Abstract
Mammalian target of rapamycin (mTOR) has been implicated as a sensor of nutrient sufficiency for dividing cells and is activated by essential amino acids and glucose. However, cells also require lipids for membrane biosynthesis. A central metabolite in the synthesis of membrane phospholipids is phosphatidic acid (PA), which is required for the stability and activity of mTOR complexes. While PA is commonly generated by the phospholipase D-catalyzed hydrolysis of phosphatidylcholine, PA is also generated by diacylglycerol kinases and lysophosphatidic acid acyltransferases, which are at the center of phospholipid biosynthesis. It is proposed that the responsiveness of mTOR/TOR to PA evolved as a means for sensing lipid precursors for membrane biosynthesis prior to doubling the mass of a cell and dividing.
Keywords: mTOR, phosphatidic acid, DG kinase, LPAAT, phospholipase D
Lipid sensing by TOR
A critical need for cell growth is the presence of the raw materials needed for doubling the mass of a cell during cell division. Target of rapamycin (TOR) is an evolutionarily conserved sensor of essential nutrients needed for the synthesis of biological molecules (1, 2). TOR has long been known to respond to amino acids, glucose, and energy (3, 4). However, over the last decade, the mammalian TOR (mTOR) has been shown to require PA for the stability of mTOR complexes and their activity (5–8). A rationale for the involvement of PA has been missing, and hence the role of PA in regulating mTOR has been controversial (9). Of significance, PA is at the center of membrane glycero-phospholipid synthesis. Based on this central position of PA generation, it is speculated that the PA requirement for mTOR and evolutionally more primitive TOR proteins represents a means for TOR to sense the presence of sufficient lipid precursors for membrane biosynthesis, cell growth and proliferation. Phospholipase D (PLD), which is activated in response to a variety of extra-cellular stimuli also generates PA and represents an alternative mechanism for generating PA needed for mTOR activation (6, 7). Importantly, PA generation via PLD is commonly elevated in human cancers where active mTOR provides signals that promote cancer cell survival (10). In this perspective, it is proposed that in addition to amino acids, glucose and energy status, TOR responds to PA as an indicator of lipid sufficiency in dividing cells. It is also proposed that the generation of PA by PLD represents a means for TOR activation in response to extracellular signals, in multicellular organisms.
Nutrient sensing by mTOR and cell cycle progression
G1 cell cycle progression has been divided into two parts G1-pm (post-mitotic) and G1-ps (pre-S) separated by a growth factor dependent restriction point (11). During G1-pm, the cell determines whether there are instructions to divide – growth factors. During G1-ps, the cell determines whether the resources needed for doubling the mass of the cells are available (12). There are likely several checkpoints in late G1 that sense the presence of sufficient nutrients for cell growth. mTOR is a key sensor of nutrients and is sensitive to the presence of amino acids, glucose, ATP, and insulin (1–4). mTOR also regulates passage through late G1, and significantly, rapamycin treatment leads to the accumulation of small cells with G1 DNA content (13, 14). Given the critical role that mTOR plays in promoting G1 cell cycle progression, it is no surprise that it has been suggested that signals that regulate mTOR are the most commonly dysregulated signals in cancer (15, 16). Although activating gain-of-function mTOR mutations have been reported in human cancers (17), more commonly, there are mutations in genes that encode proteins that regulate mTOR activity (9). The most common mutations that result in elevated mTOR activity are to phosphatidylinositol (PI)-3-kinase (PI3K), which phosphorylates PI-4,5-bisphosphate at position 3 of the inositol ring to generate PI-3,4,5-trisphosphate; and loss-of-function mutations for PTEN, which dephosphorylates PI-3,4,5-trisphosphate at the 3 position. These mutations result in elevated signaling through Akt, the tuberous sclerosis complex (TSC), and Rheb – leading to the activation of mTOR complex1 (mTORC1) (Figure 1). This pathway is activated physiologically by insulin and insulin-like growth factor (9). Insulin also leads to an increase in glucose transporters on the plasma membrane and increased uptake of glucose (18). Increased uptake of glucose is a hallmark of cancer cells that was first observed by Otto Warburg more than 80 years ago (19). Importantly, mTOR is required for much of the metabolic reprogramming that takes place in cancer cells where oxidative phosphorylation is reduced even in the presence of O2 (9, 20). Thus, mTOR not only responds to the presence of nutrients and energy, it also promotes metabolic reprogramming to promote cell growth by enhancing greater utilization of glucose for generating the biological molecules needed for a cell to double its mass upon cell division.
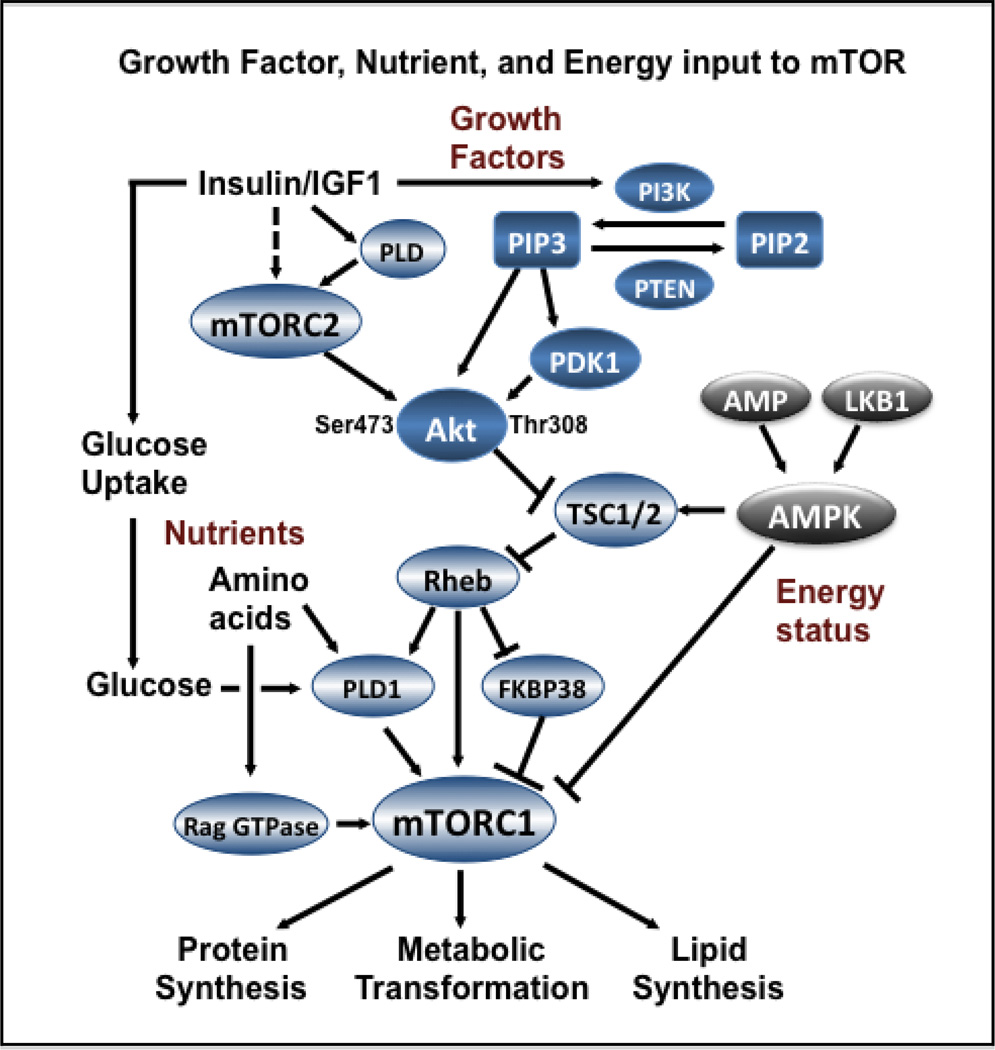
Figure 1. Nutrient signals to mTOR.
