Abstract
Fetal alcohol syndrome (FAS) is a significant problem in human reproductive medicine. Maternal alcohol administration alters maternal amino acid homeostasis and results in acidemia in both mother and fetus, causing fetal growth restriction. We hypothesized that administration of glutamine, which increases renal ammoniagenesis to regulate acid-base balance, may provide an intervention strategy. This hypothesis was tested using sheep as an animal model. On day 115 of gestation, ewes were anesthetized and aseptic surgery was performed to insert catheters into the fetal abdominal aorta as well as the maternal abdominal aorta and vena cava. On day 128 of gestation, ewes received intravenous administration of saline, alcohol [1.75 g/kg body weight (BW)/h], a bolus of 30 mg glutamine/kg BW, alcohol + a bolus of 30 mg glutamine/kg BW, a bolus of 100 mg glutamine/kg BW, alcohol + a bolus of 100 mg glutamine/kg BW, or received CO2 administration to induce acidemia independent of alcohol. Blood samples were obtained simultaneously from the mother and the fetus at times 0 and 60 min (the time of peak blood alcohol concentration) of the study. Administration of alcohol to pregnant ewes led to a reduction in concentrations of glutamine and related amino acids in plasma by 21–30%. An acute administration of glutamine to ewes, concurrent with alcohol administration, improved the profile of most amino acids (including citrulline and arginine) in maternal and fetal plasma. We suggest that glutamine may have a protective effect against alcohol-induced metabolic disorders and FAS in the ovine model.
Keywords: fetal alcohol syndrome, glutamine, amino acids, acidemia
Introduction
Alcohol consumption during pregnancy can result in Fetal Alcohol Syndrome (FAS) or Fetal Alcohol Spectrum Disorders (FASD), which encompass a wide range of physical, behavioral, learning, emotional and social disturbances. FAS (the most severe form of FASD) is estimated to affect 0.5–2.0 per 1000 live births, but when including the broader ranges of damage from prenatal alcohol exposure (FASD) the estimate is as high as 10 per 1000 live births (May and Gossage 2001). From the time that risks to the unborn baby were initially documented, considerable efforts have been applied to educate women about the dangers of drinking during pregnancy. In spite of these widespread efforts, the incidence of FAS has not diminished (Caetano et al. 2006) and prenatal alcohol exposure is the leading preventable cause of retardation in the United States (Abel and Sokol 1991). The estimated cost of FASD in the United States for the approximately 40,000 children per year born with FASD is $6 billion (Lupton et al. 2004). Since the magnitude of this problem is so great and educational efforts have failed to reduce the incidence, it is necessary to explore development of intervention/amelioration strategies or therapeutics.
A key diagnostic feature of FAS is growth restriction (Sokol and Clarren 1989). In children, prenatal alcohol exposure has been associated with fetal growth restriction that can persist into adolescence (Day et al. 2002) and low birth weight, which can lead to altered development and programming with subsequent lifelong consequences (Barker 1994; Wu et al. 2004). The mechanisms for the growth deficits that occur with prenatal alcohol exposure are not well understood. The fetus depends on a steady supply of maternal/placental nutrients for normal growth and development, and disturbances in this supply is a mechanism by which development could be altered and fetal growth restriction could result.
There are three possible ways that the fetal nutrient supply could be disrupted. First, alcohol could lead to fetal undernutrition (including reduced concentrations of amino acids) by reducing maternal dietary intake (Schenker et al. 1990) and it is known that fetal growth is vulnerable to maternal protein deficiency (Wu et al. 1998). Detrimental effects from prenatal alcohol exposure have been documented when maternal intake of nutrients appears to be adequate, and it is noteworthy that a chronic binge pattern administration of alcohol during the third trimester of pregnancy reduced the concentration of multiple amino acids in the maternal plasma of ewes fed an adequate diet (Ramadoss et al. 2008c). Second, alcohol has been shown to alter the placental transport and/or placental metabolism of nutrients, therefore leading to malnutrition in the fetus (Fisher et al. 1981; Lin 1981; Henderson et al. 1981). Alcohol also differentially alters the proteome of the uterine endothelium, including effects on proteins that regulate epigenetic, transcriptional, and translational processes, thus potentially effecting uteroplacental vascular development (Ramadoss and Magness 2012). Third, an alteration in maternal and/or fetal metabolism and compartmentalization of nutrients could have a net result of fetal undernutrition. There is a lack of data on nutrient and metabolite levels measured in both the maternal and fetal compartment, which will be required to fully assess disturbances in fetal nutrient supply.
A reduction of multiple amino acids in response to prenatal alcohol exposure has also been documented in the rodent model. Maternal plasma concentrations of threonine, serine, glutamine, glycine, alanine, and methionine were reduced in response to acute alcohol exposure in the mouse (Padmanabhan et al. 2002) and chronic alcohol exposure in the rat reduced maternal plasma proline concentrations (Marquis et al. 1984) and increased fetal plasma glutamate concentrations (Karl et al. 1995). These findings characterize the effect of alcohol on maternal amino acids, while there is a paucity of information on the effect to the fetus. This study exploits the unique strengths of the sheep model, in that pregnant ewes and their fetus can be instrumented to allow simultaneous sampling of maternal and fetal blood and allows, for the first time, comparison of maternal and fetal amino acid concentrations concurrently.
Alcohol-induced acidemia has been proposed as the central mechanism governing the changes in maternal amino acid concentrations that occur with alcohol administration (Ramadoss et al. 2008c). Disturbance in pH was hypothesized to be a mechanism underlying the teratogenic effects of alcohol even before FAS was widely recognized or characterized (Horiguchi et al. 1971). A mixed respiratory and metabolic acidosis occurs in humans in response to alcohol consumption, and the drop in blood pH is directly proportional to the blood alcohol concentration (Zehtabchi et al. 2005; Lamminpaa and Vilska 1991; Sahn et al. 1975). Transient increases in the arterial partial pressure of carbon dioxide, resulting in a reduction of both maternal and fetal arterial pH occur with every bout of alcohol exposure (Cudd et al. 2001b; Ramadoss et al. 2007; West et al. 2001; Ramadoss et al. 2008a). In addition to characterizing the amino acid profile in the fetus in response to alcohol, we evaluated this candidate mechanism of alcohol-induced acidosis (which governs the maternal alterations in amino acids) in the fetus.
Glutamine is a major nutrient required by the fetus for growth (Kwon et al. 2003). It is an abundant amino acid in the ovine allantoic and amniotic fluid and is essential for the synthesis of nucleotides, NAD(P)+, and aminosugars (Kwon et al. 2003) as well as playing an important role in fetal nitrogen and carbon metabolism (Vaughn et al. 1995). Importantly, glutamine is the precursor for the brain neurotransmitter glutamate, which is likewise used for the biosynthesis of the cellular antioxidant glutathione (Mates et al. 2002). Glutamine has also been implicated as having an important role as an apoptosis suppressor (Mates et al. 2002) and is a precursor for the synthesis of other amino acids, including ornithine, citrulline, and arginine (Wu and Morris 1998). Acute pH changes are known to alter glutamine/glutamate metabolism (Curthoys and Watford 1995; Nissim 1999) and a reduction of maternal plasma glutamine concentration by approximately 40 percent in response to alcohol (Ramadoss et al. 2008c) has been reported in pregnant sheep. Because it is evident that glutamine has a role in several processes that are disrupted by alcohol, the effect of an acute administration of glutamine given to the ewe, concurrent with alcohol administration, was also tested in this study to evaluate its potential as an intervention strategy. The metabolism and inter-organ fluxes of glutamine in the sheep resemble that seen in humans (Heitmann and Bergman 1980), allowing ease of extrapolation from the sheep model to the human condition.
Methods
Animals
The experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at Texas A&M University. Pregnant Suffolk ewes aged 2–5 years with a known date of conception (pregnancy was confirmed by ultrasound) were divided into seven groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg body weight BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID). All ewes had similar age and BW, and were assigned randomly into one of the treatment groups. Each group consisted of 8 ewes. All animals were fed 4 lb/day of a “complete” ration (TAMU Ewe Ration, Nutrena) (Satterfield et al. 2012) and consumed all of the feed offered. Composition of amino acids (including glutamine, glutamate, and arginine) in the diet, as analyzed by Li et al. (Li et al. 2011), was previously provided by Satterfield et al. (Satterfield et al. 2011). Food was withheld the morning of the experiment.
