Abstract
This study was designed to test the hypothesis that in vivo Magnetic Resonance Imaging (MRI) and Spectroscopy (MRS) can detect in adulthood the neurotoxic effects of a single exposure of prepubertal guinea pigs to the organophosphorus pesticide chlorpyrifos. Twelve female guinea pigs were given either a single dose of chlorpyrifos (0.6xLD50 or 300 mg/kg, sc) or peanut oil (vehicle; 0.5 ml/kg, sc) at 35–40 days of age. One year after the exposure, the animals were tested in the Morris water maze. Three days after the end of the behavioral testing, the metabolic and structural integrity of the brain of the animals was examined by means of MRI/MRS. In the Morris water maze, the chlorpyrifos-exposed guinea pigs showed significant memory deficit. Although no significant anatomical differences were found between the chlorpyrifos-exposed guinea pigs and the control animals by in vivo MRI, the chlorpyrifos-exposed animals showed significant decreases in hippocampal myo-inositol concentration using MRS. The present results indicate that a single sub-lethal exposure of prepubertal guinea pigs to the organophosphorus pesticide chlorpyrifos can lead to long-term memory deficits that are accompanied by significant reductions in the levels of hippocampal myo-inositol.
Keywords: Chlorpyrifos, Magnetic resonance imaging, 1H magnetic resonance spectroscopy, Myo-inositol, Hippocampus
1. Introduction
One of the drawbacks of an increasingly modernized agricultural society is the exposure of the human population to the chemical agents used to maintain it. Though banned for residential use, organophosphorus compounds such as chlorpyrifos are widely used as agricultural insecticides, and are known to have toxic effects on the developing human brain (Marks, et al., 2010; Rauh et al., 2006). Chlorpyrifos acts as an irreversible inhibitor of the enzyme acetylcholinesterase (AChE), which catalyzes the hydrolysis of the neurotransmitter acetylcholine, and as such, is vital for regulating the function of the cholinergic system in the peripheral and central nervous systems. In humans, exposure to low levels of chlorpyrifos can occur via ingestion of contaminated food, inhalation of airborne particles, contact with household dust and residues, and living near agricultural fields treated with the compound (Fenske, et al., 2002).
The presence of chlorpyrifos in the environment poses a particular danger to children. In the general US population, urine metabolites associated with chlorpyrifos are higher in children (6 to 11 years) than in adults (Crinnion, 2010; Lambert et al., 2005). Children exposed to chlorpyrifos while in the womb have an increased risk of delays in mental and motor development at age three and an increased occurrence of pervasive developmental disorders such as attention deficit hyperactivity disorder (ADHD) (Rauh et al., 2006). Recent studies in rodents exposed to chlorpyrifos show that altered neurogenesis and neurotransmission may occur even without overt signs of cholinergic toxicity (Aldridge et al., 2005; Betancourt et al., 2006; Howard et al., 2005; Ricceri et al., 2006; Roy et al., 2005; Slotkin et al., 2006a, 2006b). Chlorpyrifos can also disrupt the developing brain during glial cell proliferation and differentiation which can further contribute to alterations in myelin synthesis, changes in synaptic plasticity, and in general lead to abnormalities in morphology (Garcia et al., 2005). Prenatal exposure to low level organophosphorus pesticides and in particular chlorpyrifos have been shown to be associated with poor intellectual development in 7-year old children and increased frontal and parietal cortical thinning with increased exposure (Bouchard et al. 2011; Rauh et al., 2012). The evidence of increased exposure of children to organophosphorus pesticides correlating with increased incidence of neurological deficits highlights the importance of detecting abnormalities in brain metabolites that may be used as prognostic measures of the severity of the intoxication. Given the near ubiquitous presence of these pesticides in the modern environment (Crinnion, 2010), it is recognized that there is a need to improve the detection and monitoring of their subtle, yet detrimental neurological effects.
The sensitivity, precision, and non-invasive nature of MRI makes it a valuable in vivo method to analyze the temporal and spatial evolution of brain pathology following exposure of guinea pigs to organophosphorus compounds (Gullapalli et al, 2010). Specifically, it has been demonstrated that T2 is significantly shortened in different brain regions of guinea pigs exposed to this nerve agent. Measurements of transverse relaxation time (T2 or T2*) were proven to be very sensitive in detecting disruption of the structural integrity of the brain following a single exposure to soman. T2* mapping technique is a preferred magnetic resonance imaging (MRI) method used to measure iron concentrations indirectly, with higher T2* relaxation times being associated with lower iron levels (House, et al., 2007; Langkammer, et al., 2010). Abnormal iron accumulation in brain tissue is strongly linked to oxidative stress and neurodegenerative disorders such as Parkinson's and Alzheimer's diseases (Brass, et al., 2006; Berg, et al., 2006). It is possible that neurodegeneration stemming from chlorpyrifos exposure may be detectable with this method, with affected brain areas being characterized by shortened T2* relaxation times.
In addition to MRI having high sensitivity to detect subtle structural brain changes induced by neurotoxins, localized in vivo 1H MRS provides a unique opportunity for non-invasively measuring neurotoxicant-induced alterations in brain metabolism (Xu et al., 2011). A wealth of neurochemical information can be obtained from high resolution 1H MRS of the brain.
N-acetylaspartate (NAA), one of the most salient metabolites in the spectrum, is an amino acid specific to neurons, and as such, is indicative of their presence and level of function (Tallan et al., 1956, Birken et al. 1989). In a similar manner, myo-inositol (mI) is a vital component of the phosphatidylinositol second messenger system and is a reliable marker of astrocytes (Kim, et al., 2005; Brand, et al., 1993). Choline (glycerophosphocholine + phosphocholine, GPC+PCh) is known to be associated with cellular density and membrane turnover (Miller, et al., 1996), in addition to being the precursor of acetylcholine, a neurotransmitter greatly affected by chlorpyrifos (Cohen & Wurtman, 1975). Glutamate (Glu) and glutamine (Gln) are associated with neurotransmission, with Glu being the most abundant excitatory neurotransmitter. Glu plays a key role in long-term potentiation, a cellular mechanism underlying learning and memory (Riedel, et al., 2003; McEntee & Crook, 1993), though excess of Glu in the brain has also been associated with excitotoxicity. Gln is vital to cerebral function, being involved in detoxification and regulation of neurotransmitter activities. Gln is synthesized from Glu by glutamine synthetase in astrocytes (Ross, et al., 1991). Together, these common and reliably detectable metabolites along with structural assessment can provide important information on the metabolic and functional integrity of the brain.