Regulation of mTOR has many inputs. The PI3K input involves the generation of PIP3 from PIP2, which recruits and activates phosphoinositide-dependent kinase 1 (PDK1), which then phosphorylates Akt at Thr308. Subsequently, Akt phosphorylates and suppresses the GAP activity of the tuberous sclerosis complex (TSC) consisting of TSC1 and TSC2 (TSC1/2). Suppression of TSC1/2 results in elevated activation of the GTPase Rheb, which leads to a complex activation of mTORC1 via the activation of PLD1 and suppression of FKBP38 whereby elevated PLD activity generates the PA necessary for the formation of mTORC1 complex, and FKBP38 dissociates from mTORC1(49). This pathway is also impacted by AMPK, which in combination with the tumor suppressor LKB1 activates TSC1/2, which suppresses Rheb and thus mTOR – under conditions where ATP levels are low and AMP levels are high. Akt is also phosphorylated by mTORC2 at Ser473 in response to insulin and insulin-like growth factor 1 (IGF1), in a PLD-dependent manner. Phosphorylation at this site has been correlated with altered substrate specificity and kinase activity for Akt. Insulin also increases the level of glucose transporters and increased uptake of glucose (18). A common theme in this complex signaling network leading to mTORC1 activation is that it is highly sensitive to the presence of glucose and amino acids – nutrients needed for cell growth.
Regulation of mTOR by phosphatidic acid
While much is known about the regulation of mTOR via the PI3K/Akt/TSC/Rheb pathway, mTOR is also dependent on PA, which interacts with mTOR in a manner that is competitive with rapamycin (5, 21) and is required for the stability of both mTORC1 and mTORC2 complexes (8). mTORC1 is much more sensitive than mTORC2 to the levels of PA, which stabilizes mTOR, and to rapamycin, which disrupts the mTOR complexes (8). Consequently, it is mTORC1 that is more likely than mTORC2 to be responsive to changes in PA levels. The PA most commonly associated with mTOR regulation is generated by the hydrolysis of phosphatidylcholine by PLD (7, 10). However, recent reports have revealed that knockout of both PLD1 and PLD2 yields viable mice (22, 23). In contrast, mTOR knockouts are embryonic lethal (24, 25). Thus, if PA is essential for mTOR activity, then PA from other sources must be used.
Multiple sources of PA
PA synthesis is at the center of membrane phospholipid and triglyceride synthesis (26–29) and therefore represents a potential indicator of the capability for generating the membrane phospholipids needed for doubling cell mass. The PA targeted for membrane biogenesis is most commonly synthesized from glycerol 3-phosphate (G3P) and newly synthesized fatty acids (FAs). G3P gets acylated twice by distinct acyltransferases – the last step being catalyzed by a lysophosphatidic acid acyltransferase (LPAAT) (Figure 2a). Interestingly, this pathway is dependent on the glycolytic pathway intermediate dihydroxyacetone phosphate (DHAP), which gets reduced to G3P and is then acylated to generate PA. The Acyl-CoA needed for the acylation of G3P comes from both dietary FAs and newly synthesized palmitic acid, which is catalyzed by fatty acid synthase (FAS). Thus, the generation of PA via the LPAAT pathway involves nutrient input from glucose, dietary FAs, and de novo FA synthesis. PA can be converted by PA phosphatase to diacylglycerol (DG), which can be acylated to form triglycerides for fat storage. DG is also an intermediate for the synthesis of a subset of membrane glycerol-phospholipids. In the reverse process, PA can be generated from stored triglycerides by deacylation to DG, which can be either fed directly into membrane phospholipid biosynthesis or be phosphorylated by a DG kinase to generate PA (Figure 2a). Thus, the central position of PA in phospholipid metabolism makes PA an ideal indicator of lipid sufficiency to proceed with membrane biogenesis in a dividing cell. Importantly, LPAAT and DG kinase-θ, which generate PA, have been shown to stimulate mTOR (30, 31), although there are also reports that DG kinases can suppress mTOR (32, 33), which will be addressed below. Thus, there is a connection between the enzymes that generate the PA critical for phospholipid and membrane biosynthesis and the activation of mTOR. Intriguingly, suppression of LPAAT suppressed mTOR activity and disrupted survival and proliferative signals in several cancer cell lines (34).
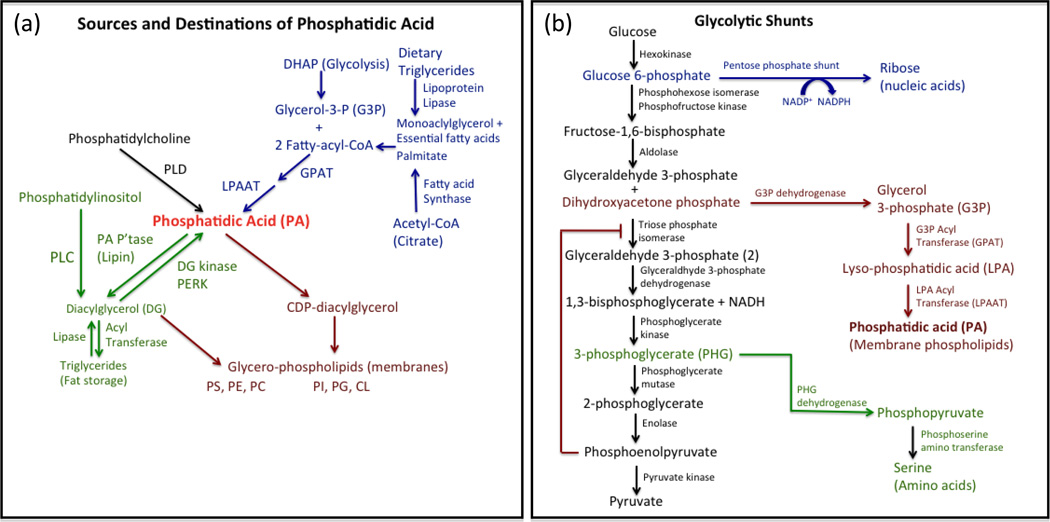
Figure 2. Phosphatidic acid metabolism.
