Abstract
We demonstrate a new compact CN-PPV dot, which emits in the orange wavelength range with high brightness. The small particle size, high brightness, and the ability to highly specifically target subcellular structures make the CN-PPV dots promising probes for biological imaging and bioanalytical applications.
Semiconducting polymer dots (Pdots) possess large absorption cross-sections, high quantum yields, and fast emission rates1–5. These optical properties make them well-suited for biological detection and imaging,6 high-speed single-particle tracking,7 and various biosensing platforms.8–11 We have recently developed a general method to form Pdot-bioconjugates and have demonstrated their applications in specific cellular imaging,12 bioorthogonal labeling,13 and in vivo tumor targeting.14 Successful bioconjugation opens up a new and practical way to employ the highly fluorescent and non-toxic Pdot-bioconjugates for a wide variety of biological applications.
Despite the field’s progress, there is still a great need to explore new polymer species that form highly fluorescent Pdots, especially ones that complement the absorption and emission properties of currently available Pdots. In general, Pdots with small hydrodynamic diameters (e.g. ~10 nm) are highly desirable for many applications because they bypass issues presented by large particle sizes (>20–30 nm), which may alter the biological function and transport of the attached biomolecules.15 Small Pdots are particularly useful for subcellular imaging because large-sized particles may cause problems such as poor mass transfer and tissue penetration as well as non-specific adsorption. But most Pdot-bioconjugates developed so far show hydrodynamic diameters larger than 20 nm.
In this communication, we report a new Pdot species based on Poly[2-methoxy-5-(2-ethylhexyloxy)-1,4-(1-cyanovinylene-1,4-phenylene)] (CN-PPV). CN-PPV Pdots emit in the orange wavelength range, a region that currently lacks Pdots with high brightness. These CN-PPV Pdots, when formed by nanoprecipitation10–13, are compact and small with an average hydrodynamic diameter of ~10 nm. The CN-PPV dots exhibit bright orange fluorescence with a quantum yield of ~60%. Successful functionalization and bioconjugation produces CN-PPV Pdot-streptavidin probes that specifically label cell-surface markers and subcellular microtubule structures in mammalian cells. These results indicate the highly fluorescent CN-PPV Pdots are promising probes for cellular imaging and bioanalytical assays.
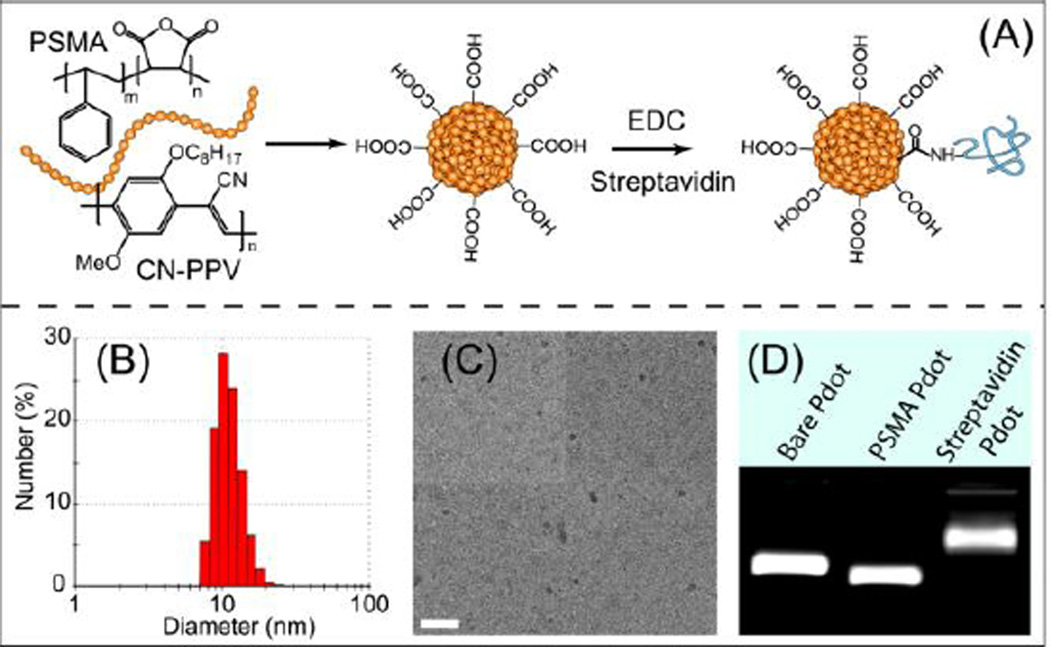
The CN-PPV Pdot bioconjugates were prepared by nanoprecipitation, a method we have previously described. 13, 14 Briefly, we blended poly(styrene-co-maleic anhydride) (PSMA) with CN-PPV during Pdot formation, which resulted in carboxyl groups on the Pdot surface after the maleic anhydride groups were spontaneously hydrolyzed in aqueous solution (Figure 1A). The CN-PPV-streptavidin bioconjugation step (electronic supplementary information; ESI†) was then completed in 20mM HEPES buffer containing 0.1 % PEG in the presence of ethyl(dimethylaminopropyl) carbodiimide (EDC). The product was purified by column filtration and concentrated by membrane spin filtration. 10–13
Figure 1.