In the present study we examined the effect of a single sub-lethal exposure of prepubertal guinea pigs to chlorpyrifos on the structural and biochemical integrity of the brain in adulthood. Guinea pigs (Cavia porcellus) were selected as the ideal animal model in this study, due both to their similarities to humans in terms of relative brain development after birth, and the sensitivity of their cholinergic system to organophosphorus pesticides. Specifically, rats and mice have higher levels of circulating carboxylesterases, enzymes that hydrolyze and inactivate organophosphorus agents, making them a far less sensitive model of exposure to organophosphorus toxicants (Albuquerque et al., 2006 ; Fonnum, et al., 1985; Ecobichon, Dykeman, & Hansell, 1978). Guinea pigs also give birth to young that are neuroanatomically mature and are a closer match to humans in the timing of prenatal and postnatal development (Dobbing & Sands, 1970).
2. Methods
2.1 Animal Model
A total of 12 female Hartley Guinea Pigs ([Crl(HA)Br]; 35–40 days old) were obtained from Charles River Laboratories (Wilmington, MA) and acclimated for a period of seven days before treatment. Animals were kept in a light- and temperature-controlled animal care facility, with food and water provided ad libitum. Individual animals were randomly and equally assigned to either the chlorpyrifos treatment group or the vehicle group. The treatment group was injected subcutaneously between the shoulder blades with chlorpyrifos (ChemService, West Chester, PA) dissolved in peanut oil (300 mg/kg, from Sigma Aldrich, St.Louis, MO), and the vehicle group was injected with peanut oil (0.5 ml/kg, sc).
The oral LD50 of chlorpyrifos in guinea pigs is 504 mg/kg, and in general, the oral and subcutaneous LD50s of organophosphorus compounds are very similar (McCollister, et al, 1974). Thus, the dose of chlorpyrifos used here corresponds to approximately 0.6×LD50, which has been reported to be below the threshold for organophosphorus-induced seizures (Shih et al., 1997) Animals were dosed with chlorpyrifos subcutaneously rather than orally because the soft palate of the guinea pig is continuous with the base of the tongue, and the opening through which to pass a feeding tube is very small. Despite all the care to avoid damage to the oral cavity, gavage is very stressful to guinea pigs and interferes with their normal feeding behavior.
Animals were monitored every 15 minutes after treatment for the first 2 hours, and then every hour for the next 8 hours. No animals displayed signs of overt toxicity. The Morris Water Maze (MWM) test and in vivo MRI/MRS scans were performed one year after administration. All experiments were carried out in accordance with the rules and regulations set forth by the University of Maryland School of Medicine Institutional Animal Care and Use Committee regarding the care and use of animals under a protocol approved by the committee, and complied with the principles of the '1996 Guide for the Care and Use of Laboratory Animals.
2.2 Morris Water Maze Test
At 11 months after chlorpyrifos or vehicle administration, the MWM, a behavioral task that is dependent on the integrity of hippocampal functioning (Morris, 1984), was used to assess the cognitive performance of the animals. Animals were tested according to the protocol described in Mamczarz et al, 2011. A large circular galvanized tub (180-cm diameter, 60-cm height) was filled with tap water mixed with non-toxic black tempera paint to a depth of 40 cm. The tub was divided virtually into four quadrants. Upon placement in the water, the animals were allowed to swim to find a hidden and submerged platform (27 cm in diameter) placed in the center of one of the quadrants. Navigation to the platform was aided by visual cues in the room visible from the animal's perspective in the maze.
Animals underwent five consecutive training sessions consisting of four swimming trials per day, with the probe test performed 72 hours after the last learning trial. During the training sessions, a maximum of 90 seconds was allowed for the animals to find the submerged platform. After the animal remained on the platform for 15s, it was removed to a shredded paper-bedded cage with a ceramic heat lamp until dry. The starting quadrant was assigned pseudo-randomly for each trial, while the submerged platform was kept in a constant position. During the probe test, the platform was removed from the tub and the animals were allowed to swim for 90s. A video camera coupled with the video tracking program Any-Maze (Stoelting Co, Wood Dale IL) was used to record and track the location of the animals during the experiment. The time to reach the submerged platform (referred to as escape latency) was recorded for each trial in each training session. The mean escape latency was taken as a learning index as described inMamczarz et al. (2011). In addition, the pattern of swimming, including the number of crossings in the area where the platform used to be located, was analyzed during the probe test. If the animals retain memory of the platform position acquired during training, they will show bias toward swimming close to area where the platform used to be located.
2.3 In vivo MRI/MRS Procedures
At the end of the behavioral tests, animals were subjected to in vivo imaging. All MRI/MRS experiments were performed on a Bruker Biospec 7.0 Tesla 30 cm horizontal bore scanner (Bruker Biospin MRI GmbH, Germany). The system was equipped with a BGA20S gradient system capable of producing 200-mT/m gradient field and interfaced to a Bruker Paravision 5.0 console. A Bruker fourelement 1H surface coil array was used as the receiver and a Bruker 154 mm inner diameter circular coil as the transmitter. Each guinea pig was anesthetized in an animal chamber using a gas mixture of O2 (1 L/min) and isoflurane (4%; IsoFlo, Abbot Laboratories, North Chicago, IL). The animal was then placed prone in an animal holder and the radiofrequency (RF) coil was positioned and fixed over the brain of the animal. The animal holder was moved to the center of the magnet for imaging and the isoflurane level was changed to 3%. The level of isofluorane was further adjusted based on the respiration rate changes of the animal for the remainder of the experiment. An MR-compatible small-animal monitoring and gating system (SA Instruments, Inc., New York, USA) was used to monitor the animal respiration rate and body temperature. The animal body temperature was maintained at 36–37°C using warm water circulation through a heating pad.