The saline control (SAL) group was surgically cannulated and given physiological saline in a dose that was isovolumetric to the alcohol dose to control for manipulations associated with cannulation and infusion. The alcohol group (ALC) received a 1.75 g/kg BW alcohol infusion. The two glutamine control groups received a dose of saline that was isovolumetric to the saline control and alcohol groups, as well as a single bolus of glutamine dosed at 30 mg/kg BW (GLN30) or 100 mg/kg BW (GLN100). Glutamine was infused into the mother and not into the fetus, as it is difficult to envision a practical protective/ameliorative strategy that would allow direct administration to a fetus and not have to employ provision of glutamine to the fetus via the mother. Alcohol + glutamine dosed at 30 mg glutamine/kg BW (ALC+GLN30) and alcohol + glutamine dosed at 100 mg glutamine/kg BW groups (ALC+GLN100) received an intravenous bolus of glutamine at the beginning of the experiment when the alcohol infusion was started, at a glutamine dose of 30 mg/kg BW or 100 mg/kg BW, respectively. In the acidemia group (ACID), the maternal pH was manipulated to create an acidemia of the same magnitude as that caused by alcohol in previous studies (Ramadoss et al. 2008b; Cudd et al. 2001b; Ramadoss et al. 2008c; Ramadoss et al. 2007) by altering inspired CO2 concentration.
Experiment protocol
The ewes and fetuses were surgically instrumented on gestational day (GD) 115±2 and the experiment was performed on GD 128±2, during the third trimester equivalent, a period of high vulnerability (Cudd 2005; Wilson and Cudd 2011). Blood samples from the mother and fetus were collected at 0 and 60min (the time of peak blood alcohol concentration) from the beginning of the treatment in order to examine the changes in both maternal and fetal and amino acid concentrations that occur during this time. Maternal and fetal arterial pH, pCO2, and O2, were also measured, and blood alcohol concentration (BAC) was determined at 60 min in the subjects receiving alcohol (the end of infusion).
Instrumentation
On day GD 115±2, the pregnant sheep were anesthetized and aseptic surgery was performed Anesthesia was induced by using intravenous administration of diazepam (0.2 mg/kg BW, Abbott Laboratories, North Chicago, IL) and ketamine (4 mg/kg BW, Ketaset®, Fort Dodge, Fort Dodge, IA). The ewes were intubated and ventilated, and anesthesia was maintained with isoflurane (0.5–2.5%, IsoFlo®, Abbott Laboratories) and oxygen. A ventral midline laparotomy was performed, the uterus was externalized and incised, and a fetal hind limb was exteriorized. An incision was made over the craniolateral aspect of the fetal hind limb, midway between the hock and stifle. A catheter (0.030″ inner diameter, 0.050″ outer diameter polyvinyl chloride) was advanced from the cranial tibial artery into the abdominal aorta to the level of the diaphragm. The fetus was returned to the uterus, and the uterus and maternal midline were closed. Catheters (0.050″ inner diameter, 0.090″ outer diameter polyvinyl chloride) were advanced from the maternal femoral artery and vein to the level of the diaphragm of the abdominal aorta and vena cava, respectively. Fetal and maternal catheters were passed through the abdominal wall in the flank region, where they were stored in a pouch attached to the skin. The average duration of surgery was 1.5 h. At the conclusion of anesthesia, ewes were given a single intramuscular injection of flunixin meglumine (1.1 mg/kg BW, Banamine®, Schering-Plough, Union, NJ), a prostaglandin synthase inhibitor, to reduce postoperative pain. Animals were treated postoperatively for 5 days with twice daily subcutaneous administration of ampicillin trihydrate (25 mg/kg BW; Polyflex®, Aveco, Fort Dodge, IA) and intramuscular administration of gentamicin sulfate (2 mg/kg BW; Gentavet® 100, Velco, St. Louis, MO).
Manipulation of maternal blood gases
Maternal blood gases were measured and controlled by varying maternal inspired gases. On the day of the experiment, ewes were placed in a modified metabolism cart so that the animal’s head was inside a plexiglass chamber. A vinyl diaphragm attached to the open side of the chamber was drawn around the animal’s neck to isolate the atmosphere in the chamber from ambient air. Subjects in the acidemic group were exposed to increased inspired fractional concentrations of CO2 over six hours to create a similar magnitude and pattern of reduction in the arterial pH compared to that produced by alcohol in previous studies (Cudd et al. 2001a; Ramadoss et al. 2007; Ramadoss et al. 2008c; Ramadoss et al. 2008b). The rate at which CO2 was introduced into the chamber in the acidemic group was determined by monitoring maternal arterial pH; the CO2 inflow rate was adjusted so that maternal arterial pH in the acidemic and alcohol groups was matched over the duration of the experimental period. The percentage of oxygen and carbon dioxide in the chamber was measured using a gas monitor (oxygen, model S-3A; carbon-dioxide, model CD-3A, Applied Technologies, Pittsburgh, PA). Normoxemic conditions were maintained throughout the experiment in the acidemic group. Subjects in the other groups also had their heads inside a plexiglass chamber, but the chamber bottom was removed to allow them to breathe room air.
Alcohol and glutamine dosing protocol
Infusions in the groups receiving alcohol were administered at a dose of 1.75 g/kg BW of a 40% (w/v) solution of alcohol in sterile saline administered intravenously over one hour. All other groups received 0.9% saline of a volume and at an infusion rate equivalent to that of the alcohol dose. Infusion solutions were delivered intravenously using a Grady Medical VetFlo® 7701B IV automated infusion pump.
L-Glutamine powder from Sigma Aldrich (Cat #5792) was reconstituted with sterile water at a concentration of 20 ml sterile water per gram of powder and passed through a 0.7 μm bacteriostatic filter. The solution was kept at room temperature and prepared no sooner than 1 to 2 h prior to administration. The dosage of glutamine was 30 mg/kg BW in the GLN30 and ALC+GLN30 groups, and was 100 mg/kg BW in the GLN100 and ALC+GLN 100 groups. The glutamine dose was based on extrapolation from the human glutamine turnover rate of 350 μmol/h/kg BW (Kuhn et al. 1999). The glutamine solution was given as an intravenous bolus at the 0th hour.
Blood alcohol concentration (BAC) measurement
Blood was drawn from the maternal femoral vein catheter one hour following the commencement of alcohol infusions for the measurement of BAC. A 20 μl aliquot of blood was collected into microcapillary tubes and transferred into vials that contained 0.6 N perchloric acid and 4 mM n-propyl alcohol (internal standard) in distilled water. The vials were tightly capped with a septum sealed lid and were stored at room temperature until analysis by headspace gas chromatography (Varian Associates model 3900, Palo Alto, CA) at least 24 h after collection. The basic gas chromatographic parameters were similar to those reported by Penton (Penton 1985), with the exception of the column (DB-wax, Megabore, J&W Scientific Folsum, CA) and the carrier gas (helium) used (West et al. 2001).
Collection of blood samples and blood gas analysis
Blood samples for amino acid measurements were collected in chilled polystyrene tubes containing lithium heparin. Tubes were kept on ice until they were centrifuged at 4°C for 20 minutes at 3200 rpm. Plasma was then obtained and stored in separate aliquots to avoid the need to assay refrozen samples. Plasma samples were stored at −80°C. Blood samples for fetal and maternal blood gas analysis (0.3 ml) were drawn anaerobically into heparinized syringes. Samples were capped and placed on ice until analysis. Blood gases were measured at 37°C using a blood gas analyzer (ABL 5; Radiometer, Westlake, OH).
Amino acid analysis
Plasma (1 ml) was acidified with 1 ml of 1.5 mM HClO4 and then neutralized with 0.5 ml of 2 mM K2CO3 (Go et al. 2012). The supernatant fluid was used for amino acid analysis by HPLC, as described previously (Wu et al. 1997). Concentrations of amino acids in samples were quantified on the basis of authentic standards from Sigma Chemicals (St. Louis, MO, USA) using the Waters Millenium-32 workstation (Dai et al. 2012a, b).
Glucose, lactate and urea analysis
Plasma L-lactate was analyzed by spectrophotometric enzymatic method (Sunrise™ absorbance reader, Tecan, Switzerland) using EnzyChrome™ L-lactate assay kit (BioAssay Systems, Hayward, CA, USA). Plasma glucose and urea were analyzed by automated hexokinase and photometry test, respectively (Texas Veterinary Medical Diagnostic Laboratory, Amarillo, TX, USA).
Statistical analysis
The percentage change in the concentrations of maternal and fetal pH and amino acids at one hour (the end of the alcohol or saline infusion) compared to that at the 0 hour (baseline), were analyzed using a one-way analysis of variance (ANOVA) with treatment group as the sole independent factor. Post-hoc tests were conducted using the Student-Newman-Keuls test. The α level was established a priori at P<0.05 for all analyses.
