry A, Lin A, Fan X, Schreck K, Matsui W, Piccirillo S, Vescovi AL, DiMeco F, Olivi A, Eberhart CG. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25:2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bettegowda C, Agrawal N, Jiao Y, Sausen M, Wood LD, Hruban RH, Rodriguez FJ, Cahill DP, McLendon R, Riggins G, Velculescu VE, Oba-Shinjo SM, Marie SKM, Vogelstei B, Bigner D, Yan H, Papadopoulos N, Kinzler KW. Mutations in CIC and FUBP1 contribute to human oligodendroglioma. Science. 2011;333:1453–1455. doi: 10.1126/science.1210557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bultje RS, Castaneda-Castellanos DR, Jan LY, Jan YN, Kriegstein AR, Shi SH. Mammalian Par3 regulates progenitor cell asymmetric division via notch signaling in the developing cortex. Neuron. 2009;63:189–202. doi: 10.1016/j.neuron.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buntinx M, Vanderlocht J, Hellings N, Vandenabeele F, Lambrichts I, Raus J, Ameloot M, Stinissen P, Steels P. Characterization of three human oligodendroglial cell lines as a model to study oligodendrocyte injury: morphology and oligodendrocyte-specific gene expression. Journal of Neurocytology. 2003;32:25–38. doi: 10.1023/a:1027324230923. [DOI] [PubMed] [Google Scholar]
- 7.Caraglia M, De Rosa G, Salzano G, Santini D, Lamberti M, Sperlongano P, Lombardi A, Abbruzzese A, Addeo R. Nanotech revolution for the anti-cancer drug delivery through blood-brain barrier. Curr Cancer Drug Targets. 2012;12:186–196. doi: 10.2174/156800912799277421. [DOI] [PubMed] [Google Scholar]
- 8.Chew LJ, Shen W, Ming X, Senatorov VVJ, Chen H-L, Cheng Y, Hong E, Knoblach S, Gallo V. SRY-box containing gene 17 regulates the Wnt/beta-catenin signaling pathway in oligodendrocyte progenitor cells. J Neurosci. 2011;31:13921–13935. doi: 10.1523/JNEUROSCI.3343-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chimonidou M, Strati A, Tzitzira A, Sotiropoulou G, Malamos N, Georgoulias V, Lianidou ES. DNA methylation of tumor suppressor and metastasis suppressor genes in circulating tumor cells. Clin Chem. 2011;57:1169–1177. doi: 10.1373/clinchem.2011.165902. [DOI] [PubMed] [Google Scholar]
- 10.Clement V, Sanchez P, de Tribolet N, Radovanovic I, Ruiz i Altaba A. Hedgehog-GM1 signaling regulates human glioma growth cancer stem cell self-renewal and tumorigenicity. Curr Biol. 2007;17:165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Danesin C, Agius E, Escalas N, Ai X, Emerson C, Cochard P, Soula C. Ventral neural progenitors switch toward an oligodendroglial fate in response to increased sonic hedgehog (Shh) activity: involvement of sulfatase 1 in modulating Shh signaling in the ventral spinal cord. J Neurosci. 2006;26:5037–5048. doi: 10.1523/JNEUROSCI.0715-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dissanayake K, Toth R, Blakey J, Olsson O, Campbell DG, Prescott AR, MacKintosh C. ERK/p90(RSK)/14-3-3 signalling has an impact on expressio of PEA3 Ets transcription factors via the transcirptional repressor capicua. Biochem J. 2011;433:515–525. doi: 10.1042/BJ20101562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du YC, Oshima H, Oguma K, Kitamura T, Itadani H, Fujimura T, Piao YS, Yoshimoto T, Minamoto T, Kotani H, Taketo MM, Oshima M. Induction and down-regulation of Sox17 and its possible roles during the course of gastrointestinal tumorigenesis. Gastroenterology. 2009;137:1346–1357. doi: 10.1053/j.gastro.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 14.Ehteshm M, Sarangi A, Valadez JG, Chanthaphaychith S, Becher MW, Abel TW, Thompson RC, Cooper MK. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26:5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 15.Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, Taylor RE, Bailey S, Clifford SC, Gilbertson RJ. Medulloblastoma: clinicopathological correlates of SHH WNT and non-SHH/WNT molecular subgroups. Acta Neuropathol. 2011;121:381–396. doi: 10.1007/s00401-011-0800-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fancy SPJ, Baranzini SE, Zhao C, Yuk D-l, Irvine K-A, Kaing S, Sanai N, Franklin RJM, Rowitch DH. Dysregulation of the Wnt pathway inhibits timely myelination and remyelination in the mammalian CNS. Genes and Development. 2009;23:1571–1585. doi: 10.1101/gad.1806309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feigenson K, Reid M, See J, Crenshaw EBr, Grinspan JB. Wnt signaling is sufficient to perturb oligodendrocyte maturation. Mol Cell Neurosci. 2009;42:255–265. doi: 10.1016/j.mcn.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 18.Flahaut M, Meier R, Coulon A, Nardou KA, Niggli FK, Martinet D, Beckmann JS, Joseph JM, Muhlethaler-Mottet A, Gross N. The Wnt receptor FZD1 mediates chemoresistance in neuroblastoma through activation of the Wnt/beta-catenin pathway. Oncogene. 2009;28:2245–2256. doi: 10.1038/onc.2009.80. [DOI] [PubMed] [Google Scholar]
- 19.Fu D-Y, Wang Z-M, Chen L, Wang B-L, Shen Z-Z, Huang W, Shao Z-M. Sox17 the canonical Wnt antagonist, is epigenetically inactivated by promoter methylation in human breast cancer. Breast Cancer. 2010 doi: 10.1007/s10549-009-0339-8. [DOI] [PubMed] [Google Scholar]
- 20.Givogri MI, Bongarzone ER, Schonmann V, Campagnoni AT. Expression and regulation of golli products of myelin basic protein gene during in vitro development of oligodendrocytes. J Neurosci Res. 2001;66:679–690. doi: 10.1002/jnr.10031. [DOI] [PubMed] [Google Scholar]
- 21.Gubbay J, Collignon J, Koopman P, Capel B, Economou A, Munsterberg A, Vivian N, Goodfellow P, Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- 22.He S, Kim I, Lim MS, Morrison SJ. Sox17 expression confers self-renewal potential and fetal stem cell characteristics upon adult hematopoietic progenitors. Genes and Development. 2011;25:1613–1627. doi: 10.1101/gad.2052911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hudson C, Clements D, Friday RV, Stott D, Woddland HR. XSox17alpha and -beta mediate endoderm formation in Xenopus. Cell. 1997;91:397–405. doi: 10.1016/s0092-8674(00)80423-7. [DOI] [PubMed] [Google Scholar]
- 24.Jia Y, Yang Y, Liu S, Herman JG, Lu F, Guo M. Sox17 antagonizes WNT/beta-catenin signaling pathway in hepatocellular carcinoma. Epigenetics. 2010;5:743–749. doi: 10.4161/epi.5.8.13104. [DOI] [PubMed] [Google Scholar]
- 25.Kanai-Azuma M, Kanai Y, Gad JM, Tajima Y, Taya C, Kurohmaru M, Sanai Y, Yonekawa H, Yazaki K, Tam PPL, Hayashi Y. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development. 2002;129:2367–2379. doi: 10.1242/dev.129.10.2367. [DOI] [PubMed] [Google Scholar]
- 26.Katoh Y, Katoh M. WNT antagonist, SFRP1, is Hedgehog signaling target. Int J Mol Med. 2006;17:171–175. [PubMed] [Google Scholar]
- 27.Kelly JJ, Blough MD, Stechishin OD, Chan JA, Beauchamp D, Perizzolo M, Demetrick DJ, Steele L, Auer RN, Hader WJ, Westgate M, Parney IF, Jenkins R, Cairncross JG, Weiss S. Oligodendroglioma cell lines containing t(1;19)(q10;p10) Neuro Oncol. 2010;12:745–755. doi: 10.1093/neuonc/noq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim I, Saunders TL, Morrison SJ. Sox17 dependence distinguishes the transcriptional regulation of fetal from adult hematopoietic stem cells. Cell. 2007;130:470–483. doi: 10.1016/j.cell.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsui T, Kanai-Azuma M, Hara K, Matoba S, Hiramatsu R, Kawakami H, Kurohmaru M, Koopman P, Kanai Y. Redundant roles of Sox17 and Sox18 in postnatal angiogenesis in mice. J Cell Sci. 2006;119:3513–3526. doi: 10.1242/jcs.03081. [DOI] [PubMed] [Google Scholar]