A scout image consisting of three slices (one each in the axial, mid-sagittal, and coronal plane) was used to localize the guinea pig brain. A rapid shimming protocol (FASTMAP) was used to reduce the external magnetic field inhomogeneity within the region of interest (Gruetter, 1993). A Rapid Acquisition with Relaxation Enhancement (RARE) sequence was used to obtain T2-weighted MR images with repetition time/effective echo time (TR/TEeff) = 6197/60 ms, echo train length = 8, field of view (FOV) = 35 × 35 mm2, matrix size = 256 × 256, slice thickness = 1 mm, number of slices = 24, and number of averages = 2, in the coronal plane.
T2* Images were obtained on three vehicle treated and four chlorpyrifos treated guinea pigs using thirteen gradient refocused echoes with the TE of the first echo being 4.5 ms and the remaining echoes being spaced 5.5 ms apart. Four contiguous coronal slices were placed to cover the bulk of the striatum, hippocampus, and thalamus. These images were obtained at a TR of 1.5 s covering 35 mm FOV using 2 mm slice thickness at a matrix resolution of 112 × 112 using four averages in the coronal plane.
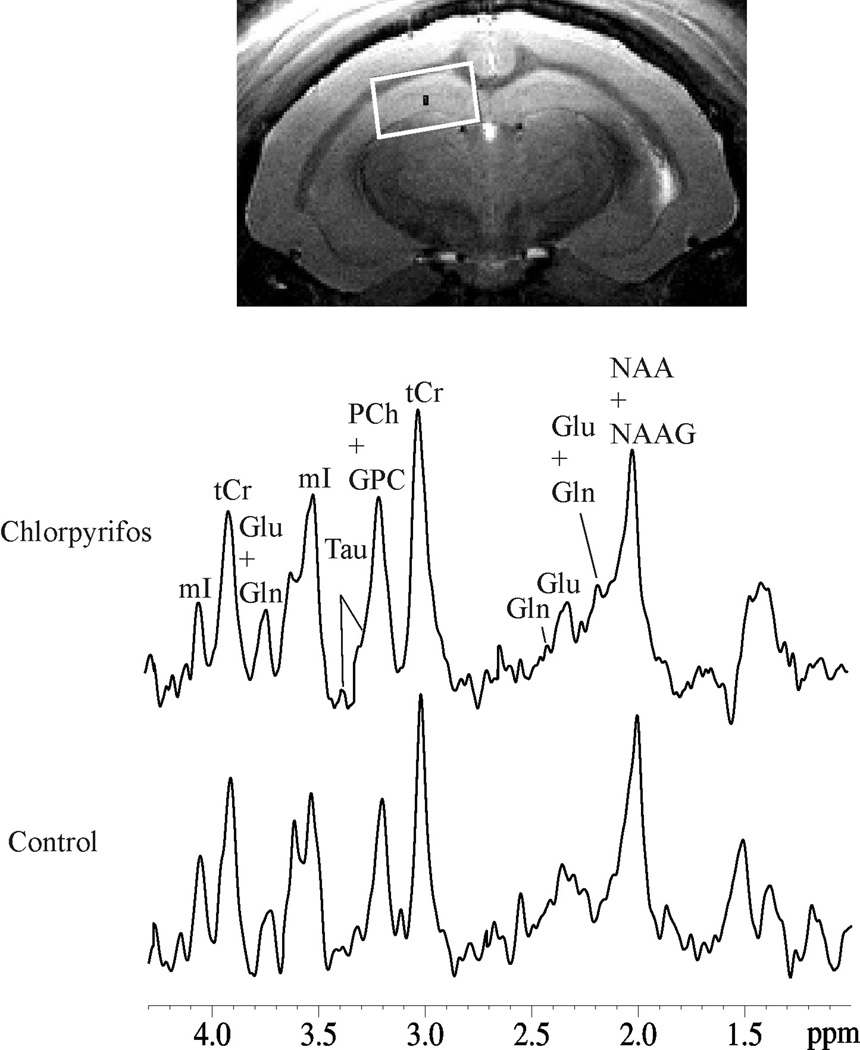
For 1H MRS, adjustments of all first- and second-order shims over the voxel of interest were accomplished with the FASTMAP procedure. Typically, the in vivo shimming procedure resulted in approximately 10.8 to 11.7 Hz full-width half maximum line-width of the unsuppressed water peak over the spectroscopy voxel. The water signal was suppressed by variable power RF pulses with optimized relaxation delays. Outer volume suppression combined with point-resolved spectroscopy (PRESS) sequence was used for signal acquisition, with TR/TE = 2500/20 ms, spectral bandwidth = 4 kHz, number of data points = 2048, number of averages = 600. Localized 1H MRS were acquired from the right hippocampus (3 × 5 × 2 mm3; Figure 2) for each of the guinea pigs. MRS data were acquired immediately following the structural MRI acquisition.
Figure 2.
Localized in vivo 1H spectra in hippocampus from the control and chlorpyrifos-treated guinea pigs. The spectroscopy voxel is indicated by the white box in the T2-weighted images. Creatine (tCr), glutamate (Glu), glutamine (Gln), glycerophosphorylcholine (GPC), myo-inositol (mI), N-acetylaspartate (NAA), phosphocreatine (PCr), phosphocholine (PCh), and taurine (Tau).