Results
Blood alcohol concentration
The mean peak BAC for the groups receiving alcohol was 218±29 mg/dl in the ALC group, 207±18 mg/dl in the ALC+GLN30 group, and 190±7 mg/dl in the ALC+GLN100 group. There was no statistical difference among groups (P=0.406).
Maternal and fetal arterial pH, PCO2, and PO2
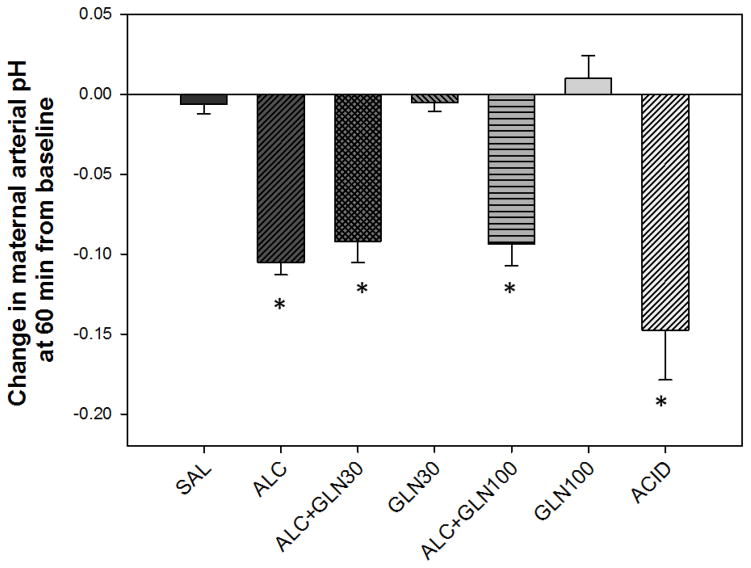
There were statistically significant differences among groups for change in maternal arterial pH from the baseline (P<0.001) (Figure 1). There was a statistically significant reduction in maternal arterial pH at 60 min from the baseline (the time of peak blood alcohol concentration) in the alcohol and acidemia groups as well as in both alcohol + glutamine groups compared to the saline and glutamine control groups (all at P<0.05). The saline control and two glutamine control groups did not differ from each other. The alcohol and acidemia groups did not differ from each other, and neither differed from the ALC+GLN30 or ALC+GLN100 groups.
Figure 1. Change in maternal arterial pH at 60 min from baseline.
Maternal arterial pH at 60 min was significantly reduced in the alcohol receiving groups and acidemia group compared to saline control and glutamine control groups. * indicates statistically significant difference compared to the saline control and glutamine control groups with P < 0.05. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
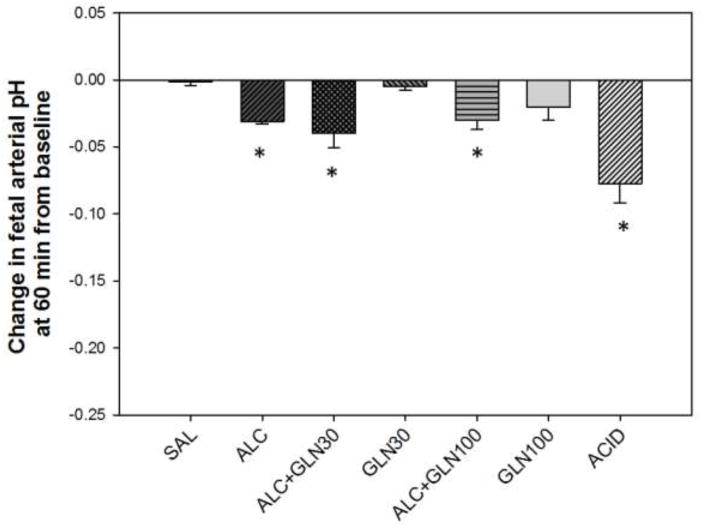
There were statistically significant differences among groups for change in fetal arterial pH at 60 min from the baseline (P<0.001) (Figure 2). There was a statistically significant reduction in fetal arterial pH at 60 min from the baseline in the alcohol and acidemia groups as well as in both alcohol + glutamine groups compared to the saline and glutamine control groups (all at P<0.05). The saline control and two glutamine control groups did not differ from each other. The alcohol and acidemia groups did not differ from each other, and neither differed from the ALC+GLN30 or ALC+GLN100 groups.
Figure 2. Change in fetal arterial pH at 60 min from baseline.
Fetal arterial pH at 60 min was significantly reduced in the alcohol receiving groups and acidemia group compared to saline control and glutamine control groups. * indicates statistically significant difference compared to the saline control and glutamine control groups with P < 0.05. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
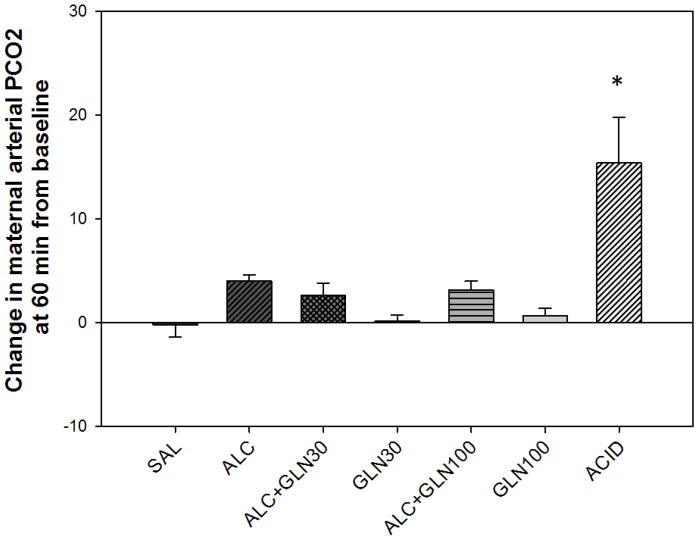
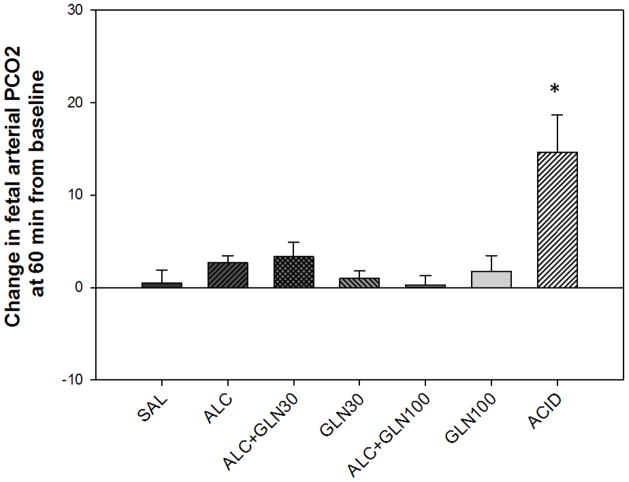
Changes in maternal (Figure 3) and fetal (Figure 4) arterial PCO2 were significantly different between the groups (P<0.001), and changes in maternal and fetal arterial PCO2 at 60 min from the baseline for the ACID group were significantly elevated compared to all other groups (P<0.001). Neither the fetal nor maternal PO2 fell below normal at any time in any group.
Figure 3. Change in maternal arterial PCO2 at 60 min from baseline.
Maternal inspired fractional concentration of PCO2 in the acidemia group was modulated to achieve a similar decrease in maternal pH as that observed in the alcohol group (Figure 1). Maternal arterial PCO2 was significantly elevated in the acidemia group compared to all other groups. * indicates a statistically significant difference with P < 0.05. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
Figure 4. Change in fetal arterial PCO2 at 60 min from baseline.
Fetal arterial PCO2 was significantly elevated in the acidemia group compared to all other groups. * indicates a statistically significant difference with P < 0.05. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
Maternal plasma concentration of amino acids
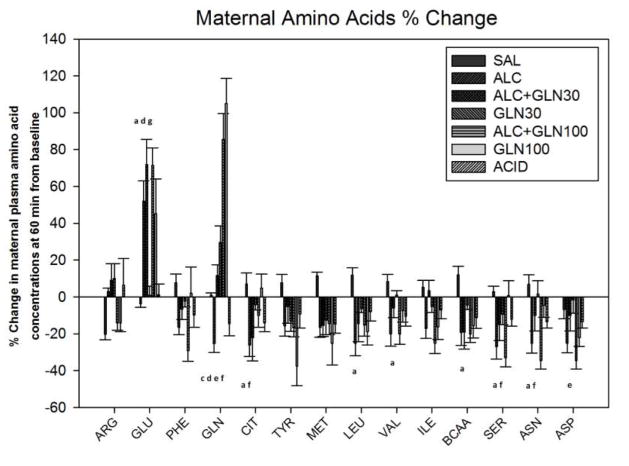
There was a statistically significant difference in the percent change from 0 to 60 min among groups for the following amino acids: arginine, glutamate, phenylalanine, glutamine, citrulline, tyrosine, methionine, leucine, valine, isoleucine, branched chain amino acids, serine, aspartic acid and asparagine. The values at 60 min are reported in Table 1 and depicted in Figure 5, with the details of which groups were statistically different for each amino acid summarized in Supplemental Table 1.