(a) PA can be generated by three major mechanisms – first by the de novo synthesis pathway that involves the acylation of G3P by GPAT and LPAAT (Blue). G3P is generated by the reduction of the glycolytic intermediate DHAP. The FAs that acylate G3P can be synthesized by FAS and then elongated and mono-desaturated in mammalian cells. However, dietary essential FAs are required in mammalian cells for the synthesis of poly-unsaturated FAs needed for the acylation of membrane phospholipids. The second pathway involves the phosphorylation of DG by DG kinases (Green). The DG required for this pathway must come from either deacylated triglyceride or PLC-generated DG derived primarily from phosphatidylinositol-4,5-trisphosphate. Thus, DG kinase can generate PA in response to growth factor induced PLC, or from stored lipids via triglyceride lipases. The third pathway involves the hydrolysis of phosphatidylcholine by PLD (Black). This pathway is not likely involved in the generation of PA for membrane biosynthesis since the PA is derived from a membrane phospholipid. The same is true for the PLC pathway that hydrolyzes phosphatidylinositol to generate DG. These pathways likely represent growth factor-dependent stimulation of PA production that occurs in the absence of membrane biosynthesis and is restricted to multicellular organisms. As indicated, PA is a substrate for the synthesis of phosphatidylinositol (PI), phsophatidylglycerol (PG) and cardiolipin (CL). DG generated by PA phosphatase (PA P’tase) is the substrate for the synthesis phosphatidylserine (PS), phosphatidylethanolamine (PE), and phosphatidylcholine (PC) (Red). (b) PA is generated from the glycolytic intermediate DHAP. Glycolysis represents the conversion of the 6-carbon glucose to two molecules of the 3-carbon pyruvate. The last step is catalyzed by pyruvate kinase (PK), which in dividing cells involves an embryonic isoform known as PKM2, which has a slower catalytic rate (41) that can be made even slower by tyrosine phosphorylation (42). The outcome of the reduced pyruvate kinase activity coupled with increased glucose uptake is the accumulation of glycolytic intermediates (43). The elevated level of glycolytic intermediates is the conversion to molecules for anabolic synthesis of biological molecules that are needed to double the mass of a dividing cell. The most understood utilization of a glycolytic intermediate is the pentose phosphate shunt, which generates ribose that can be utilized in the synthesis of nucleic acids (Blue). The pentose phosphate shunt also leads to the generation of NADPH that can be utilized in anabolic reactions – especially FA synthesis. Amino acids, most notably serine, are generated from 3-phosphoglycerate (PHG) via the conversion to phosphopyruvate by PHG dehydrogenase and then to serine by phosphoserine amino transferase (Green). Glycerol-3-phosphate, the substrate for the acyltransferases that generate PA, is generated from DHAP by reduction to G3P by G3P dehydrogenase (Red). Of interest, the enzyme that converts DHAP to glyceraldhyde-3-phosphate, triose phosphate isomerase, is suppressed by phosphoenolpyruvate (45).
An alternative pathway for growth factor induced PLD-induced PA production is via a phospholipase C (PLC)-mediated production of DG followed by the conversion of DG to PA by DG kinase as described previously (29). Like PLD, PLC is commonly activated by growth factors and could account for ability of PLD null mice to survive. It will therefore be of interest to determine whether in the absence of PLD, there is a compensatory increase in the level of PA generated by PLC and DG kinase in response to growth factors.
In addition to the known DG kinases (29), it was recently reported that ER-localized PKR-like ER kinase (PERK), a kinase that responds to ER stress, has an intrinsic DG kinase activity (35). Importantly, the PA produced in response to PERK stimulated both mTORC1 and mTORC2. ER stress or the unfolded protein response (UPR) that occurs on the ER induces different responses depending on nutrient availability (36). The outcome can be apoptosis under nutritional stress, or a homeostatic response that restores ER function. Thus, the ability of PERK to generate PA and stimulate Akt phosphorylation at Ser473 – a site phosporylated by mTORC2 – may be part of the UPR that leads to restoration of ER function. The stimulation of mTOR by the UPR and PERK would promote the uptake of glucose and the generation of anabolic intermediates needed to alleviate ER stress. Interestingly, loss of either TSC1 or TSC2, which leads to hyperactive mTOR, also triggers ER stress and the UPR (37) – indicating that hyperactive mTOR leads to the activation of PERK and generates the PA to support increased mTOR activity.
Altered metabolism in proliferating cells leads to increased utilization of metabolites for anabolic needs and cell growth – including PA production
When a cell commits to dividing there is a “metabolic transformation” that takes place whereby there is a shift from catabolic metabolism that favors the mitochondrial production of ATP via the electron transport chain to anabolic metabolism that favors the production of NADPH, which is used for the synthesis of biological molecules – especially FAs (38, 39). Glucose metabolism is highly impacted in proliferating cells, most significantly through increased glucose transport (40). Interestingly, dividing cells express an embryonic form of the enzyme pyruvate kinase M2 (PKM2) that catalyzes the last step of glycolysis – the conversion of phosphoenolpyruvate (PEP) to pyruvate (41). PKM2 is inefficient in converting PEP to pyruvate and is suppressed further by growth factor-induced tyrosine phosphorylation (42). The reduced PKM2 activity combined with increased glucose uptake results in the increase of glycolytic intermediates (43). These glycolytic intermediates are shunted off into pathways for the synthesis of nucleotides and amino acids (Figure 2a). Glucose-6-phosphate (G6P) can be converted to ribose via the pentose phosphate shunt, and 3-phosphoglycerate converted to serine and other amino acids via phosphoglycerate dehydrogenase. This last pathway, which leads to serine synthesis, is required for certain breast cancers (44). While these two shunts have been discussed in recent reviews (15, 43), there is another critical shunt leading to the synthesis of membrane phospholipids involving the conversion of DHAP to G3P. Interestingly, triose phosphate isomerase, the glycolytic enzyme that converts DHAP to glyceraldehyde-3-P is suppressed by PEP – the substrate of PKM2 (45). Thus PKM2, by slowing the conversion of PEP to pyruvate, further enhances the accumulation of DHAP during glycolysis and the production of G3P – the substrate for the acyl-transferases that generate PA (Figure 2b). Enhancement of G3P levels by suppression of both pyruvate kinase and triose phosphate isomerase activity strongly indicates that generating G3P, a precursor of PA, is critical in dividing and cancerous cells.