2.4 MR Image and Spectrum Processing
Brain volume measurements were made in the hippocampus, parenchyma, and ventricular regions. The volumes of parenchymal (white + gray) and ventricular areas were calculated with the userguided tools available within Medical Image Processing, Analysis, and Visualization software (MIPAV v5.3.1, CIT, NIH, Bethesda, MD; McCauliffe et al., 2001). Hippocampal volume was outlined manually on coronal T2-weighted MR images. Anatomical areas were verified with the guinea pig atlas published by Rapisarda & Bacchelli (1977). Manual tracing on coronal T2-weighted MR images was used to differentiate cerebrospinal fluid (CSF) and sinus areas from ventricles, and to delineate the borders of the hippocampus. T2* values were computed in select ROIs (amygdala, cortex, hippocampus, striatum, and thalamus) were measured with a home-made MATLAB procedure. 1H MRS data were fitted using the Linear Combination of Model spectra (LCModel) package (Provencher, 2001). The mean metabolite concentration relative to total Creatine (tCr) was calculated for later statistical analysis of spectroscopy data.
2.5 Statistical Analysis
Results obtained from chlorpyrifos- and peanut oil-exposed guinea pigs were compared using unpaired Student's t-test. Pearson's ‘r’ was used to assess correlations between Morris Water Maze latencies and MRI modalities. For spectroscopy data, Cramér-Rao lower bounds (CRLB) as reported from the LCModel analysis were used for assessing the reliability of the major metabolites. Only metabolites with a CRLB ≤ 20 % were included in the analysis.
3. Results and Discussion
Figure 1 illustrates the anatomic T2-weighted images which covered the main regions of interest in this study from a chlorpyrifos-injected and a vehicle-injected guinea pig, respectively. The T2- weighted MR images did not display any apparent qualitative abnormalities between the animals injected with chlorpyrifos and vehicle. No gross volumetric differences existed between the whole brain parenchyma of animals in either group (3397.1 ± 48.5 mm3 in chlorpyrifos treated vs 3308.7 ± 51.3 mm3 in control animals; p = 0.24). Segmentation of the ventricular areas also revealed no significant difference (56.8 ± 4.9 mm3 in chlorpyrifos treated vs 56.5 ± 5.1 mm3 in control animals; p = 0.97). There were no group differences (225.1 ± 15.4 mm3 in chlorpyrifos treated vs 229.5 ± 3.1 mm3 in control animals; p = 0.5) between the volumes of the hippocampus using manual ROI-based delineation or when the hippocampus was normalized to the whole brain volume (Parenchyma + Ventricular areas). T2* values were also comparable between chlorpyrifos- and saline-injected guinea pigs (Table 1). However, a trend towards shortened T2* relaxation times was observed in the hippocampus, striatum, and amygdala in the group of chlorpyrifos-injected animals which may suggest an accumulation of iron in these regions.
Figure 1.
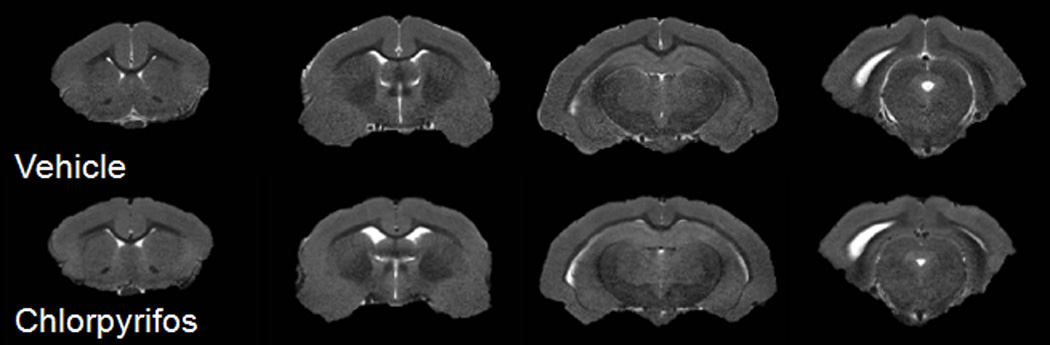
Axial T2-weighted images from the brain of a vehicle (top row) and a chlorpyrifos treated guinea pig (bottom row).
Table 1.
T2* values (mean ± SE) in the different regions of the guinea pig brains in vehicle- (n=3) and chlorpyrifos- (n=4) treated groups.
| Region | Vehicle Treated (ms) |
Chlorpyrifos Treated (ms) |
p-value |
|---|---|---|---|
| Amygdala | 17.3±2.7 | 15.0±2.0 | 0.26 |
| Cortex | 21.4±2.2 | 24.8±2.0 | 0.30 |
| Hippocampus | 29.8±6.8 | 25.4±4.5 | 0.60 |
| Striatum | 32.9±1.6 | 28.7±1.8 | 0.48 |
| Thalamus | 25.2±3.6 | 24.4±3.2 | 0.86 |
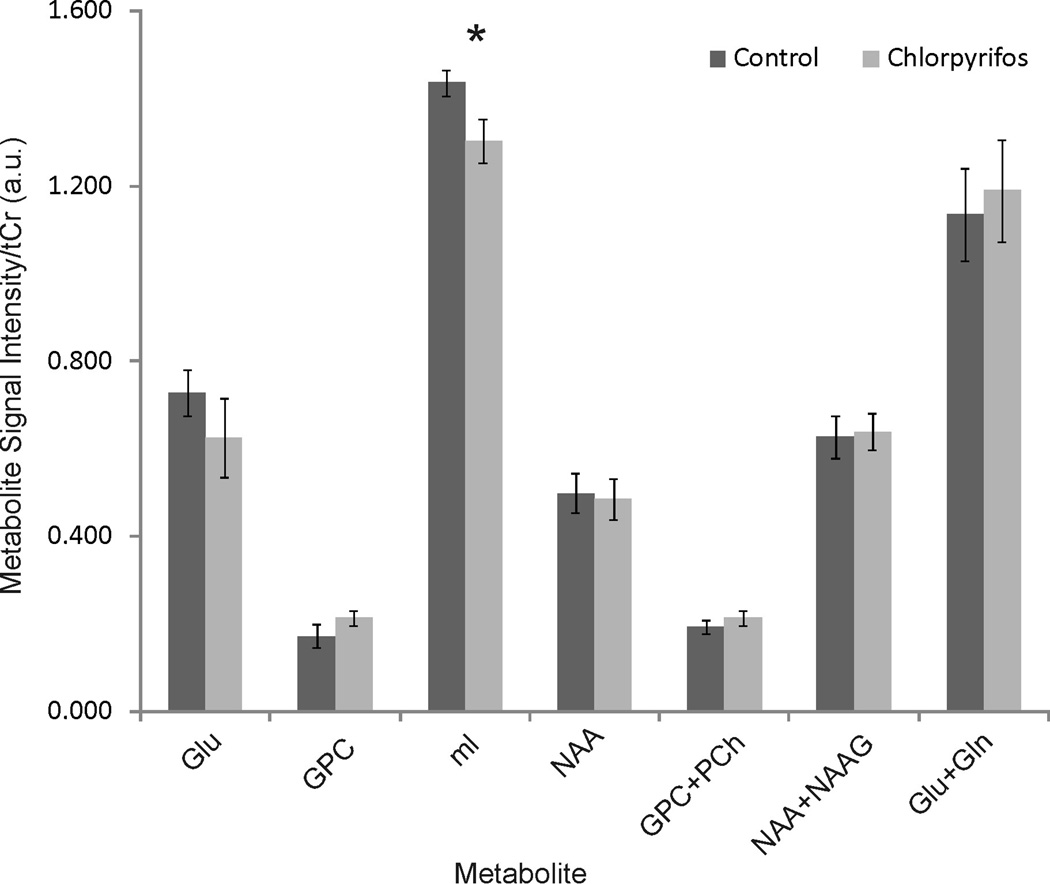
Examples of typical in vivo 1H MR spectra of the chlorpyrifos- and vehicle-injected guinea pigs are shown for the hippocampus in Figure 2. The spectroscopic voxel predominantly covers the hippocampus as shown in this figure indicated by the white box in the anatomic MR image. The spectra provided neurochemical profile of 10 metabolites, and only those metabolites with CRLB below 20 % were quantified. There were no statistically significant differences in Gln, Glu, Glu + Gln, GPC, GPC + PCh, NAA, and NAA + N-acetylaspartate glutamate (NAAG) levels in the hippocampus (Figure 3). In addition, levels of these metabolites in the striatum were not significantly different between the two groups. However, quantitative analysis of the spectral data showed that levels of myo-inositol (mI) were significantly lower in the hippocampi of chlorpyrifos-injected animals compared to control (vehicle-injected) animals (Figure 3).