Figure 5. Change in maternal plasma amino acid concentrations at 60 min from baseline.
Percent change in maternal plasma amino acid concentrations at 60 min after an acute alcohol, glutamine, alcohol+glutamine or acidemia exposure. Significant alterations in maternal plasma amino acid concentrations were observed among groups. a, c, d, e, f, g indicate a statistically significant difference (P < 0.05) in the ALC group compared to the SAL, ALC+GLN30, GLN30, ALC+GLN100, GLN100 and ACID groups respectively. More detailed significant differences among groups are provided in the supplementary section. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
Fetal plasma concentration of amino acids
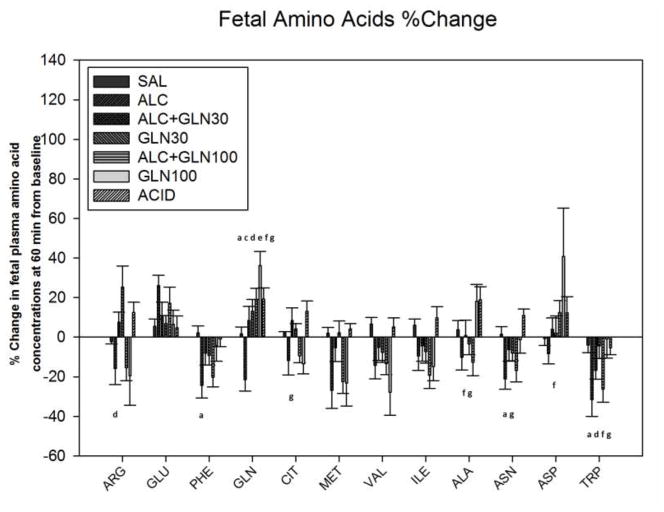
There was a statistically significant difference in the percent change from 0 to 60 min among groups for the following amino acids: arginine, glutamate, phenylalanine, glutamine, citrulline, methionine, valine, isoleucine, alanine, aspartic acid, asparagine and tryptophan. The values at 60 min are reported in Table 1 and depicted in Figure 6, with the details of which groups were statistically different for each amino acid summarized in Supplemental Table 2.
Figure 6. Change in fetal plasma amino acid concentrations at 60 min from baseline.
Percent change in fetal plasma amino acid concentrations at 60 min after an acute alcohol, glutamine, alcohol+glutamine or acidemia exposure. Significant alterations in fetal plasma amino acid concentrations were observed among groups. a, c, d, e, f, g indicate a statistically significant difference (P < 0.05) in the ALC group compared to the SAL, ALC+GLN30, GLN30, ALC+GLN100, GLN100 and ACID groups respectively. More detailed significant differences among groups are provided in the supplementary section. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
Maternal and fetal plasma concentration of glucose, lactate and urea
There was a statistically significant difference in the percent change from 0 to 60 min among groups for maternal plasma glucose concentrations (P=0.002) (Figure 7). Maternal plasma glucose levels for the alcohol group were significantly decreased compared to the acidemia and both glutamine control groups (P<0.05). There was a statistically significant difference in the percent change from 0 to 60 min among groups for the maternal plasma lactate levels (P<0.001) (Figure 7). Maternal plasma lactate levels for the alcohol receiving groups were significantly elevated at 60 min compared to the saline control, acidemia and both the glutamine control groups (P<0.05). Blood urea nitrogen levels (BUN) did not differ among groups (Figure 7).
Figure 7. Change in maternal plasma glucose, lactate and blood urea nitrogen (BUN) concentrations at 60 min from baseline.
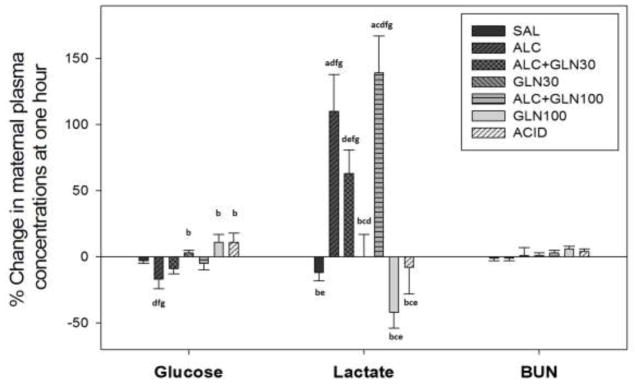
Percent change in maternal plasma glucose, lactate and blood urea nitrogen (BUN) concentrations at 60 min after an acute alcohol, glutamine, alcohol+glutamine or acidemia exposure. Alcohol exposure reduced maternal plasma glucose level compared to the glutamine control groups and acidemia group (P < 0.05). Maternal lactate levels were elevated in all alcohol receiving groups at 60 min (P < 0.05) compared to the saline control, glutamine control groups and acidemia group. Maternal blood urea nitrogen levels were not different among groups. a, b, c, d, e, f, g indicate a statistically significant difference (P < 0.05) in the group compared to the SAL, ALC, ALC+GLN30, GLN30, ALC+GLN100, GLN100 and ACID groups respectively. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
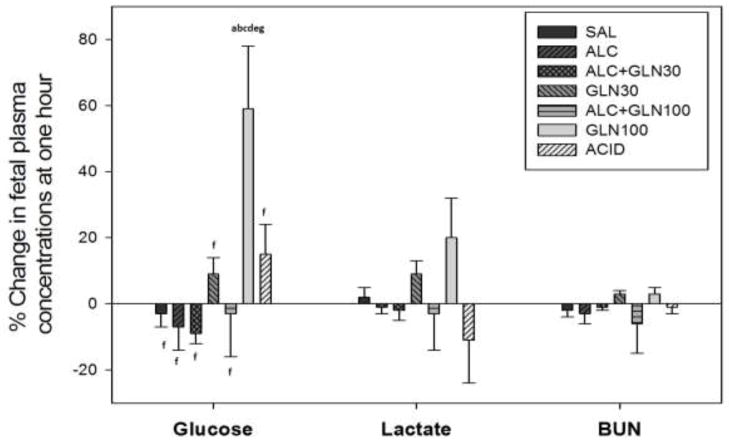
There was a statistically significant difference in the percent change from 0 to 60 min among groups for the fetal plasma glucose concentrations (P<0.001) (Figure 8). Fetal plasma glucose levels for the GLN100 group was significantly elevated at 60 min compared to all other groups (P<0.05). Fetal lactate and blood urea nitrogen levels did not differ among groups (Figure 8).
Figure 8. Change in fetal plasma glucose, lactate and blood urea nitrogen (BUN) concentrations at 60 min from baseline.
Percent change in fetal plasma glucose, lactate and blood urea nitrogen (BUN) concentrations at 60 min after an acute alcohol, glutamine, alcohol+glutamine or acidemia exposure. Maternal administration of a glutamine 100 mg/kg BW dose in the GLN100 control group elevated fetal plasma glucose level at 60 min compared to all other groups (P < 0.05). Fetal plasma lactate and blood urea nitrogen levels were not different among groups. a, b, c, d, e, f, g indicate a statistically significant difference (P < 0.05) in the group compared to the SAL, ALC, ALC+GLN30, GLN30, ALC+GLN100, GLN100 and ACID groups respectively. Groups: saline control (SAL), alcohol (ALC), alcohol + 30 mg glutamine/kg BW (ALC+GLN30), 30 mg glutamine/kg BW (GLN30), alcohol + 100 mg glutamine/kg BW (ALC+GLN100), 100 mg glutamine/kg BW (GLN100), and acidemia (ACID).
Discussion
The important findings from this study are 1) significant amino acid perturbations occur in response to a single binge episode of alcohol exposure or acidemia, 2) significant amino acid perturbations are present in the fetus as well as the mother, 3) the maternal and fetal response to acidemia are different, and while acidosis governs the maternal change in amino acids in response to alcohol, in the fetus alcohol mediates changes in addition to or independent of acidosis, 4) a single bolus of glutamine at the two different doses tested in this study was not enough to completely prevent the fall in pH seen in response to alcohol, and 5) maternal administration of a single dose of glutamine concurrent with alcohol attenuated the drop in fetal amino acid concentrations seen with alcohol alone.