(A) Surface functionalization of semiconducting polymer dots and subsequent streptavidin bioconjugation via EDC-catalyzed coupling. (B) Hydrodynamic diameter of functionalized CN-PPV dots measured by DLS. (C) Representative TEM image of functionalized CN-PPV dots. The scale bar represents 100 nm. (D) Gel electrophoresis of Pdots with different surface functional groups.
We characterized the size of the CN-PPV Pdots using dynamic light scattering (DLS, Figure 1B) and transmission electron microscopy (TEM, Figure 1C). The CN-PPV Pdots exhibited a hydrodynamic diameter of ~10 nm based on DLS measurements, which is consistent with the result from TEM, The carboxyl functionalization did not cause any noticeable change in the particle size. Under the same preparation conditions, the yellow-emitting carboxyl PFBT dots from our previous reports showed a hydrodynamic diameter larger than 15 nm.13, 14 The size difference between the two Pdot species may be caused by differences in the rigidity of the polymer backbone and the molecular weight of the original polymer precursors. After streptavidin bioconjugation, the average hydrodynamic diameter of CN-PPV-streptavidin conjugates showed a slight increase of ~2 nm in diameter.
We performed gel electrophoresis to verify the formation of functional groups on the Pdot surface. Figure 1D shows that the carboxyl-functionalized Pdot (PSMA-Pdot, middle column) exhibited an apparent increase in mobility compared to a bare Pdot (left column) in an agarose gel. The streptavidin-conjugated Pdot showed slower mobility than bare Pdot because of the decrease in negative surface charge together with the slight increase in the size of the Pdot-bioconjugates. These results suggest successful carboxyl functionalization of the Pdots as well as the surface bioconjugation with streptavidin.
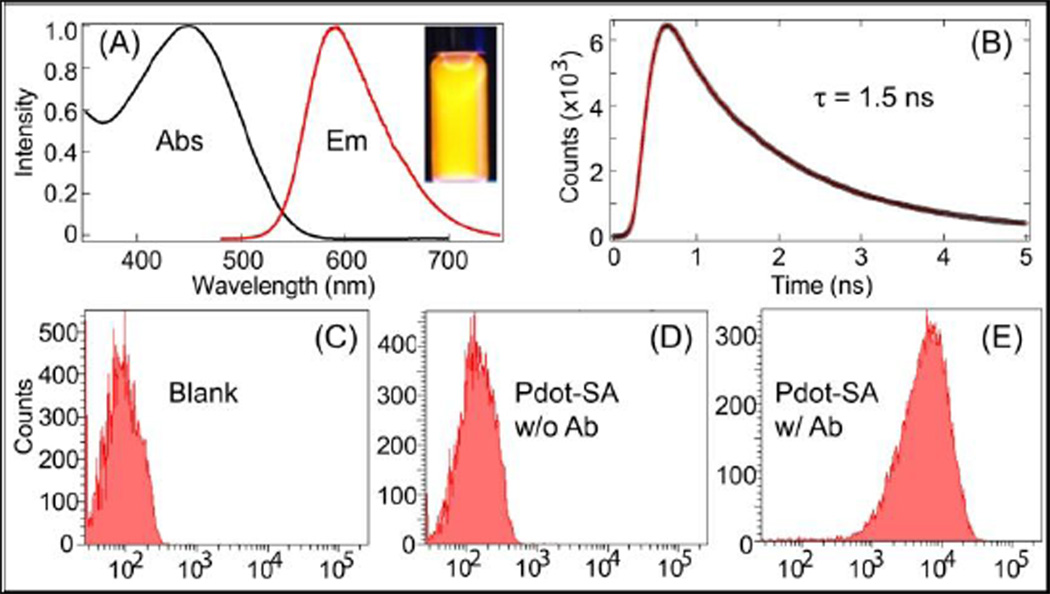
Importantly, the brightness of the CN-PPV dot’s fluorescence remained unchanged after carboxyl surface modification and streptavidin bioconjugation. Both the bare Pdots and surface-modified Pdots showed a similar quantum yield of ~ 60% with a peak absorption cross section as large as 2.3×10−13 cm2 for a 10-nm Pdot. This combination of high quantum yield and high cross section makes CN-PPV Pdots brighter by several orders of magnitude than conventional organic dyes, such as Alexa. Figure 2A shows the absorption and emission spectra of the CN-PPV Pdots. This Pdot can be conveniently excited by the 488-nm line of a blue laser, matching the CN-PPV Pdot well with most instruments currently available for biological imaging or flow cytometry. Using a time-correlated single-photon counting instrument (TCSPC) (Figure 2B), we determined CN-PPV dots have a fluorescence lifetime of ~1.5 ns, which is about 2–3 times shorter than most dyes that emit in the visible range. The CN-PPV Pdots also possessed high photostability; their fluorescence persisted for ~5 minutes under continuous exposure (power density at sample was ~9.0 × 103 W/cm−2) of the 488-nm laser of the confocal microscope (Zeiss LSM 510), while Alexa-labeled samples photobleached within 1 min under the same imaging conditions.