Figure 3.
Metabolite to total creatine ratios (Mean ± SD) measured in the hippocampus from animals injected with peanut oil or chlorpyrifos. Error bars represent standard deviation. Gln: glutamine, Glu: glutamate, GPC: Glycerophosphocholine, mI: myo-inisotol, NAA: Nacetylaspartate, NAAG: N-acetylaspartyl glutamate, and PCh: Phosphocholine.
* indicates p< 0.05.
Performance in the MWM was significantly different between chlorpyrifos- and peanut oilinjected animals and the chlorpyrifos treated animals also showed significant memory retention impairment in the MWM test. The learning index of chlorpyrifos-injected animals was significantly larger than that measured with saline-injected animals (72.4 ± 16.5 s vs. 51.2 ± 8.6 s, p < 0.05). In addition, during the probe test, chlorpyrifos-injected guinea pigs showed significantly less bias towards the platform area in the training quadrant than did control animals. Significant reduction (p < 0.05) in the number of crossings of the target platform area in the group of animals injected with chlorpyrifos (1.5± 0.5) was observed compared to the control group (3.8±0.9). Taken together, these results suggest an impairment of hippocampal-dependent cognitive processing in chlorpyrifos-injected animals. Bivariate partial correlations revealed a trend between the learning indices and mI values from 1H MRS on both hippocampus (r = −.56, p = .06) and striatum (r = .50, p = .099) for the chlorpyrifos-injected animals.
Myo-inositol (mI) is actively used in neurons, but is primarily stored and thus detected in astrocytes. While pragmatically viewed as a useful marker of glial cells in 1H MR spectroscopy, mI actually serves several important biological roles: as an osmolyte (Thurston et al., 1989), as a growth factor (Ross, 1991), as a precursor of membrane phospholipids (Holub, 1986), and as a vital precursor molecule in the phosphatidoinositol (PI) signaling system (Berridge, et al., 1989; Kim et al., 2005). It is the role of mI in the PI cycle and PI3K/Akt signal transduction that best explains the results from this study.
In neurons, the PI cycle causes a signal cascade that results in activation of protein kinase C (PKC) and the release of the intracellular calcium stores, easing depolarization and subsequent neuronal firing. Impairment of this cycle due to lack of mI precursors, may result in inactivation of the affected neurons, as they have less access to the calcium stores used to sensitize their firing threshold. An example of this same phenomenon is seen in the effects of lithium and valproic acid (VPA) acting to deplete mI in the cerebral cortex of patients with bipolar symptoms (Harwood, 2005). The effects of such neuronal inactivation in the hippocampus could explain the deficits in the hippocampal-dependent water maze task seen in the current study. It is also noteworthy that mI is phosphorylated by the enzyme phosphoinositide 3-kinase (PI3K). PI3K is a family of signal transducer enzymes that promote cell survival by phosphorylating and inhibiting proapoptotic proteins. PI3K mediates the prosurvival action of neurotoxic N-methyl-D-aspartate in cerebellar granule neurons (Zhang et al., 1998). The decreased mI in hippocampus of chlorpyrifos-injected animals could lead to alterations in PI level, and potential alterations in the PI3K signaling pathways. Lithium has been shown to act on PI3K (Kang et al., 2003) and protect phencyclidine-induced neurotoxicity in the developing brain (Xia et al. 2008). In addition, PI3K has also been implicated in long-term potentiation which is widely considered one of the major cellular mechanisms that underlies learning and memory (Bliss and Collingridge, 1993; Cooke and Bliss, 2006). The alteration of the PI3K signaling pathway by decreased mI level may be important determinants of the memory deficits seen in chlorpyrifos-injected animals.