Maternal amino acid disturbances
The decreases in maternal amino acid concentrations in response to alcohol and acidemia in this study parallel findings in previous work done with a chronic exposure pattern followed by an acute alcohol or acidemic challenge (Ramadoss et al. 2008c), indicating that disturbances in amino acid disturbances occur even with a single binge episode of alcohol. The decreases in some of the amino acids were more profound in the chronic followed by acute exposure than what was found in this study with a single acute challenge, which suggests that compensation may not be occurring with the chronic binge exposure paradigm. Acidemia and the resulting changes in glutamine-dependent pathways (Ramadoss et al. 2008c) may be the cause of the maternal amino acid reduction for both the alcohol and acidemia maternal groups seen in our acute paradigm. This is supported by the less severe reduction of several amino acids observed when glutamine was administered concurrently with alcohol (Figure 5).
Glutamate concentration in maternal plasma significantly increased in response to alcohol. This could be a result of an inhibitory effect by alcohol on the oxidation of glutamate, thus increasing its concentration as it builds up. The effect of alcohol plus the conversion of the increased concentration of plasma glutamine (from the acute administration of glutamine) into glutamate by phosphate-activated glutaminase likely explains the higher increase in glutamate seen in the alcohol plus glutamine groups compared to the alcohol group and in the 100 mg glutamine/kg BW group.
Fetal amino acid disturbances
The fetal arterial pH dropped in response to maternal alcohol administration or induction of maternal acidemia, just as a decrease in pH was seen maternally in these groups. Concentrations of multiple amino acids in fetal plasma were significantly reduced in response to alcohol. One exception was glutamate, which showed an increasing trend that did not reach statistical significance. The reductions in fetal amino acids could be explained by a combination of factors, including impaired placental transfer and a change in the fetal metabolic balance between synthesis and degradation of amino acids (for example, inhibition of protein degradation would decrease plasma amino acid levels). Maternal uterine blood flow, umbilical blood flow, placental uptake and bioavailability of amino acids in the maternal compartment are major factors regulating fetal amino acids bioavailability (Chung et al. 1998). Studies in a pregnant sheep model have shown that maternal hypercapnea increases umbilical blood flow and elevates maternal mean arterial pressure, which leads to an increase in uterine artery blood flow (Walker et al. 1976; Berman et al. 1976). Another study in pregnant pigs found that mild hypercapnea leads to a decrease in systemic and uterine vascular resistance, and it eventually leads to an increase in uterine blood flow (Hanka et al. 1975). These reports support our finding that the acidemia group did not have the same general pattern of change in fetal plasma amino acids as that seen in the alcohol group; instead an increase in multiple amino acid concentrations was observed as a result of acidemia independent of alcohol. This is also in contrast to the reduction in most amino acids found in maternal plasma in response to acidemia. This suggests that while acidemia and the resulting changes in glutamine-dependent pathways may be the cause of the maternal amino acid reduction for both the alcohol and acidemia maternal groups (Ramadoss et al. 2008c), alcohol mediates changes in amino acid concentrations in the fetus by mechanisms in addition to or independent of acidemia alone. Acidemia likely causes a disturbance in the balance between protein synthesis and degradation, resulting in a net increase of amino acid concentrations, and acute metabolic acidemia in fetal lambs has been shown to stimulate protein degradation (Milley 1997).
Maternal administration of a single dose of glutamine concurrent with alcohol attenuated the drop in fetal amino acid concentrations seen with alcohol alone, and for some amino acids, such as arginine and citrulline, the 30 mg/kg glutamine dose actually raised plasma concentrations. The higher dose of glutamine (100 mg/kg) tested demonstrated a potential anabolic effect, thus explaining the decrease in plasma concentrations of several amino acids in the groups that received alcohol plus glutamine. Glutamine is known to have trophic, anabolic effects and changes in tissue glutamine concentrations have been shown to correlate with net protein turnover; there is evidence that glutamine may both stimulate protein synthesis and inhibit protein degradation (Smith and Wilmore 1990). Thus, the net effect of glutamine administration could result in a decrease in plasma amino acids from anabolic stimulation rather than nutrient deprivation. This is supported by the observation that there were reductions in the plasma amino acid concentrations of many amino acids in the glutamine control group. Since plasma amino acid concentrations depend on a number of complex factors, including protein turnover, synthesis, oxidation, placental transport, and absorption, dissecting out each of these steps will require additional experiments.
Glutamine and pH
Acute pH changes are known to alter glutamine metabolism, and acidosis has been shown to decrease plasma glutamine levels (Heitmann and Bergman, 1980; Ramadoss et al., 2008c). However, an acute administration of a single bolus of glutamine at the doses used in this study was not sufficient to completely prevent the fall in maternal or fetal arterial pH seen with alcohol administration. Since glutamine turnover is rapid, it is possible that a longer infusion period, repeating current dosages but administering the glutamine more than once, or further increasing the dosage could have a greater effect on preventing the acidemia associated with alcohol. Regardless of its ability to prevent a change in pH, glutamine may still be protective through mechanisms other than prevention of acidemia because this amino acid has a role in several processes that are disrupted by alcohol.
Maternal and fetal plasma concentration of glucose, lactate and urea
In-vivo and in-vitro studies have shown that glutamine is a very effective substrate for gluconeogenesis (Brockman and Bergman 1975; Bergman and Heitmann 1978; Heitmann and Bergman 1978). The carbon skeleton of glutamine is used for new glucose molecule synthesis, and glutamine is the major gluconeogenesis source in the kidney (Nurjhan et al. 1995). Infusion of 28 g of glutamine over 4 h in humans resulted in a 3 fold increase in plasma glutamine concentrations and it led to a 7 fold increase in glucose formation, without changes in plasma insulin and glucagon levels (Perriello et al. 1997). These reports support our findings of an increase in maternal and fetal glucose levels in glutamine control groups.
The increase in maternal lactate levels in alcohol receiving groups, but not the acidemia group, is consistent with earlier reports (Ramadoss et al. 2008c; Kreisberg et al. 1971) and explains why a higher increase in PCO2 in the acidemia group was required to lower the pH to match that seen in the alcohol group. The acidemia group had only a respiratory acidosis, while there was both a respiratory and metabolic acidosis in the alcohol receiving groups. Manipulation of inspired CO2 was chosen as the means to induce acidosis to avoid the confound of volume and electrolyte disturbances involved with acid infusion, and because the CO2 readily crosses the placenta and decreases pH in both the maternal and fetal compartments. Glutamine uptake has been demonstrated to be similar whether the acidosis is respiratory or metabolic in origin (Gougoux et al. 1982; Windus et al. 1984).
Glutamine administration and functions
Maternal administration of two different doses of glutamine was tested in this study, and when administered concurrently with alcohol as a single dose, both the maternal and fetal amino acid profiles improved compared to the alcohol group. Glutamine, now considered a conditionally essential amino acid (Smith and Wilmore 1990), is the most abundant amino acid in the blood and the free amino acid pool in the body (Kalhan et al. 2005). It plays an important role in the inter-organ shuttle of nitrogen and carbon as well as serving as the primary oxidative fuel for enterocytes and lymphocytes (Kalhan et al. 2005). Glutamine is also a precursor for the de novo synthesis of arginine and proline (Wu et al. 2011a), and both glutamine and arginine have beneficial effects to the recovery of seriously ill patients (Li et al. 2007; Xi et al. 2011); it is possible that glutamine exerts some of its actions by enhancing the availability of arginine (Ligthart-Melis et al. 2008; Wu and Morris 1998). Other mechanisms of actions for the beneficial effects of glutamine include a modulatory effect on gene expression. For example, glutamine supplementation in weanling piglets increased intestinal expression of genes necessary for cell growth and removal of oxidants, while reducing expression of genes that promote oxidative stress and immune activation which resulted in improved growth and weight gain (Wang et al. 2008). In addition, glutamine has been shown to be a stimulus/regulator to mTOR (Xi et al. 2012), a protein kinase that phosphorylates its down-stream target proteins leading to enhanced protein synthesis and reduced protein degradation in cells (Nicklin et al. 2009; Fumarola et al. 2005; Dennis et al. 1999).