Figure 2.
(A) Absorption and emission of CN-PPV dot, inset shows the fluorescence image of a Pdot solution under a UV lamp. (B) Fluorescence decay lifetime (1.5 ns) of CN-PPV dots measured by a time-correlated single-photon counting instrument (TCSPC). The black dots represent experimental data, and the red line is the curve fit obtained using an iterative deconvolution method. Fluorescence intensity distributions of (C) unlabeled MCF-7 cells, (D) MCF-7 cells incubated with Pdot-streptavidin (Pdot-SA) in the absence of antibody (Ab), and (E) MCF-7 cells labeled with Pdot-SA and the biotinylated primary antibody against EpCAM. In the flow-cytometry experiment, a 488-nm laser excitation and a 582/42nm band pass filter was used.
We conducted flow cytometry to examine the specific binding of the CN-PPV bioconjugates. In this experiment, the Pdot-streptavidin probes and biotinylated primary anti - EpCAM antibody were used to label the cell-surface EpCAM receptors on MCF-7 cells (ESI†). The fluorescence intensity of MCF-7 cells incubated with only Pdot-streptavidin (no antibody) was similar to that of blank MCF-7 cells, suggesting that nonspecific binding was absent or extremely low for CN-PPV Pdot probes. The positive labeling with Pdot-streptavidin and biotinylated primary antibody exhibited strong fluorescence compared to the two control samples, indicating the high brightness of CN-PPV probes and the high specificity they have for their targets.
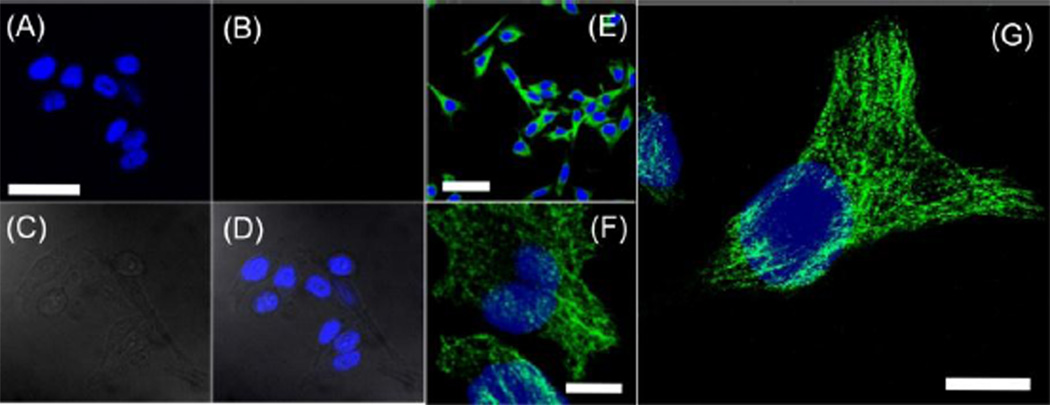
Finally, we carried out subcellular microtubule labeling in HeLa cells. Hela cells were fixed with 4% paraformaldehyde in phosphate buffered saline (PBS) and then permeabilized with 0.25% Triton-X 100/PBS solution. Biotinylated monoclonal anti-α-tubulin antibody and CN-PPV-streptavidin probes were then added to label microtubule structures in HeLa cells (ESI†). The HeLa cells incubated with Pdots in the presence or absence of biotinylated antibody were imaged by confocal microcopy (ESI†). Figure 3A–D shows the confocal images of HeLa cells incubated with Pdot-streptavidin without antibody where fluorescence wasn’t detected (Fig 3B). The absence of fluorescence in Panel 3B indicates nonspecific binding of Pdot was extremely low in these subcellular labelling experiments. In contrast, Figure 3E–G show representative confocal images at different magnifications of cells incubated with both Pdot-streptavidin and biotinylated anti-α-tubulin; specific and extremely bright fluorescence was observed from the labeled microtubules. Individual tubular structures were clearly seen and were well-resolved in the images. Under the same labeling conditions, similarly high-quality images of subcellular structures were not observed in our previous report when we used PFBT-streptavidin probes.12 The primary reason for this difference may be that the compact and small size of CN-PPV Pdot conjugates exhibited ultralow nonspecific binding in the subcellular environment compared to the larger PFBT probes. This feature of low nonspecific binding is advantageous for most biological applications, such as imaging cellular and subcellular targets.