Does mTOR sense dietary essential FAs?
FA synthesis generates palmitic acid – a 16 carbon saturated FA, which can be incorporated into membrane lipids via acyl-transferases (see Figure 2). In mammalian cells, palmitic acid can be elongated, but mammals are limited in their ability to desaturate FAs between C-10 and the methyl terminal end. Hence, they need exogenously supplied linoleic and linolenic acids, 18 carbon poly-unsaturated FAs with 2 and 3 double bonds respectively that have double bonds at the omega 3 and 6 positions. These FAs are needed for the synthesis of critical poly-unsaturated FAs such as arachidonic acid (46, 47). Hence, linoleic and linolenic acids are considered “essential fatty acids” – analogous to the essential amino acids not synthesized by mammalian cells. In this regard, it is of interest that PA with two saturated palmitates actually inhibits mTORC2 (48), whereas 1-palmitoyl, 2-steroyl-PA is stimulatory for both mTORC1 and mTORC2 (8, 49). These data suggest that PA with some degree of unsaturation is required for a functional interaction with mTOR. Or alternatively, that di-palmitoyl-PA, with two saturated FAs is targeted for energy storage, and thusly, is dephosphorylated and acylated to triacylglycerol. This is shown schematically in Figure 3a, where there are two outcomes from FA and PA synthesis: 1) in storage mode, newly synthesized palmitate is converted into triglycerides for energy storage; and 2) in proliferation mode, there is the generation of longer chain FAs with some degree of unsaturation that are incorporated into PA targeted for membrane phospholipids. It is PA with at least one FA containing an unsaturated FA that interacts with mTOR to promote complex stability and activity. Whether there is a mechanism for mTOR to distinguish PA species with poly-unsaturated FAs derived from the essential linoleic and linolenic acids remains to be determined. However, there does appear to be a means for distinguishing PA with only saturated palmitate, which inhibits mTORC2, from PA with two saturated FAs (48). This effect may explain the observation alluded to above that under some conditions, DG kinase can suppress mTOR (32, 33). If the DG species being phosphorylated contains two saturated FAs, then it would inhibit rather than stimulate mTOR. mTOR responds to essential amino acids specifically (50, 51) and it will therefore be of interest to determine if mTOR can sense essential FAs and whether this involves PA.
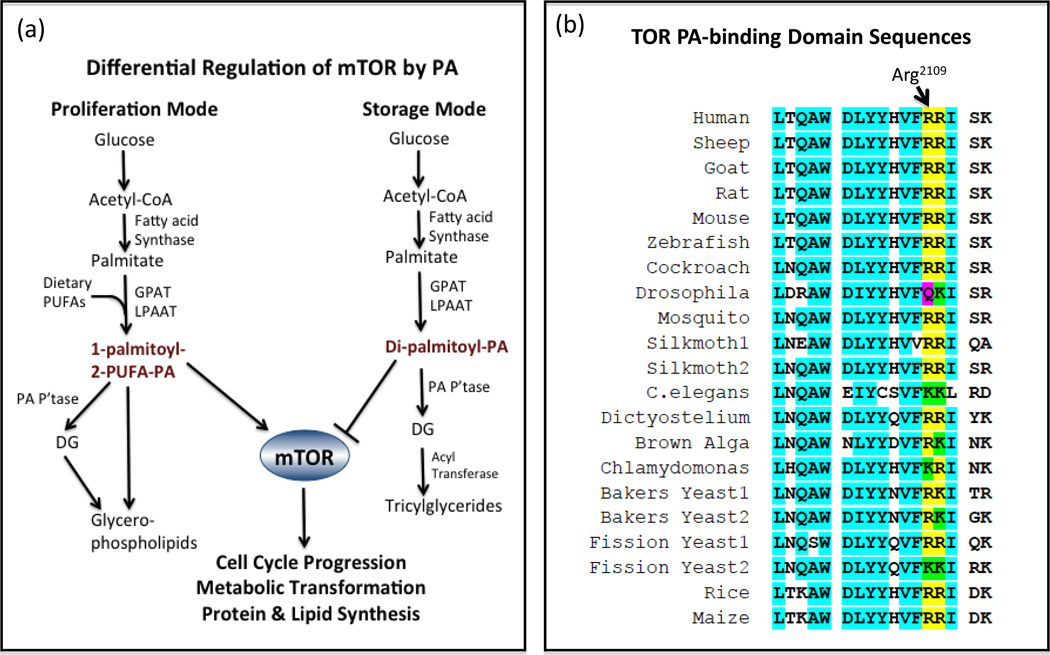
Figure 3. Regulation of TOR by phosphatidic acid.
(a) There are two major destinations for newly synthesized PA – membrane phospholipids when cells are in a proliferative mode, and triglycerides when cells are in a storage mode. In storage mode it is postulated that there is more newly synthesized saturated palmitic acid used for triglyceride synthesis; whereas in proliferation mode, there are more elongated and desaturated FAs utilized to maintain membrane fluidity and provide poly-unsaturated FAs (PUFAs) for eicosanoid synthesis. PA with two saturated FAs was reported to suppress mTORC2 (48). Thus, there appears to a mechanism whereby mTOR can distinguish between PA directed towards membrane biosynthesis and PA directed to triglyceride storage. (b) The PA-binding domain of TOR is within the region of TOR that also binds rapamycin (5, 52). This sequence contains a critical Arg residue at position 2109 (human sequence), critical for PA binding (5). There is also a conserved positively charged amino acid at the adjacent position at 2110, and highly conserved regions flanking these two positive charges all the way from yeast to humans. Sequence number is relative to Arg 2109 of the human sequence. Many of these sequences were assembled previously (53).