Because of its critical role in a number of physiological systems, and because there is a rapid depletion of whole body glutamine pools during acute illness, trauma, and burns, glutamine has been studied extensively as a nutrient supplement, both parenterally and enterally (Ligthart-Melis et al. 2008; Smith and Wilmore 1990; Garlick 2001). In low birth weight infants, parenteral glutamine supplementation reduced whole-body proteolysis and increased protein accretion (Kalhan et al. 2005). An anabolic effect of glutamine has also been reported for piglets with supplementation ameliorating intrauterine growth restriction (Wu et al. 2011b). Administration of corticosteroids in humans to mimic stress conditions increased the uptake of glutamine from the splanchnic bed and could thus be a contributor to glutamine depletion under these conditions (Thibault et al. 2008). This is noteworthy since alcohol has been demonstrated to increase both fetal and maternal glucocorticoid levels (Cudd et al. 2001b; Washburn et al. 2012).
Although glutamine is stable as a dry solid, it is known to be unstable in solution, resulting in the toxic product pyroglutamate (Furst et al. 1997); such problems can be avoided by preparing solutions freshly from the dry solid or by administering it in the form of a dipeptide (Garlick 2001; Furst et al. 1997). The ewes in the glutamine control groups and alcohol + glutamine groups received on average an approximate total dose of 2.5 g or 8 g of glutamine for the 30 or 100 mg/kg BW doses respectively. No adverse effects of glutamine have been demonstrated when given orally to adult humans in doses of 50–60 g/day (Garlick 2001), so this should be well within a possible safe range of administration.
Conclusion and perspectives
Results of this study support the conclusion that alcohol exposure alters amino acid homeostasis in both the mother and the fetus, and that even a single binge alcohol exposure causes both a maternal and fetal disturbance. These findings may help explain the intra-uterine growth restriction and structural damage to the nervous system observed in FASD. We have demonstrated that amino acid disturbances in the fetus and the ewe are similar for alcohol exposure, and are in direct contrast to in response to acidemia, thus indicating that alcohol mediates changes in amino acid concentrations in the fetus by mechanisms in addition to or independent of acidemia alone. This finding illustrates the importance of doing fetal experiments such as this one, where both maternal and fetal measurements can be taken simultaneously. Maternal administration of a single dose of glutamine concurrent with alcohol attenuated the drop in fetal amino acid concentrations seen with alcohol alone, and demonstrated a potential anabolic effect at the higher dose. Glutamine has great potential as a new nutritional means to ameliorate alcohol-induced metabolic disorders and thus serve as an intervention or therapeutic strategy for FASD. Results of this study support the notions that glutamine is a functional amino acid for improving animal and human health (Wu 2010). Our findings are also consistent with the proposition that, under conditions (e.g., FAS in this study) when rates of utilization of amino acids are greater than rates of their endogenous synthesis, there should be a dietary requirement for glutamine and other traditionally classified “nonessential amino acids” to maintain whole-body homeostasis (e.g., acid-base balance) and adequate rates of tissue protein synthesis (Lei et al. 2012; Rezaei et al. 2012; Wu et al. 2012; Yao et al. 2012).
Supplementary Material
Supplemental Table 1. Detailed significances among groups for change in maternal plasma amino acid concentrations at 60 min from baseline (Figure 5)
Supplemental Table 2. Detailed significances among groups for change in fetal plasma amino acid concentrations at 60 min from baseline (Figure 6)
Acknowledgments
This study was supported by NIAAA Grant AA10940 (TAC and GW) and K08AA18166 (SEW). We thank the research assistants in our laboratories for technical assistance.
Footnotes
The authors declare that they have no conflict of interest.
References
- Abel EL, Sokol RJ. A revised estimate of the economic impact of fetal alcohol syndrome. Recent Dev Alcohol. 1991;9:117–125. [PubMed] [Google Scholar]
- Barker DJ. Maternal and fetal origins of coronary heart disease. Journal of the Royal College of Physicians of London. 1994;28(6):544–551. [PMC free article] [PubMed] [Google Scholar]
- Bergman EN, Heitmann RN. Metabolism of amino acids by the gut, liver, kidneys, and peripheral tissues. Federation proceedings. 1978;37(5):1228–1232. [PubMed] [Google Scholar]
- Berman W, Jr, Goodlin RC, Heymann MA, Rudolph AM. Relationships between pressure and flow in the umbilical and uterine circulations of the sheep. Circ Res. 1976;38(4):262–266. doi: 10.1161/01.res.38.4.262. [DOI] [PubMed] [Google Scholar]
- Brockman RP, Bergman EN. Effect of glucagon on plasma alanine and glutamine metabolism and hepatic gluconeogenesis in sheep. The American journal of physiology. 1975;228(6):1628–1633. doi: 10.1152/ajplegacy.1975.228.6.1627. [DOI] [PubMed] [Google Scholar]
- Caetano R, Ramisetty-Mikler S, Floyd LR, McGrath C. The epidemiology of drinking among women of child-bearing age. Alcoholism, clinical and experimental research. 2006;30(6):1023–1030. doi: 10.1111/j.1530-0277.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- Chung M, Teng C, Timmerman M, Meschia G, Battaglia FC. Production and utilization of amino acids by ovine placenta in vivo. The American journal of physiology. 1998;274(1 Pt 1):E13–22. doi: 10.1152/ajpendo.1998.274.1.E13. [DOI] [PubMed] [Google Scholar]
- Cudd TA. Animal model systems for the study of alcohol teratology. Exp Biol Med (Maywood) 2005;230(6):389–393. doi: 10.1177/15353702-0323006-06. [DOI] [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, Parnell SE, West JR. Third trimester binge ethanol exposure results in fetal hypercapnea and acidemia but not hypoxemia in pregnant sheep. Alcoholism, clinical and experimental research. 2001a;25(2):269–276. [PubMed] [Google Scholar]
- Cudd TA, Chen WJ, West JR. Fetal and maternal sheep hypothalamus pituitary adrenal axis responses to chronic binge ethanol exposure during the third trimester equivalent. Alcoholism, clinical and experimental research. 2001b;25(7):1065–1071. [PubMed] [Google Scholar]
- Curthoys NP, Watford M. Regulation of glutaminase activity and glutamine metabolism. Annual review of nutrition. 1995;15:133–159. doi: 10.1146/annurev.nu.15.070195.001025. [DOI] [PubMed] [Google Scholar]
- Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Metabolism of select amino acids in bacteria from the pig small intestine. Amino acids. 2012a;42(5):1597–1608. doi: 10.1007/s00726-011-0846-x. [DOI] [PubMed] [Google Scholar]
- Dai ZL, Li XL, Xi PB, Zhang J, Wu G, Zhu WY. Regulatory role for L-arginine in the utilization of amino acids by pig small-intestinal bacteria. Amino acids. 2012b;43(1):233–244. doi: 10.1007/s00726-011-1067-z. [DOI] [PubMed] [Google Scholar]
- Day NL, Leech SL, Richardson GA, Cornelius MD, Robles N, Larkby C. Prenatal alcohol exposure predicts continued deficits in offspring size at 14 years of age. Alcoholism, clinical and experimental research. 2002;26(10):1584–1591. doi: 10.1097/01.ALC.0000034036.75248.D9. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Fumagalli S, Thomas G. Target of rapamycin (TOR): balancing the opposing forces of protein synthesis and degradation. Current opinion in genetics & development. 1999;9(1):49–54. doi: 10.1016/s0959-437x(99)80007-0. [DOI] [PubMed] [Google Scholar]
- Fisher SE, Atkinson M, Holzman I, David R, Van Thiel DH. Effect of ethanol upon placental uptake of amino acids. Progress in biochemical pharmacology. 1981;18:216–223. [PubMed] [Google Scholar]