Figure 3.
Two-color confocal fluorescence microscopy images of microtubules in HeLa cells labeled with Pdot-streptavidin. The blue channel was for the nucleus stain while the green channel showed emission from CN-PPV Pdots. For (A–D), cells were incubated with Pdot-streptavidin but in the absence of biotinylated primary antibody, clearly showing the absence of non-specific binding. (A) Image of nucleus; (B) image of microtubules (no fluorescence was detected due to the absence of biotinylated primary antibody);(C) bright-field image of cells; (D) two-color image obtained by merging panels (A) and (B). The scale bar in (A) is 50 µm. (E–G) are two-color images showing cells that were incubated with both Pdot-streptavidin and biotinylated anti-α-tubulin. The scale bars represent 50 µm, 10 µm and 10 µm for (E), (F) and (G), respectively.
In conclusion, we report a new Pdot that is compact and extremely bright. It spectrally complements existing Pdots because there currently aren’t any Pdots that emit in the orange wavelength range with high brightness. The CN-PPV Pdots didn’t show noticeable nonspecific binding during subcellular imaging. The small particle size, high brightness, and the ability to highly specifically target subcellular structures make the CN-PPV dots promising probes for biological imaging and bioanalytical applications.
Supplementary Material
Acknowledgments
We gratefully acknowledge support of this work by the National Institutes of Health (GM085485 and NS052637) and the National Science Foundation (CHE-0924320)
Footnotes
Electronic Supplementary Information (ESI) available: detailed experimental description. See DOI:
Notes and references
- 1.Wu C, McNeill J. Langmuir. 2008;24:5855–5861. doi: 10.1021/la8000762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wu C, Peng H, Jiang Y, McNeill J. J. Phys. Chem. B. 2006;110:14148–14154. doi: 10.1021/jp0618126. [DOI] [PubMed] [Google Scholar]
- 3.Wu C, Szymanski C, Cain Z, McNeill J. J. Am. Chem. Soc. 2007;129:12904–12905. doi: 10.1021/ja074590d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pu KY, Li K, Shi JB, Liu B. Chem Mater. 2009;21:3816–3822. [Google Scholar]
- 5.Rahim NAA, McDaniel W, Bardon K, Srinivasan S, Vickerman V, So PTC, Moon JH. Advanced Materials. 2009;21:3492–3496. [Google Scholar]
- 6.Wu C, Bull B, Szymanski C, Christensen K, McNeill J. ACS Nano. 2008;2:2415–2423. doi: 10.1021/nn800590n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Wu C, Sahu SP, Fernando LP, Szymanski C, McNeill J. J. Am. Chem. Soc. 2009;131:18410–18414. doi: 10.1021/ja907228q. [DOI] [PubMed] [Google Scholar]
- 8.Wu C, Bull B, Christensen K, McNeill J. Angew. Chem., Int. Ed. 2009;48:2741–2745. doi: 10.1002/anie.200805894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan Y-H, Wu C, Ye F, Jin Y, Smith PB, Chiu DT. Anal. Chem. 2011;83:1448–1455. doi: 10.1021/ac103140x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ye F, Wu C, Jin Y, Chan Y-H, Zhang X, Chiu DT. J. Am. Chem. Soc. 2011;133:8146–8149. doi: 10.1021/ja202945g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan Y-H, Jin Y, WU C, Chiu DT. Chem Comm. 2010;47:2820–2822. doi: 10.1039/c0cc04929h. [DOI] [PubMed] [Google Scholar]
- 12.Wu C, Schneider T, Zeigler M, Yu J, Schiro PG, Burnham DR, McNeill JD, Chiu DT. J. Am. Chem. Soc. 2010;132:15410–15417. doi: 10.1021/ja107196s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu C, Jin Y, Schneider T, Burnham DR, Smith PB, Chiu DT. Angew. Chem., Int. Ed. 2010;49:9436–9440. doi: 10.1002/anie.201004260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu C, Hansen SJ, Hou Q, Yu J, Zeigler M, Jin Y, Burnham DR, McNeill JD, Olson JM, Chiu DT. Angew. Chem., Int. Ed. 2010;50:3430–3434. doi: 10.1002/anie.201007461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu W, Howarth M, Greytak AB, Zheng Y, Nocera DG, Ting AY, Bawendi MG. J. Am. Chem. Soc. 2008;130:1274–1284. doi: 10.1021/ja076069p. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.