The metabolic changes observed at one year after exposure to chlorpyrifos treated animals without any accompanying morphological changes is particularly interesting. The lack of morphological changes is consistent with the observation of Aldridge, et al., (2005), who demonstrated no significant changes in the weights of various brain regions weights at 5 months after exposure following administration of 1 mg/kg chlorpyrifos daily to neonatal rats on postnatal days 1–4. Furthermore, they found global upregulation of 5HT-related synaptic proteins and suggest that the effect of chlorpyrifos exposure on the 5HT system may be permanent. Although, unrelated, a relatively recent study demonstrated an inverse relationship between 5HT activity and mI using proton MRS upon 3,4- methylenedioxymethamphetamine (MDMA) administration in rats (Perrine et al., 2010). Decreases in mI, one year after pre-pubertal exposure to chlorpyrifos in this study may be a result of the long-term effect on the serotonin system which indirectly affects the phosphotidyl inositol signaling pathways.
Although it was only possible to obtain T2* measurement on a few animals (3 controls, 4 chlorpyrifos), and no conclusive statements can be made, the idea of a relation between iron content and mI is intriguing because of the known action of some inositol phosphates in reducing cellular iron uptake by chelation (Skoglund, et al., 1999, Brune et al., 1992). Inositol tri- and tetraphosphates similar to those found in the PI cycle are also known to modulate iron uptake and absorption (Han et al., 1994). The current study showed a non-significant trend towards increased iron concentrations coinciding with the significantly decreased mI concentration. Accumulation of iron has been implicated in various neurological disorders including multiple sclerosis, Parkinson’s, and Alzheimer’s disease (Du et al., 2011; Khalil et al., 2011; Railey et al., 2011), While any conclusion from the observation of the link between mI and possible increased brain iron would be premature, the relationship between the two should be explored further with a larger sample size as it may provide important insights into the long-term predisposition for the development of neurological deficits following an exposure to pesticides at early ages.
Conclusion
In this study, we demonstrated a decrease of mI content in the hippocampus one year after a single administration of chlorpyrifos (0.6×LD50) to young guinea pigs using in vivo 1H MR spectroscopy. The reduction of hippocampal mI levels correlated with the severity of the memory deficits that were detected in the MWM, suggesting that even a single exposure to a sublethal dose of chlorpyrifos during adolescence can lead to long lasting memory impairments. Our findings suggest that the effects of an exposure to chlorpyrifos exposure are most evident in the astrocytic glial cells of the hippocampus and may affect the phosphotidyl inositol signaling pathways. As there were no volumetric differences between the control and chlorpyrifos groups, the mI decrease may be a result of a deficit in astrocytic function rather than loss of astrocytes. This notion is further supported by the trend to reduced T2* in several brain regions, including the hippocampus. Further studies on the time course of changes in brain metabolites in vivo, and its relationship to T2* changes may provide important information on the pathophysiology of CNS changes following single and continuous sub-lethal exposure to pesticides.
Acknowledgements
This study was partly supported by a grant 5R01ES019282 from the National Institute of Environmental Health Sciences. RJM is supported by a Training grant in Molecular and Mechanistic Toxicology (T32 ES00726; PI: Albuquerque)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albuquerque EX, Pereira EFR, Aracava Y, et al. Effective countermeasure against poisoning by organophosphorus insecticides and nerve agents. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(35):13220–13225. doi: 10.1073/pnas.0605370103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in Central Nervous System Serotonergic and Dopaminergic Synaptic Activity in Adulthood after Prenatal or Neonatal Chlorpyrifos Exposure. Environmental Health Perspectives. 2005;113(8):1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Youdim MBH. Role of iron in neurodegenerative disorders. Topics in magnetic resonance imaging : TMRI. 2006;17(1):5–17. doi: 10.1097/01.rmr.0000245461.90406.ad. [DOI] [PubMed] [Google Scholar]
- Berridge MJ, Irvine RF. Inositol phosphates and cell signalling. Nature. 1989;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Betancourt AM, Burgess SC, Carr RL. Effect of developmental exposure to chlorpyrifos on the expression of neurotrophin growth factors and cell-specific markers in neonatal rat brain. Toxicological sciences : an official journal of the Society of Toxicology. 2006;92(2):500–506. doi: 10.1093/toxsci/kfl004. [DOI] [PubMed] [Google Scholar]
- Birken DL, Oldendorf WH. N-acetyl-L-aspartic acid: a literature review of a compound prominent in 1H-NMR spectroscopic studies of brain. Neuroscience and biobehavioral reviews. 1989;13(1):23–31. doi: 10.1016/s0149-7634(89)80048-x. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361(6407):31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Brand A, Richter-Landsberg C, Leibfritz D. Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Developmental neuroscience. 1993;15(3–5):289–298. doi: 10.1159/000111347. [DOI] [PubMed] [Google Scholar]
- Brass SD, Chen N, Mulkern RV, Bakshi R. Magnetic resonance imaging of iron deposition in neurological disorders. Topics in magnetic resonance imaging : TMRI. 2006;17(1):31–40. doi: 10.1097/01.rmr.0000245459.82782.e4. [DOI] [PubMed] [Google Scholar]