- 30.Milovanovic T, Planutis K, Nguyen A, Marsh JL, Lin F, Hope C, Holcombe RF. Expression of Wnt genes and frizzled 1 and 2 receptors in normal breast epithelium and infiltrating breast carcinoma. Int J Oncol. 2004;25:1337–1342. [PubMed] [Google Scholar]
- 31.Niakan KK, Ji K, Maehr R, Vokes SA, Rodolfa KT, Sherwood RI, Yamaki M, Dimos JT, Chen AE, Melton DA, McMahon AP, Eggan K. Sox17 promotes differentiation in mouse embryonic stem cells by directly regulating extraembryonic gene expression and indirectly antagonizing self-renewal. Genes and Development. 2010;24:312–326. doi: 10.1101/gad.1833510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pannone G, Bufo P, Santoro A, Franco R, Aquino G, Longo F, Botti G, Serpico R, Cafarelli B, Abbruzzese A, Caraglia M, Papagerakis S, Lo Muzio L. WNT pathway in oral cancer: epigenetic inactivation of WNT-inhibitors. Oncol Rep. 2010;24:1035–1041. doi: 10.3892/or.2010.1035. [DOI] [PubMed] [Google Scholar]
- 33.Persson AI, Petritsch C, Swartling FJ, Itsara M, Sim FJ, Auvergne R, Goldenberg DD, Vandenberg SR, Nguyen KN, Yakovenko S, Ayers-Ringler J, Nishiiyama A, Stallcup WB, Berger MS, Bergers G, McKnight TR, Goldman SA, Weiss WA. Non-stem cell origin for oligodendroglioma. Cancer Cell. 2010;18:669–682. doi: 10.1016/j.ccr.2010.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Post GR, Dawson G. Characterization of a cell line derived from a human oligodendroglioma. Mol Chem Neuropathol. 1992;16:303–317. doi: 10.1007/BF03159976. [DOI] [PubMed] [Google Scholar]
- 35.Post GR, Dawson G. Regulation of carbachol- and histamine-induced inositol phospholipid hydrolysis in a human oligodendroglioma. Glia. 1992;5:122–130. doi: 10.1002/glia.440050206. [DOI] [PubMed] [Google Scholar]
- 36.Reis-Filho JS, Faoro LN, Carrilho C, Bleggi-Torres LF, Schmitt FC. Evaluation of cell proliferation epidermal growth factor receptor and bcl-2 immunoexpression as prognostic factors for patients with World Health Organization grade 2 oligodendroglioma. Cancer. 2000;88:862–869. doi: 10.1002/(sici)1097-0142(20000215)88:4<862::aid-cncr17>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 37.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of Sox: extent homology and nomenclature of the mouse and human Sox transcription factor gene families. Developmental Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 38.Schwalbe EC, Lindsey JC, Straughton D, Hogg TL, Cole M, Megahed H, Ryan SL, Lusher ME, Taylor MD, Gilbertson RJ, Ellison DW, Bailey S, Clifford SC. Rapid diagnosis of medulloblastoma molecular subgroups. Clin Cancer Res. 2011;17:1883–1894. doi: 10.1158/1078-0432.CCR-10-2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin S-CJ, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Molecular and Cellular Biology. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinner D, Rankin S, Lee M, Zorn AM. Sox17 and beta-catenin cooperate to regulate the transcription of endodermal genes. Development. 2004;131:3069–3080. doi: 10.1242/dev.01176. [DOI] [PubMed] [Google Scholar]