Conservation of the PA requirement of TOR
Jie Chen and colleagues established that positively charged Arg2109 in the rapamycin-binding domain of mTOR was critical for interaction with PA (5). This interaction was subsequently verified in an NMR structural study (52). Significantly, this site is highly conserved evolutionarily – with an Arg or Lys at this site in a wide variety of species from yeast to humans (53). The only exception to a positively charged amino acid at position 2109 is Drosophila, where there is Gln instead (Figure 3b). This piece of evidence along with the finding that PLD knockout in Drosophila did not impact on TOR signaling (54), led Sun and Chen (7) to suggest that the regulation of mTOR by PLD might be restricted to mammals. However, while there is a Gln instead of an Arg or Lys at position 2109, a conserved positive charge exists at the adjacent amino acid at 2110. Moreover, although Gln does not carry a positive charge, it does have amide hydrogens capable of forming hydrogen-bonds with electrons on the phosphate oxygens of PA. Thus, while PLD may not be required to generate PA for TOR activation in Drosophila, it is still quite possible that there is a PA requirement for Drosophila TOR that is met by LPAAT, DG kinase, or a PLC/DG kinase mechanism. However, there is indirect evidence that PA may regulate TOR in Dictyostelium. Both over expression of PLD and exogenously supplied PA suppresses the starvation response in Dyctyostelium (55), and starvation in Dictyostelium inhibits 4E–BP1 phosphorylation (56). Thus, PA suppresses the starvation response that involves inhibition of phosphorylation of an mTORC1 substrate. Thus, it is highly likely that PA is regulating mTOR in an evolutionarily primitive organism. These studies not only implicate PA as an activator of TOR in a primitive organism, they also implicate PLD as activator of TOR at an early stage of evolution. Since the sequence in the PA-binding region of TOR is so highly conserved, there is a compelling evidence that PA is critical for TOR in distant species including yeast, Dictyostelium, and C. elegans, – all of which have the highly conserved PA-binding region of TOR with two positively charged amino acids at position 2109 and 2110, as well as several other conserved amino acids flanking this site (Figure 3b). The critical amino acids for binding of Raf to PA have also been mapped and while there are differences between the Raf and TOR sequences, the two consecutive positively charged amino acids are conserved (57).
PA generated for membrane lipid biosynthesis versus PLD
During proliferation, there is substantial membrane phospholipid synthesis going on, and as a consequence, there is significant PA production that is targeted for membrane phospholipid biosynthesis. PLD-generated PA, which is derived from a membrane phospholipid, is therefore not involved in cell growth. However, mTOR is not stimulated solely by nutrients; it is also stimulated by a variety of growth factors – perhaps most significantly by insulin and insulin-like growth factor1 (9). Importantly, these and other growth factors also activate PLD (10), and the stimulation of mTORC2 by insulin is dependent on PLD-generated PA (8). Thus, it is tempting to speculate that growth factor-induced PLD activity evolved as a means to promote TOR complex stabilization in the absence of anabolic PA synthesis by DG kinase and LPAAT. Growth factor signals to PLD activation involve the Ras-related protein RalA (58), which is constitutively associated with PLD1 (59). Of significance, RalA has also been implicated in amino acid-induced mTOR activation (60). Thus, the generation of PA by PLD has apparently been integrated into nutrient-induced mTOR activation. Consistent with this hypothesis, it was recently reported that the activation of mTOR with both amino acids and glucose is dependent on PLD (50, 51, 61). Thus, at least in mammalian cells, nutrient-induced signals that stimulate mTOR have utilized PLD-derived PA – indicating that PLD-generated PA contributes to nutrient sensing by mTORC1.
Phosphatidic acid and cancer
PLD activity is commonly elevated in human cancer and human cancer cells (10). Importantly, elevated PLD activity is essential for the survival of cancer cells (62–69). Cancer cells are particularly sensitive to suppression of PLD and mTOR activity in the absence of serum (14). Intriguingly, there is a rapid increase in PLD activity in most human cancer cells, when serum is withdrawn (67–69). Thus, cancer cells may have co-opted PLD in order to generate PA to keep mTOR intact in the absence of exogenously supplied lipids. This would indicate that PLD could be a good therapeutic target for what is likely a large number of human cancers, that have elevated PLD activity. Interestingly, the plant compound honokiol, which suppresses PLD activity in cancer cells, only suppresses the PLD that is activated in response to the loss of serum lipids (69). Honokiol is highly toxic to cancer cells deprived of serum, while having minimal effects on normal cells (69, 70). Since PLD knockout mice are viable (22, 23), targeting PLD as an anti-cancer therapeutic approach might be less toxic than other approaches that target mTOR. There are currently several drugs that target both PLD1 and PLD2 specifically, and more are in development (71–73). Thus, it may be possible to target a non-essential cellular function to treat human cancers by inhibiting the elevated PLD activity observed in human cancers.
Concluding remarks
While the generation of PA from PLD seems critical for mTOR activity in cancer cells, and perhaps under other stressful conditions, clearly PLD is not essential for development. However, mTOR is essential and requires PA for maintaining the stability of the mTOR complexes. Thus, there must be other means for generating the PA needed to stabilize mTOR complexes and for mTOR kinase activity. Four mechanisms for doing this are described: 1) the acylation of G3P; 2) the phosphorylation of DG generated from triglycerides; 3) the phosphorylation of DG generated from PLC; and lastly 4) the generation of PA from DG by PERK in response to ER stress and the UPR. The first two mechanisms are central to membrane phospholipid synthesis and it is proposed that the PA requirement of TOR complexes represents a means for TOR to sense sufficient PA for membrane biosynthesis, cell growth, and division. The ability to generate PA by phospholipases, PLD and PLC, likely evolved as a means for multi-cellular organisms to generate PA in response to inter-cellular signals, such as insulin, that stimulate mTOR. Of significance, PLD activity, which is commonly activated by growth factors and insulin, is elevated in many human cancers and promotes survival in an mTOR-dependent manner. A significant role for elevated PA levels in cancer cells generated by DG kinase and LPAAT activity has not been observed. However, it is still possible that interfering with PA and membrane lipid synthesis could have an impact on the survival of cancer cells. Targeting FA synthesis, which generates FAs that contribute to the synthesis of PA, has been proposed as a promising clinical target for several human cancers (74). TOR functions as a nutrient sensor and is responsive to amino acids and glucose that are important for the synthesis of proteins and many other biological molecules needed for cel growth. Since PA is central to the metabolic pathways that generate membrane phospholipids, there is a strong rationale for why TOR is dependent on PA – namely to sense the presence of sufficient phospholipids for cell growth. The strong conservation of the PA-binding domain in TOR – from yeast to mammals – suggests that the PA requirement for TOR was a very early evolutionary event.
Outstanding Questions.
Is there a PA requirement for TOR proteins from more primitive species?
When did organisms start generating PA for mTOR activation via PLD? Was this an adaptation to multicellularity?
Are there compensatory increases in alternative pathways for PA production in PLD null cells?