- Brune M, Rossander-Hultén L, Hallberg L, Gleerup A, Sandberg AS. Iron absorption from bread in humans: inhibiting effects of cereal fiber, phytate and inositol phosphates with different numbers of phosphate groups. The Journal of nutrition. 1992;122(3):442–449. doi: 10.1093/jn/122.3.442. [DOI] [PubMed] [Google Scholar]
- CDC. Fourth National Report on Human Exposure to Environmental Chemicals. Atlanta, GA: 2009. [PubMed] [Google Scholar]
- Cohen EL, Wurtman RJ. Brain acetylcholine: increase after systemic choline administration. Life sciences. 1975;16(7):1095–1102. doi: 10.1016/0024-3205(75)90194-0. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Bliss TVP. Plasticity in the human central nervous system. Brain : a journal of neurology. 2006;129(Pt 7):1659–1673. doi: 10.1093/brain/awl082. [DOI] [PubMed] [Google Scholar]
- Crinnion WJ. The CDC fourth national report on human exposure to environmental chemicals: what it tells us about our toxic burden and how it assist environmental medicine physicians. Alternative medicine review : a journal of clinical therapeutic. 2010;15(2):101–109. [PubMed] [Google Scholar]
- Dobbing J, Sands J. Growth and development of the brain and spinal cord of the guinea pig. Brain research. 1970;17(1):115–123. doi: 10.1016/0006-8993(70)90311-2. [DOI] [PubMed] [Google Scholar]
- Du G, Lewis MM, Styner M, et al. Combined R2* and diffusion tensor imaging changes in the substantia nigra in Parkinson’s disease. Movement disorders : official journal of the Movement Disorder Society. 2011;26(9):1627–1632. doi: 10.1002/mds.23643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecobichon DJ, Dykeman RW, Hansell MM. The development of hepatic drug-metabolizing enzymes in perinatal guinea pigs: a biochemical and morphological study. Canadian journal of biochemistry. 1978;56(7):738–745. doi: 10.1139/o78-111. [DOI] [PubMed] [Google Scholar]
- Fenske RA, Lu C, Barr D, Needham L. Children’s exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environmental health perspectives. 2002;110(5):549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonnum F, Sterri SH, Aas P, Johnsen H. Carboxylesterases, importance for detoxification of organophosphorus anticholinesterases and trichothecenes. Fundamental and applied toxicology : official journal of the Society of Toxicology. 1985;5(6 Pt 2):S29–S38. doi: 10.1016/0272-0590(85)90112-5. [DOI] [PubMed] [Google Scholar]
- Gruetter R. Automatic, localized in Vivo adjustment of all first-and second-order shim coils. Magnetic Resonance in Medicine. 1993;29(6):804–811. doi: 10.1002/mrm.1910290613. [DOI] [PubMed] [Google Scholar]
- Gullapalli RP, Aracava Y, Zhuo J, et al. Magnetic resonance imaging reveals that galantamine prevents structural brain damage induced by an acute exposure of guinea pigs to soman. Neurotoxicology. 2010;31(1):67–76. doi: 10.1016/j.neuro.2009.09.004. [DOI] [PubMed] [Google Scholar]
- Han O, Failla ML, Hill AD, Morris ER, Smith JC. Inositol phosphates inhibit uptake and transport of iron and zinc by a human intestinal cell line. The Journal of nutrition. 1994;124(4):580–587. doi: 10.1093/jn/124.4.580. [DOI] [PubMed] [Google Scholar]
- Harwood a J. Lithium and bipolar mood disorder: the inositol-depletion hypothesis revisited. Molecular psychiatry. 2005;10(1):117–126. doi: 10.1038/sj.mp.4001618. [DOI] [PubMed] [Google Scholar]
- Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annual review of nutrition. 1986;6:563–597. doi: 10.1146/annurev.nu.06.070186.003023. [DOI] [PubMed] [Google Scholar]
- House MJ, St Pierre TG, Kowdley KV, et al. Correlation of proton transverse relaxation rates (R2) with iron concentrations in postmortem brain tissue from alzheimer’s disease patients. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2007;57(1):172–180. doi: 10.1002/mrm.21118. [DOI] [PubMed] [Google Scholar]
- Howard AS, Bucelli R, Jett DA, Bruun D, Yang D, Lein PJ. Chlorpyrifos exerts opposing effects on axonal and dendritic growth in primary neuronal cultures. Toxicology and applied pharmacology. 2005;207(2):112–124. doi: 10.1016/j.taap.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Noh JS, Bae YS, Gwag BJ. Calcium-dependent prevention of neuronal apoptosis by lithium ion: essential role of phosphoinositide 3-kinase and phospholipase Cgamma. Molecular pharmacology. 2003;64(2):228–234. doi: 10.1124/mol.64.2.228. [DOI] [PubMed] [Google Scholar]
- Khalil M, Langkammer C, Ropele S, et al. Determinants of brain iron in multiple sclerosis: a quantitative 3T MRI study. Neurology. 2011;77(18):1691–1697. doi: 10.1212/WNL.0b013e318236ef0e. [DOI] [PubMed] [Google Scholar]
- Kim H, McGrath BM, Silverstone PH. A review of the possible relevance of inositol and the phosphatidylinositol second messenger system (PI-cycle) to psychiatric disorders--focus on magnetic resonance spectroscopy (MRS) studies. Human psychopharmacology. 2005;20(5):309–326. doi: 10.1002/hup.693. [DOI] [PubMed] [Google Scholar]
- Lambert WE, Lasarev M, Muniz J, et al. Variation in Organophosphate Pesticide Metabolites in Urine of Children Living in Agricultural Communities. Environmental Health Perspectives. 2005;113(4):504–508. doi: 10.1289/ehp.6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langkammer C, Krebs N, Goessler W, et al. Quantitative MR imaging of brain iron: a postmortem validation study. Radiology. 2010;257(2):455–462. doi: 10.1148/radiol.10100495. [DOI] [PubMed] [Google Scholar]
- Mamczarz J, Kulkarni GS, Pereira EFR, Albuquerque EX. Galantamine counteracts development of learning impairment in guinea pigs exposed to the organophosphorus poison soman: clinical significance. Neurotoxicology. 2011;32(6):785–798. doi: 10.1016/j.neuro.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Harley K, Bradman A, et al. Organophosphate pesticide exposure and attention in young Mexican-American children: the CHAMACOS study. Environmental health perspectives. 2010;118(12):1768–1774. doi: 10.1289/ehp.1002056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCollister SB, Kociba RJ, Humiston CG, McCollister DD, Gehring PJ. Studies of the acute and longterm oral toxicity of chlorpyrifos (O,O-diethyl-O-(3,5,6-trichloro-2-pyridyl) phosphorothioate) Food and Cosmetics Toxicology. 1974;12(1):45–61. doi: 10.1016/0015-6264(74)90321-6. [DOI] [PubMed] [Google Scholar]