- 41.Sohn J, Natale JE, Chew LJ, Belachew S, Cheng Y, Aguirre AA, Lytle J, Nait-Oumesmar B, Kerninon C, Kanai-Azuma M, Kanai Y, Gallo V. Identification of Sox17 as a transcription factor that regulates oligodendrocyte development. Journal of Neuroscience. 2006;26:9722–9735. doi: 10.1523/JNEUROSCI.1716-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sugiarto S, Persson AI, Munoz EG, Waldhuber M, Lamagna C, Andor N, Hanecker P, Ayers-Ringler J, Phillips J, Siu J, Lim DA, Vandenberg S, Stallcup W, Berger MS, Bergers G, Weiss WA, Petritsch C. Asymmetry-defective oligodendrocyte progentiros are glioma precursors. Cancer Cell. 2011;20:328–340. doi: 10.1016/j.ccr.2011.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Takezaki T, Hide T, Takanaga H, Nakamura H, Kuratsu J, Kondo T. Essential role of the Hedgehog signaling pathway in human glioma-initiating cells. Cancer Science. 2011;102:1306–1312. doi: 10.1111/j.1349-7006.2011.01943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taylor MD, Northcott PA, Korshunov A, Remke M, Cho YJ, Clifford SC, Eberhart CG, Parsons DW, Rutkowski S, Gajjar A, Ellison DW, Lichter P, Gilbertson RJ, Pomeroy SL, Kool M, Pfister SM. Molecular subgroups of medulloglastoma: the current consensus. Acta Neuropathol. 2012;123:465–472. doi: 10.1007/s00401-011-0922-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ueno K, Hazama S, Mitomori S, Nishioka M, Suehiro Y, Hirata H, Oka M, lmai K, Dahiya R, Hinoda Y. Down-regulation of frizzled-7 decreases survival invasion and metastatic capabilities of colon cancer cells. Br J Cancer. 2009;101:1374–1381. doi: 10.1038/sj.bjc.6605307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ueno K, Hiura M, Suehiro Y, Hazama S, Hirata H, Oka M, lmai K, Dahiya R, Hinoda Y. Frizzled-7 as a potential therapeutic target in colorectal cancer. Neoplasia. 2008;10:697–705. doi: 10.1593/neo.08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.van der Meide WF, Snellenberg S, Meijer CJ, Baalgergen A, Helmerhorst TJ, van der Sluis WB, Snijders PJ, Steenbergen RD. Promoter methylation analysis of WNT/beta-catenin signaling pathway regulators to detect adenocarcinoma or its precursor lesion of the cervix. Gynecol Oncol. 2011;123:116–122. doi: 10.1016/j.ygyno.2011.06.015. [DOI] [PubMed] [Google Scholar]
- 48.Wegner M. Transcriptional Control in myelinating glia: flavors and spices. Glia. 2000;31:1–14. doi: 10.1002/(sici)1098-1136(200007)31:1<1::aid-glia10>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 49.Wegner M. Expression of Transcription Factors during oligodendroglial development. Microscopy Research and Technique. 2001;52:746–752. doi: 10.1002/jemt.1059. [DOI] [PubMed] [Google Scholar]
- 50.Xu Q, Yuan X, Liu G, Black KL, Yu JS. Hedgehog signaling regulates brain tumor-initiating cell proliferation and portends shorter survival for patients with PTEN-coexpressing glioblastomas. Stem Cells. 2008;26:3018–3026. doi: 10.1634/stemcells.2008-0459. [DOI] [PubMed] [Google Scholar]
- 51.Yasunaga M, Tada S, Torikai-Nishikawa S, Nakano Y, Okada M, Jakt LM, Nishikawa S, Chiba T, Era T. Induction and monitoring of definitive and visceral endoderm differentiation of mouse ES cells. Nat Biotechnol. 2005;23:1542–1550. doi: 10.1038/nbt1167. [DOI] [PubMed] [Google Scholar]
- 52.Yates AJ. An overview of principles for classifying brain tumors. Mol Chem Neuropathol. 1992;17:103–120. doi: 10.1007/BF03159986. [DOI] [PubMed] [Google Scholar]
- 53.Ye YW, Wu JH, Wang CM, Zhou Y, Du CY, Zheng BQ, Cao X, Zhou XY, Sun MH, Shi YQ. Sox17 regulates proliferation and cell cycle during gastric cancer progression. Cancer Lett. 2011;307:124–131. doi: 10.1016/j.canlet.2011.03.024. [DOI] [PubMed] [Google Scholar]
- 54.Zheng Y, Miskimins WK. Far upstream element binding protein 1 activates translation of p27Kip1 mRNA through its internal ribosomal entry site. Int J Biochem Cell Biol. 2011;43:1641–1648. doi: 10.1016/j.biocel.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]