- Fumarola C, La Monica S, Guidotti GG. Amino acid signaling through the mammalian target of rapamycin (mTOR) pathway: Role of glutamine and of cell shrinkage. Journal of cellular physiology. 2005;204(1):155–165. doi: 10.1002/jcp.20272. [DOI] [PubMed] [Google Scholar]
- Furst P, Pogan K, Stehle P. Glutamine dipeptides in clinical nutrition. Nutrition. 1997;13(7–8):731–737. doi: 10.1016/s0899-9007(97)83035-3. [DOI] [PubMed] [Google Scholar]
- Garlick PJ. Assessment of the safety of glutamine and other amino acids. The Journal of nutrition. 2001;131(9 Suppl):2556S–2561S. doi: 10.1093/jn/131.9.2556S. [DOI] [PubMed] [Google Scholar]
- Go G, Wu G, Silvey DT, Choi S, Li X, Smith SB. Lipid metabolism in pigs fed supplemental conjugated linoleic acid and/or dietary arginine. Amino acids. 2012;43(4):1713–1726. doi: 10.1007/s00726-012-1255-5. [DOI] [PubMed] [Google Scholar]
- Gougoux A, Vinay P, Cardoso M, Duplain M, Lemieux G. Immediate adaptation of the dog kidney to acute hypercapnia. The American journal of physiology. 1982;243(3):F227–234. doi: 10.1152/ajprenal.1982.243.3.F227. [DOI] [PubMed] [Google Scholar]
- Hanka R, Lawn L, Mills IH, Prior DC, Tweeddale PM. The effects of maternal hypercapnia on foetal oxygenation and uterine blood flow in the pig. J Physiol. 1975;247(2):447–460. doi: 10.1113/jphysiol.1975.sp010940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitmann RN, Bergman EN. Glutamine metabolism, interorgan transport and glucogenicity in the sheep. The American journal of physiology. 1978;234(2):E197–203. doi: 10.1152/ajpendo.1978.234.2.E197. [DOI] [PubMed] [Google Scholar]
- Heitmann RN, Bergman EN. Integration of amino acid metabolism in sheep: effects of fasting and acidosis. The American journal of physiology. 1980;239(4):E248–E254. doi: 10.1152/ajpendo.1980.239.4.E248. [DOI] [PubMed] [Google Scholar]
- Henderson GI, Turner D, Patwardhan RV, Lumeng L, Hoyumpa AM, Schenker S. Inhibition of placental valine uptake after acute and chronic maternal ethanol consumption. The Journal of pharmacology and experimental therapeutics. 1981;216(3):465–472. [PubMed] [Google Scholar]
- Horiguchi T, Suzuki K, Comas-Urrutia AC, Mueller-Heubach E, Boyer-Milic AM, Baratz RA, Morishima HO, James LS, Adamsons K. Effect of ethanol upon uterine activity and fetal acid-base state of the rhesus monkey. American journal of obstetrics and gynecology. 1971;109(6):910–917. doi: 10.1016/0002-9378(71)90806-4. [DOI] [PubMed] [Google Scholar]
- Kalhan SC, Parimi PS, Gruca LL, Hanson RW. Glutamine supplement with parenteral nutrition decreases whole body proteolysis in low birth weight infants. The Journal of pediatrics. 2005;146(5):642–647. doi: 10.1016/j.jpeds.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Karl PI, Kwun R, Slonim A, Fisher SE. Ethanol elevates fetal serum glutamate levels in the rat. Alcoholism, clinical and experimental research. 1995;19(1):177–181. doi: 10.1111/j.1530-0277.1995.tb01488.x. [DOI] [PubMed] [Google Scholar]
- Kreisberg RA, Owen WC, Siegal AM. Ethanol-induced hyperlacticacidemia: inhibition of lactate utilization. J Clin Invest. 1971;50(1):166–174. doi: 10.1172/JCI106470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn KS, Schuhmann K, Stehle P, Darmaun D, Furst P. Determination of glutamine in muscle protein facilitates accurate assessment of proteolysis and de novo synthesis-derived endogenous glutamine production. The American journal of clinical nutrition. 1999;70(4):484–489. doi: 10.1093/ajcn/70.4.484. [DOI] [PubMed] [Google Scholar]
- Kwon H, Spencer TE, Bazer FW, Wu G. Developmental changes of amino acids in ovine fetal fluids. Biology of reproduction. 2003;68(5):1813–1820. doi: 10.1095/biolreprod.102.012971. [DOI] [PubMed] [Google Scholar]
- Lamminpaa A, Vilska J. Acid-base balance in alcohol users seen in an emergency room. Veterinary and human toxicology. 1991;33(5):482–485. [PubMed] [Google Scholar]
- Lei J, Feng D, Zhang Y, Dahanayaka S, Li X, Yao K, Wang J, Wu Z, Dai Z, Wu G. Regulation of leucine catabolism by metabolic fuels in mammary epithelial cells. Amino acids. 2012;43(5):2179–2189. doi: 10.1007/s00726-012-1302-2. [DOI] [PubMed] [Google Scholar]
- Li P, Yin YL, Li D, Kim SW, Wu G. Amino acids and immune function. The British journal of nutrition. 2007;98(2):237–252. doi: 10.1017/S000711450769936X. [DOI] [PubMed] [Google Scholar]
- Li X, Rezaei R, Li P, Wu G. Composition of amino acids in feed ingredients for animal diets. Amino acids. 2011;40(4):1159–1168. doi: 10.1007/s00726-010-0740-y. [DOI] [PubMed] [Google Scholar]
- Ligthart-Melis GC, van de Poll MC, Boelens PG, Dejong CH, Deutz NE, van Leeuwen PA. Glutamine is an important precursor for de novo synthesis of arginine in humans. The American journal of clinical nutrition. 2008;87(5):1282–1289. doi: 10.1093/ajcn/87.5.1282. [DOI] [PubMed] [Google Scholar]
- Lin GW. Effect of ethanol feeding during pregnancy on placental transfer of alpha-aminoisobutyric acid in the rat. Life sciences. 1981;28(6):595–601. doi: 10.1016/0024-3205(81)90122-3. [DOI] [PubMed] [Google Scholar]
- Lupton C, Burd L, Harwood R. Cost of fetal alcohol spectrum disorders. Am J Med Genet C Semin Med Genet. 2004;127C(1):42–50. doi: 10.1002/ajmg.c.30015. [DOI] [PubMed] [Google Scholar]
- Marquis SM, Leichter J, Lee M. Plasma amino acids and glucose levels in the rat fetus and dam after chronic maternal alcohol consumption. Biology of the neonate. 1984;46(1):36–43. doi: 10.1159/000242030. [DOI] [PubMed] [Google Scholar]
- Mates JM, Perez-Gomez C, Nunez de Castro I, Asenjo M, Marquez J. Glutamine and its relationship with intracellular redox status, oxidative stress and cell proliferation/death. The international journal of biochemistry & cell biology. 2002;34(5):439–458. doi: 10.1016/s1357-2725(01)00143-1. [DOI] [PubMed] [Google Scholar]
- May PA, Gossage JP. Estimating the prevalence of fetal alcohol syndrome. A summary. Alcohol Res Health. 2001;25(3):159–167. [PMC free article] [PubMed] [Google Scholar]
- Milley JR. Ovine fetal leucine kinetics and protein metabolism during acute metabolic acidosis. The American journal of physiology. 1997;272(2 Pt 1):E275–281. doi: 10.1152/ajpendo.1997.272.2.E275. [DOI] [PubMed] [Google Scholar]
- Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, Myer VE, MacKeigan JP, Porter JA, Wang YK, Cantley LC, Finan PM, Murphy LO. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136(3):521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissim I. Newer aspects of glutamine/glutamate metabolism: the role of acute pH changes. The American journal of physiology. 1999;277(4 Pt 2):F493–497. doi: 10.1152/ajprenal.1999.277.4.F493. [DOI] [PubMed] [Google Scholar]
- Nurjhan N, Bucci A, Perriello G, Stumvoll M, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Glutamine: a major gluconeogenic precursor and vehicle for interorgan carbon transport in man. J Clin Invest. 1995;95(1):272–277. doi: 10.1172/JCI117651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan R, Ibrahim A, Bener A. Effect of maternal methionine pre-treatment on alcohol-induced exencephaly and axial skeletal dysmorphogenesis in mouse fetuses. Drug and alcohol dependence. 2002;65(3):263–281. doi: 10.1016/s0376-8716(01)00173-9. [DOI] [PubMed] [Google Scholar]
- Penton Z. Headspace measurement of ethanol in blood by gas chromatography with a modified autosampler. Clin Chem. 1985;31(3):439–441. [PubMed] [Google Scholar]
- Perriello G, Nurjhan N, Stumvoll M, Bucci A, Welle S, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Regulation of gluconeogenesis by glutamine in normal postabsorptive humans. The American journal of physiology. 1997;272(3 Pt 1):E437–445. doi: 10.1152/ajpendo.1997.272.3.E437. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Ouyang N, Chen WJ, Cudd TA. Acid-sensitive channel inhibition prevents fetal alcohol spectrum disorders cerebellar Purkinje cell loss. American journal of physiology Regulatory, integrative and comparative physiology. 2008a;295(2):R596–603. doi: 10.1152/ajpregu.90321.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Lunde ER, Pina KB, Chen WJ, Cudd TA. All three trimester binge alcohol exposure causes fetal cerebellar purkinje cell loss in the presence of maternal hypercapnea, acidemia, and normoxemia: ovine model. Alcoholism, clinical and experimental research. 2007;31(7):1252–1258. doi: 10.1111/j.1530-0277.2007.00422.x. [DOI] [PubMed] [Google Scholar]