- McEntee WJ, Crook TH. Glutamate: its role in learning, memory, and the aging brain. Psychopharmacology. 1993;111(4):391–401. doi: 10.1007/BF02253527. [DOI] [PubMed] [Google Scholar]
- Miller BL, Chang L, Booth R, et al. In vivo 1H MRS choline: correlation with in vitro chemistry/histology. Life sciences. 1996;58(22):1929–1935. doi: 10.1016/0024-3205(96)00182-8. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984;11(1):47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Perrine SA, Ghoddoussi F, Michaels MS, Hyde EM, Kuhn DM, Galloway MP. MDMA administration decreases serotonin but not N-acetylaspartate in the rat brain. Neurotoxicology. 2010;31(6):654–661. doi: 10.1016/j.neuro.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeuffer J, Tkác I, Provencher SW, Gruetter R. Toward an in vivo neurochemical profile: quantification of 18 metabolites in short-echo-time (1)H NMR spectra of the rat brain. Journal of magnetic resonance (San Diego, Calif. 1997) 1999;141(1):104–120. doi: 10.1006/jmre.1999.1895. [DOI] [PubMed] [Google Scholar]
- Provencher SW. Automatic quantitation of localized in vivo 1H spectra with LCModel. NMR in biomedicine. 2001;14(4):260–264. doi: 10.1002/nbm.698. [DOI] [PubMed] [Google Scholar]
- Railey AM, Groeber CM, Flinn JM. The effect of metals on spatial memory in a transgenic mouse model of Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2011;24(2):375–381. doi: 10.3233/JAD-2011-101452. [DOI] [PubMed] [Google Scholar]
- Rapisarda C, Bacchelli B. The brain of the guinea pig in stereotaxic coordinates. Archivio di scienze biologiche. 1977;61(1–4):1–37. [PubMed] [Google Scholar]
- Rauh VA, Garfinkel R, Perera FP, et al. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118(6):e1845–e1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Venerosi A, Capone F, et al. Developmental neurotoxicity of organophosphorous pesticides: fetal and neonatal exposure to chlorpyrifos alters sex-specific behaviors at adulthood in mice. Toxicological sciences : an official journal of the Society of Toxicology. 2006;93(1):105–113. doi: 10.1093/toxsci/kfl032. [DOI] [PubMed] [Google Scholar]
- Riedel G, Platt B, Micheau J. Glutamate receptor function in learning and memory. Behavioural brain research. 2003;140(1–2):1–47. doi: 10.1016/s0166-4328(02)00272-3. [DOI] [PubMed] [Google Scholar]
- Ross BD. Biochemical considerations in 1H spectroscopy. Glutamate and glutamine; myo-inositol and related metabolites. NMR in biomedicine. 1991;4(2):59–63. doi: 10.1002/nbm.1940040205. [DOI] [PubMed] [Google Scholar]
- Roy TS, Sharma V, Seidler FJ, Slotkin TA. Quantitative morphological assessment reveals neuronal and glial deficits in hippocampus after a brief subtoxic exposure to chlorpyrifos in neonatal rats. Brain research. Developmental brain research. 2005;155(1):71–80. doi: 10.1016/j.devbrainres.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Shih TM, McDonough JH. Neurochemical mechanisms in soman-induced seizures. Journal of applied toxicology : JAT. 1997;17(4):255–264. doi: 10.1002/(sici)1099-1263(199707)17:4<255::aid-jat441>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Skoglund E, Lönnerdal B, Sandberg A-S. Inositol Phosphates Influence Iron Uptake in Caco-2 Cells. Journal of Agricultural and Food Chemistry. 1999;47(3):1109–1113. doi: 10.1021/jf980745c. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Levin ED, Seidler FJ. Comparative developmental neurotoxicity of organophosphate insecticides: effects on brain development are separable from systemic toxicity. Environmental health perspectives. 2006;114(5):746–751. doi: 10.1289/ehp.8828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin TA, Tate CA, Ryde IT, Levin ED, Seidler FJ. Organophosphate insecticides target the serotonergic system in developing rat brain regions: disparate effects of diazinon and parathion at doses spanning the threshold for cholinesterase inhibition. Environmental health perspectives. 2006;114(10):1542–1546. doi: 10.1289/ehp.9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallan HH, Moore S, Stein WH. N-Acetyl-L-aspartic acid in brain. The Journal of biological chemistry. 1956;219(1):257–264. [PubMed] [Google Scholar]
- Thurston JH, Sherman WR, Hauhart RE, Kloepper RF. myo-inositol: a newly identified nonnitrogenous osmoregulatory molecule in mammalian brain. Pediatric research. 1989;26(5):482–485. doi: 10.1203/00006450-198911000-00024. [DOI] [PubMed] [Google Scholar]
- Xia Y, Wang CZ, Liu J, Anastasio NC, Johnson KM. Lithium protection of phencyclidine-induced neurotoxicity in developing brain: the role of phosphatidylinositol-3 kinase/Akt and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase signaling pathways. The Journal of pharmacology and experimental therapeutics. 2008;326(3):838–848. doi: 10.1124/jpet.107.133272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu S, Zhuo J, Racz J, et al. Early microstructural and metabolic changes following controlled cortical impact injury in rat: a magnetic resonance imaging and spectroscopy study. Journal of neurotrauma. 2011;28(10):2091–2102. doi: 10.1089/neu.2010.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FX, Rubin R, Rooney TA. N-Methyl-D-aspartate inhibits apoptosis through activation of phosphatidylinositol 3-kinase in cerebellar granule neurons. A role for insulin receptor substrate-1 in the neurotrophic action of n-methyl-D-aspartate and its inhibition by ethanol. The Journal of biological chemistry. 1998;273(41):26596–26602. doi: 10.1074/jbc.273.41.26596. [DOI] [PubMed] [Google Scholar]