Are there differences in the PA species generated via different enzymatic pathways and do different PA species impact differentially on mTORC1 and mTORC2?
Does mTOR have an ability to respond to dietary “essential fatty acids”?
To what extent do serum lipids contribute to mTOR activation?
Are there lipid sensitive cell cycle checkpoints? And if so, are they dysregulated in human cancers?
Can metabolic pathways leading to PA generation be targeted in human cancers?
Highights.
Phosphatidic acid (PA) is a central metabolite in membrane biosynthesis
TOR/mTOR is a sensor of amino acids and glucose needed for cell growth
PA is a critical regulator of mTOR and likely more primitive TOR species as well
The PA requirement for TOR may represent a means for the sensing of lipids, which are also required for cell growth
Acknowledgements
Craig Thompson is acknowledged for suggesting a broader role for phosphatidic acid as an indicator of lipid sufficiency. Derrick Brazill is acknowledged for pointing out the connection between phosphatidic acid and TOR in Dictyostelium. I would like to thank Deepak Menon for organizing the PA-binding sequences in TOR. Support by National Cancer Institute grant CA46677 is acknowledged. Research Centers in Minority Institutions (RCMI) award RR-03037 from the National Center for Research Resources of the National Institutes of Health, which supports infrastructure and instrumentation in the Biological Sciences Department at Hunter College, is also acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr. Opin. Cell Biol. 2009;21:209–218. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- 2.Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- 3.Sengupta S, Peterson TR, Sabatini DM. Regulation of the mTOR complex 1 pathway by nutrients, growth factors, and stress. Mol Cell. 2010;40:310–322. doi: 10.1016/j.molcel.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avruch J, Long X, Ortiz-Vega S, Rapley J, Papageorgiou A, Dai N. Amino acid regulation of TOR complex 1. Am. J. Phys. Endocrin. Metab. 2009;296:E592–E602. doi: 10.1152/ajpendo.90645.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Science. 2001;294:1942–1945. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- 6.Foster DA. Regulation of mTOR by phosphatidic acid? Cancer Res. 2007;67:1–4. doi: 10.1158/0008-5472.CAN-06-3016. [DOI] [PubMed] [Google Scholar]
- 7.Sun Y, Chen J. mTOR signaling: PLD takes center stage. Cell Cycle. 2008;7:3118–3123. doi: 10.4161/cc.7.20.6881. [DOI] [PubMed] [Google Scholar]
- 8.Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol. Cell. Biol. 2009;29:1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim. Biophys. acta. 2009;1791:949–955. doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zetterberg A, Larsson O, Wiman KG. What is the restriction point? Curr. Opin. Cell Biol. 1995;7:835–842. doi: 10.1016/0955-0674(95)80067-0. [DOI] [PubMed] [Google Scholar]
- 12.Foster DA, Yellen P, Xu L, Saqcena M. Regulation of G1 Cell Cycle Progression: Distinguishing the Restriction Point from a Nutrient-Sensing Cell Growth Checkpoint(s) Genes Cancer. 2010;1:1124–1131. doi: 10.1177/1947601910392989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E–BP1/eukaryotic translation initiation factor 4E. Mol. Cell. Biol. 2004;24:200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gadir N, Jackson DN, Lee E, Foster DA. Defective TGF-beta signaling sensitizes human cancer cells to rapamycin. Oncogene. 2008;27:1055–1062. doi: 10.1038/sj.onc.1210721. [DOI] [PubMed] [Google Scholar]
- 15.Ward PS, Thompson CB. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blagosklonny MV. Molecular damage in cancer: an argument for mTOR-driven aging. Aging. 2011;3:1130–1141. doi: 10.18632/aging.100422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hardt M, Chantaravisoot N, Tamanoi F. Activating mutations of TOR (target of rapamycin) Genes Cells. 2011;16:141–151. doi: 10.1111/j.1365-2443.2010.01482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leto D, Saltiel AR. Regulation of glucose transport by insulin: traffic control of GLUT4. Nat. Rev. Mol. Cell. Biol. 2012;13:383–396. doi: 10.1038/nrm3351. [DOI] [PubMed] [Google Scholar]
- 19.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 20.Cantor JR, Sabatini DM. Cancer cell metabolism: one hallmark, many faces. Cancer Discov. 2012;2:881–898. doi: 10.1158/2159-8290.CD-12-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen Y, Zheng Y, Foster DA. Phospholipase D confers rapamycin resistance in human breast cancer cells. Oncogene. 2003;22:3937–3942. doi: 10.1038/sj.onc.1206565. [DOI] [PubMed] [Google Scholar]
- 22.Dall'Armi C, Hurtado-Lorenzo A, Tian H, Morel E, Nezu A, Chan RB, Yu WH, Robinson KS, Yeku O, Small SA, Duff K, Frohman MA, Wenk MR, Yamamoto A, Di Paolo G. The phospholipase D1 pathway modulates macroautophagy. Nature Comm. 2010;1:142. doi: 10.1038/ncomms1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thielmann I, Stegner D, Kraft P, Hagedorn I, Krohne G, Kleinschnitz C, Stoll G, Nieswandt B. Redundant functions of phospholipases D1 and D2 in platelet alpha-granule release. J. Thromb. Haemos. 2012 doi: 10.1111/j.1538-7836.2012.04924.x. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 24.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, Brown M, Fitzgerald KJ, Sabatini DM. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev Cell. 2006;11:859–871. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Gangloff YG, Mueller M, Dann SG, Svoboda P, Sticker M, Spetz JF, Um SH, Brown EJ, Cereghini S, Thomas G, Kozma SC. Disruption of the mouse mTOR gene leads to early postimplantation lethality and prohibits embryonic stem cell development. Mol. Cell. Biol. 2004;24:9508–9516. doi: 10.1128/MCB.24.21.9508-9516.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Athenstaedt K, Daum G. Phosphatidic acid, a key intermediate in lipid metabolism. Eur. J. Biochem. 1999;266:1–16. doi: 10.1046/j.1432-1327.1999.00822.x. [DOI] [PubMed] [Google Scholar]