- Ramadoss J, Magness RR. Alcohol-induced alterations in maternal uterine endothelial proteome: A quantitative iTRAQ mass spectrometric approach. Reprod Toxicol. 2012;34(4):538–544. doi: 10.1016/j.reprotox.2012.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Tress U, Chen WJ, Cudd TA. Maternal adrenocorticotropin, cortisol, and thyroid hormone responses to all three-trimester equivalent repeated binge alcohol exposure: ovine model. Alcohol. 2008b;42(3):199–205. doi: 10.1016/j.alcohol.2007.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadoss J, Wu G, Cudd TA. Chronic binge ethanol-mediated acidemia reduces availability of glutamine and related amino acids in maternal plasma of pregnant sheep. Alcohol. 2008c;42(8):657–666. doi: 10.1016/j.alcohol.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, Fielder SE, Eide SJ, Lovering SL, Wu G. Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino acids. 2012 doi: 10.1007/s00726-012-1420-x. [DOI] [PubMed] [Google Scholar]
- Sahn SA, Lakshminarayan S, Pierson DJ, Weil JV. Effect of ethanol on the ventilatory responses to oxygen and carbon dioxide in man. Clinical science and molecular medicine. 1975;49(1):33–38. doi: 10.1042/cs0490033. [DOI] [PubMed] [Google Scholar]
- Satterfield CM, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in diet-induced obese sheep. Amino acids. 2012;43(4):1593–1603. doi: 10.1007/s00726-012-1235-9. [DOI] [PubMed] [Google Scholar]
- Satterfield MC, Dunlap KA, Keisler DH, Bazer FW, Wu G. Arginine nutrition and fetal brown adipose tissue development in nutrient-restricted sheep. Amino acids. 2011 doi: 10.1007/s00726-011-1168-8. [DOI] [PubMed] [Google Scholar]
- Schenker S, Becker HC, Randall CL, Phillips DK, Baskin GS, Henderson GI. Fetal alcohol syndrome: current status of pathogenesis. Alcoholism, clinical and experimental research. 1990;14(5):635–647. doi: 10.1111/j.1530-0277.1990.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Smith RJ, Wilmore DW. Glutamine nutrition and requirements. JPEN Journal of parenteral and enteral nutrition. 1990;14(4 Suppl):94S–99S. doi: 10.1177/014860719001400412. [DOI] [PubMed] [Google Scholar]
- Sokol RJ, Clarren SK. Guidelines for use of terminology describing the impact of prenatal alcohol on the offspring. Alcoholism, clinical and experimental research. 1989;13(4):597–598. doi: 10.1111/j.1530-0277.1989.tb00384.x. [DOI] [PubMed] [Google Scholar]
- Thibault R, Welch S, Mauras N, Sager B, Altomare A, Haymond M, Darmaun D. Corticosteroids increase glutamine utilization in human splanchnic bed. Am J Physiol-Gastr L. 2008;294(2):G548–G553. doi: 10.1152/ajpgi.00461.2007. [DOI] [PubMed] [Google Scholar]
- Vaughn PR, Lobo C, Battaglia FC, Fennessey PV, Wilkening RB, Meschia G. Glutamine Glutamate Exchange between Placenta and Fetal Liver. Am J Physiol-Endoc M. 1995;268(4):E705–E711. doi: 10.1152/ajpendo.1995.268.4.E705. [DOI] [PubMed] [Google Scholar]
- Walker AM, Oakes GK, Ehrenkranz R, McLaughlin M, Chez RA. Effects of hypercapnia on uterine and umbilical circulations in conscious pregnant sheep. J Appl Physiol. 1976;41(5 Pt 1):727–733. doi: 10.1152/jappl.1976.41.5.727. [DOI] [PubMed] [Google Scholar]
- Wang J, Chen L, Li P, Li X, Zhou H, Wang F, Li D, Yin Y, Wu G. Gene expression is altered in piglet small intestine by weaning and dietary glutamine supplementation. The Journal of nutrition. 2008;138(6):1025–1032. doi: 10.1093/jn/138.6.1025. [DOI] [PubMed] [Google Scholar]
- Washburn SE, Tress U, Lunde ER, Chen WJ, Cudd TA. The role of cortisol in chronic binge alcohol-induced cerebellar injury: Ovine model. Alcohol. 2012 doi: 10.1016/j.alcohol.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West JR, Parnell SE, Chen WJ, Cudd TA. Alcohol-mediated Purkinje cell loss in the absence of hypoxemia during the third trimester in an ovine model system. Alcoholism, clinical and experimental research. 2001;25(7):1051–1057. [PubMed] [Google Scholar]
- Wilson SE, Cudd TA. Focus On: The Use of Animal Models for the Study of Fetal Alcohol Spectrum Disorders. Alcohol Res Health. 2011;34(1):92–98. [PMC free article] [PubMed] [Google Scholar]
- Windus DW, Klahr S, Hammerman MR. Glutamine transport in basolateral vesicles from dogs with acute respiratory acidosis. The American journal of physiology. 1984;247(3 Pt 2):F403–407. doi: 10.1152/ajprenal.1984.247.3.F403. [DOI] [PubMed] [Google Scholar]
- Wu G. Functional amino acids in growth, reproduction, and health. Adv Nutr. 2010;1(1):31–37. doi: 10.3945/an.110.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Burghardt RC, Johnson GA, Kim SW, Knabe DA, Li P, Li X, McKnight JR, Satterfield MC, Spencer TE. Proline and hydroxyproline metabolism: implications for animal and human nutrition. Amino acids. 2011a;40(4):1053–1063. doi: 10.1007/s00726-010-0715-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Cudd TA, Meininger CJ, Spencer TE. Maternal nutrition and fetal development. The Journal of nutrition. 2004;134(9):2169–2172. doi: 10.1093/jn/134.9.2169. [DOI] [PubMed] [Google Scholar]
- Wu G, Bazer FW, Johnson GA, Knabe DA, Burghardt RC, Spencer TE, Li XL, Wang JJ. Triennial Growth Symposium: important roles for L-glutamine in swine nutrition and production. Journal of animal science. 2011b;89(7):2017–2030. doi: 10.2527/jas.2010-3614. [DOI] [PubMed] [Google Scholar]
- Wu G, Davis PK, Flynn NE, Knabe DA, Davidson JT. Endogenous synthesis of arginine plays an important role in maintaining arginine homeostasis in postweaning growing pigs. The Journal of nutrition. 1997;127(12):2342–2349. doi: 10.1093/jn/127.12.2342. [DOI] [PubMed] [Google Scholar]
- Wu G, Imhoff-Kunsch B, Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatric and perinatal epidemiology. 2012;26(Suppl 1):4–26. doi: 10.1111/j.1365-3016.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Wu G, Morris SM., Jr Arginine metabolism: nitric oxide and beyond. The Biochemical journal. 1998;336(Pt 1):1–17. doi: 10.1042/bj3360001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Pond WG, Ott T, Bazer FW. Maternal dietary protein deficiency decreases amino acid concentrations in fetal plasma and allantoic fluid of pigs. The Journal of nutrition. 1998;128(5):894–902. doi: 10.1093/jn/128.5.894. [DOI] [PubMed] [Google Scholar]
- Xi P, Jiang Z, Dai Z, Li X, Yao K, Zheng C, Lin Y, Wang J, Wu G. Regulation of protein turnover by L-glutamine in porcine intestinal epithelial cells. The Journal of nutritional biochemistry. 2012;23(8):1012–1017. doi: 10.1016/j.jnutbio.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Xi P, Jiang Z, Zheng C, Lin Y, Wu G. Regulation of protein metabolism by glutamine: implications for nutrition and health. Frontiers in bioscience: a journal and virtual library. 2011;16:578–597. doi: 10.2741/3707. [DOI] [PubMed] [Google Scholar]
- Yao K, Yin Y, Li X, Xi P, Wang J, Lei J, Hou Y, Wu G. Alpha-ketoglutarate inhibits glutamine degradation and enhances protein synthesis in intestinal porcine epithelial cells. Amino acids. 2012;42(6):2491–2500. doi: 10.1007/s00726-011-1060-6. [DOI] [PubMed] [Google Scholar]
- Zehtabchi S, Sinert R, Baron BJ, Paladino L, Yadav K. Does ethanol explain the acidosis commonly seen in ethanol-intoxicated patients? Clin Toxicol (Phila) 2005;43(3):161–166. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1. Detailed significances among groups for change in maternal plasma amino acid concentrations at 60 min from baseline (Figure 5)
Supplemental Table 2. Detailed significances among groups for change in fetal plasma amino acid concentrations at 60 min from baseline (Figure 6)