- 27.Wendel AA, Lewin TM, Coleman RA. Glycerol-3-phosphate acyltransferases: rate limiting enzymes of triacylglycerol biosynthesis. Biochim. Biophys. acta. 2009;1791:501–506. doi: 10.1016/j.bbalip.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rincon E, Gharbi SI, Santos-Mendoza T, Merida I. Diacylglycerol kinaseζ: at the crossroads of lipid signaling and protein complex organization. Prog. Lipid Res. 2012;51:1–10. doi: 10.1016/j.plipres.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 29.Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem. J. 2008;409:1–18. doi: 10.1042/BJ20071040. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Yuan J, Chen X, Gu X, Luo K, Li J, Wan B, Wang Y, Yu L. Identification of a novel human lysophosphatidic acid acyltransferase, LPAAT-theta, which activates mTOR pathway. J. Biochem. Mol. Biol. 2006;39:626–635. doi: 10.5483/bmbrep.2006.39.5.626. [DOI] [PubMed] [Google Scholar]
- 31.Avila-Flores A, Santos T, Rincon E, Merida I. Modulation of the mammalian target of rapamycin pathway by diacylglycerol kinase-produced phosphatidic acid. J. Biol Chem. 2005;280:10091–10099. doi: 10.1074/jbc.M412296200. [DOI] [PubMed] [Google Scholar]
- 32.Takita T, Konuma T, Hanazato M, Inoue H. Diacylglycerol kinase inhibitor R59022-induced autophagy and apoptosis in the neuronal cell line NG108-15. Arch. Biochem. Biophys. 2011;509:197–201. doi: 10.1016/j.abb.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Gorentla BK, Wan CK, Zhong XP. Negative regulation of mTOR activation by diacylglycerol kinases. Blood. 2011;117:4022–4031. doi: 10.1182/blood-2010-08-300731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coon M, Ball A, Pound J, Ap S, Hollenback D, White T, Tulinsky J, Bonham L, Morrison DK, Finney R, Singer JW. Inhibition of lysophosphatidic acid acyltransferase beta disrupts proliferative and survival signals in normal cells and induces apoptosis of tumor cells. Mol. Cancer Ther. 2003;2:1067–1078. [PubMed] [Google Scholar]
- 35.Bobrovnikova-Marjon E, Pytel D, Riese MJ, Vaites LP, Singh N, Koretzky GA, Witze ES, Diehl JA. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol. Cell. Biol. 2012;32:2268–2278. doi: 10.1128/MCB.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 37.Ozcan U, Ozcan L, Yilmaz E, Duvel K, Sahin M, Manning BD, Hotamisligil GS. Loss of the tuberous sclerosis complex tumor suppressors triggers the unfolded protein response to regulate insulin signaling and apoptosis. Mol. Cell. 2008;29:541–551. doi: 10.1016/j.molcel.2007.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 39.Deberardinis RJ, Sayed N, Ditsworth D, Thompson CB. Brick by brick: metabolism and tumor cell growth. Curr. Opin. Gen Dev. 2008;18:54–61. doi: 10.1016/j.gde.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Toschi A, Lee E, Thompson S, Gadir N, Yellen P, Drain CM, Ohh M, Foster DA. Phospholipase D-mTOR requirement for the Warburg effect in human cancer cells. Cancer Lett. 2010;299:72–79. doi: 10.1016/j.canlet.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christofk HR, Vander Heiden MG, Harris MH, Ramanathan A, Gerszten RE, Wei R, Fleming MD, Schreiber SL, Cantley LC. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature. 2008;452:230–233. doi: 10.1038/nature06734. [DOI] [PubMed] [Google Scholar]
- 42.Hitosugi T, Kang S, Vander Heiden MG, Chung TW, Elf S, Lythgoe K, Dong S, Lonial S, Wang X, Chen GZ, Xie J, Gu TL, Polakiewicz RD, Roesel JL, Boggon TJ, Khuri FR, Gilliland DG, Cantley LC, Kaufman J, Chen J. Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2009;2:ra73. doi: 10.1126/scisignal.2000431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Possemato R, Marks KM, Shaul YD, Pacold ME, Kim D, Birsoy K, Sethumadhavan S, Woo HK, Jang HG, Jha AK, Chen WW, Barrett FG, Stransky N, Tsun ZY, Cowley GS, Barretina J, Kalaany NY, Hsu PP, Ottina K, Chan AM, Yuan B, Garraway LA, Root DE, Mino-Kenudson M, Brachtel EF, Driggers EM, Sabatini DM. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature. 2011;476:346–350. doi: 10.1038/nature10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruning NM, Rinnerthaler M, Bluemlein K, Mulleder M, Wamelink MM, Lehrach H, Jakobs C, Breitenbach M, Ralser M. Pyruvate kinase triggers a metabolic feedback loop that controls redox metabolism in respiring cells. Cell Metab. 2011;14:415–427. doi: 10.1016/j.cmet.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Russo GL. Dietary n-6 and n-3 polyunsaturated fatty acids: from biochemistry to clinical implications in cardiovascular prevention. Biochem. Pharm. 2009;77:937–946. doi: 10.1016/j.bcp.2008.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Sardesai VM. The essential fatty acids. Nutr Clin Pract. 1992;7:179–186. doi: 10.1177/0115426592007004179. [DOI] [PubMed] [Google Scholar]
- 48.Zhang C, Wendel AA, Keogh MR, Harris TE, Chen J, Coleman RA. Glycerolipid signals alter mTOR complex 2 (mTORC2) to diminish insulin signaling. Proc. Natl. Acad. Sci. U.S.A. 2012;109:1667–1672. doi: 10.1073/pnas.1110730109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoon MS, Sun Y, Arauz E, Jiang Y, Chen J. Phosphatidic acid activates mammalian target of rapamycin complex 1 (mTORC1) kinase by displacing FK506 binding protein 38 (FKBP38) and exerting an allosteric effect. J. Biol. Chem. 2011;286:29568–29574. doi: 10.1074/jbc.M111.262816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu L, Salloum D, Medlin PS, Saqcena M, Yellen P, Perrella B, Foster DA. Phospholipase D mediates nutrient input to mammalian target of rapamycin complex 1 (mTORC1) J. Biol. Chem. 2011;286:25477–25486. doi: 10.1074/jbc.M111.249631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoon MS, Du G, Backer JM, Frohman MA, Chen J. Class III PI-3-kinase activates phospholipase D in an amino acid-sensing mTORC1 pathway. J. Cell Biol. 2011;195:435–447. doi: 10.1083/jcb.201107033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Veverka V, Crabbe T, Bird I, Lennie G, Muskett FW, Taylor RJ, Carr MD. Structural characterization of the interaction of mTOR with phosphatidic acid and a novel class of inhibitor: compelling evidence for a central role of the FRB domain in small molecule-mediated regulation of mTOR. Oncogene. 2008;27:585–595. doi: 10.1038/sj.onc.1210693. [DOI] [PubMed] [Google Scholar]
- 53.Rodriguez Camargo DC, Link NM, Dames SA. The FKBP-rapamycin binding domain of human TOR undergoes strong conformational changes in the presence of membrane mimetics with and without the regulator phosphatidic acid. Biochemistry. 2012;51:4909–4921. doi: 10.1021/bi3002133. [DOI] [PubMed] [Google Scholar]
- 54.Bjorklund M, Taipale M, Varjosalo M, Saharinen J, Lahdenpera J, Taipale J. Identification of pathways regulating cell size and cell-cycle progression by RNAi. Nature. 2006;439:1009–1013. doi: 10.1038/nature04469. [DOI] [PubMed] [Google Scholar]
- 55.Ray S, Chen Y, Ayoung J, Hanna R, Brazill D. Phospholipase D controls Dictyostelium development by regulating G protein signaling. Cellular signalling. 2011;23:335–343. doi: 10.1016/j.cellsig.2010.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosel D, Khurana T, Majithia A, Huang X, Bhandari R, Kimmel AR. TOR complex 2 (TORC2) in Dictyostelium suppresses phagocytic nutrient capture independently of TORC1-mediated nutrient sensing. J. Cell Sci. 2012;125:37–48. doi: 10.1242/jcs.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ghosh S, Moore S, Bell RM, Dush M. Functional analysis of a phosphatidic acid binding domain in human Raf-1 kinase: mutations in the phosphatidate binding domain lead to tail and trunk abnormalities in developing zebrafish embryos. J. Biol. Chem. 2003;278:45690–45696. doi: 10.1074/jbc.M302933200. [DOI] [PubMed] [Google Scholar]
- 58.Goi T, Shipitsin M, Lu Z, Foster DA, Klinz SG, Feig LA. An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. The EMBO J. 2000;19:623–630. doi: 10.1093/emboj/19.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jiang H, Luo JQ, Urano T, Frankel P, Lu Z, Foster DA, Feig LA. Involvement of Ral GTPase in v-Src-induced phospholipase D activation. Nature. 1995;378:409–412. doi: 10.1038/378409a0. [DOI] [PubMed] [Google Scholar]
- 60.Maehama T, Tanaka M, Nishina H, Murakami M, Kanaho Y, Hanada K. RalA functions as an indispensable signal mediator for the nutrient-sensing system. J. Biol. Chem. 2008;283:35053–35059. doi: 10.1074/jbc.M805822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wiczer BM, Thomas G. Phospholipase D and mTORC1: nutrients are what bring them together. Sci. Signal. 2012;5:pe13. doi: 10.1126/scisignal.2003019. [DOI] [PubMed] [Google Scholar]
- 62.Zhong M, Shen Y, Zheng Y, Joseph T, Jackson D, Foster DA. Phospholipase D prevents apoptosis in v-Src-transformed rat fibroblasts and MDA-MB-231 breast cancer cells. Biochem. Biophys. Res. Comm. 2003;302:615–619. doi: 10.1016/s0006-291x(03)00229-8. [DOI] [PubMed] [Google Scholar]
- 63.Chen Y, Rodrik V, Foster DA. Alternative phospholipase D/mTOR survival signal in human breast cancer cells. Oncogene. 2005;24:672–679. doi: 10.1038/sj.onc.1208099. [DOI] [PubMed] [Google Scholar]
- 64.Rodrik V, Zheng Y, Harrow F, Chen Y, Foster DA. Survival signals generated by estrogen and phospholipase D in MCF-7 breast cancer cells are dependent on Myc. Mol. Cell. Biol. 2005;25:7917–7925. doi: 10.1128/MCB.25.17.7917-7925.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Rodrik V, Gomes E, Hui L, Rockwell P, Foster DA. Myc stabilization in response to estrogen and phospholipase D in MCF-7 breast cancer cells. FEBS Lett. 2006;580:5647–5652. doi: 10.1016/j.febslet.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hui L, Rodrik V, Pielak RM, Knirr S, Zheng Y, Foster DA. mTOR-dependent suppression of protein phosphatase 2A is critical for phospholipase D survival signals in human breast cancer cells. J. Biol. Chem. 2005;280:35829–35835. doi: 10.1074/jbc.M504192200. [DOI] [PubMed] [Google Scholar]
- 67.Zheng Y, Rodrik V, Toschi A, Shi M, Hui L, Shen Y, Foster DA. Phospholipase D couples survival and migration signals in stress response of human cancer cells. J. Biol. Chem. 2006;281:15862–15868. doi: 10.1074/jbc.M600660200. [DOI] [PubMed] [Google Scholar]
- 68.Shi M, Zheng Y, Garcia A, Xu L, Foster DA. Phospholipase D provides a survival signal in human cancer cells with activated H-Ras or K-Ras. Cancer Lett. 2007;258:268–275. doi: 10.1016/j.canlet.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Garcia A, Zheng Y, Zhao C, Toschi A, Fan J, Shraibman N, Brown HA, Bar-Sagi D, Foster DA, Arbiser JL. Honokiol suppresses survival signals mediated by Ras-dependent phospholipase D activity in human cancer cells. Clin. Cancer Res. 2008;14:4267–4274. doi: 10.1158/1078-0432.CCR-08-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wolf I, O'Kelly J, Wakimoto N, Nguyen A, Amblard F, Karlan BY, Arbiser JL, Koeffler HP. Honokiol, a natural biphenyl, inhibits in vitro and in vivo growth of breast cancer through induction of apoptosis and cell cycle arrest. Int. J. Oncol. 2007;30:1529–1537. [PubMed] [Google Scholar]
- 71.Lavieri RR, Scott SA, Selvy PE, Kim K, Jadhav S, Morrison RD, Daniels JS, Brown HA, Lindsley CW. Design, synthesis, and biological evaluation of halogenated N-(2-(4-oxo-1-phenyl-1,3,8-triazaspiro[4.5]decan-8-yl)ethyl)benzamides: discovery of an isoform-selective small molecule phospholipase D2 inhibitor. J. Med. Chem. 2010;53:6706–6719. doi: 10.1021/jm100814g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lewis JA, Scott SA, Lavieri R, Buck JR, Selvy PE, Stoops SL, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity. Bioorg. Med. Chem. Lett. 2009;19:1916–1920. doi: 10.1016/j.bmcl.2009.02.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lavieri R, Scott SA, Lewis JA, Selvy PE, Armstrong MD, Brown HA, Lindsley CW. Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part II. Identification of the 1,3,8-triazaspiro[4,5]decan-4-one privileged structure that engenders PLD2 selectivity. Bioorg. Med. Chem. Lett. 2009;19:2240–2243. doi: 10.1016/j.bmcl.2009.02.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nature Rev. Cancer. 2007;7:763–777. doi: 10.1038/nrc2222. [DOI] [PubMed] [Google Scholar]