Abstract
Transtympanic administration of gentamicin is a widely accepted and effective approach for treating patients with intractable vertigo. Previous studies have demonstrated the uptake, distribution and effects of gentamicin in peripheral vestibular and cochlear structures after transtympanic injection. However, little is known about whether transtympanically administered gentamicin is trafficked into more central auditory and vestibular structures and its effect on these structures. In this study, we used immunofluorescence to determine the distribution of gentamicin within the auditory and vestibular brainstem. We observed gentamicin immunolabeling bilaterally in the vestibular efferent neurons, and in the superior olivary complex, and ipsilaterally in the cochlear nucleus 24 h after transtympanic administration of gentamicin, and that the drug could still be detected in these locations 30 days after injection. In contrast, no gentamicin labeling was detected in the vestibular nuclear complex. In the vestibular efferent neurons and superior olivary complex, gentamicin labeling was detected in the cytoplasm and cell processes, while in the cochlear nucleus gentamicin is mainly localized outside and adjacent to the cell bodies of neurons. Nerve fibers in cochlear nucleus, root of eighth nerve, as well as descending pathways from the superior olivary complex, are also immunolabeled with gentamicin continuously. Based on these data, we hypothesize that retrograde axonal transport of gentamicin is responsible for the distribution of gentamicin in these efferent nuclei including vestibular efferent neurons and superior olivary complex and anterograde axonal transport into the ipsilateral cochlear nucleus.
1. Introduction
Schuknecht first reported applying transtympanic administration of aminoglycoside in clinical management of intractable Meniere’s disease (Schuknecht, 1956). Since then, this clinical strategy has been extensively tested using low or high doses of transtympanically injected gentamicin, by continuous or titrated delivery, and clinical protocols have improved to achieve better control of vertigo with fewer adverse effects (Chia et al., 2004; Bodmer et al., 2007; Postema et al., 2008; Zhai et al., 2010). However, adverse effects such as hearing loss, constant disequilibrium as well as skew deviation, induced by transtympanic administration of gentamicin, and individual variation in responsiveness to gentamicin, remain problems in the efficacious clinical application of transtympanic administration of gentamicin (Ng et al., 2011).
Gentamicin has been detected throughout the peripheral cochlear and vestibular organs for up to 1 month after transtympanic administration of gentamicin (Imamura and Adams, 2003; Roehm et al., 2007). Lyford-Pike et al. reported that the selective loss of type I hair cells may occur due to preferential uptake of gentamicin by these cells after transtympanic administration of gentamicin. Type II hair cells and support cells take up substantially less gentamicin (Lyford-Pike et al., 2007).
Aminoglycosides damage the sensory hair cells in the inner ear after systemic or local administration. Damage to the peripheral organs of hearing and balance may produce changes in cellular metabolism, morphology and activity in the central nervous system. Lippe (1991) reported that gentamicin caused a significant and reversible reduction in the size of the rostral cochlear nucleus that varied as a function of location and survival time after systemic administration. Rosen et al. (1998) demonstrated that transtympanic administration of gentamicin resulted in abnormal astroglia in the cochlear nucleus and decreased cytochrome oxidase staining along the auditory neural pathway as far as the superior olivary complex as late as 30 days after injection in guinea pigs. Xu et al. (2009) reported that gentamicin-induced pathological alterations in the cochlear nucleus and are not exclusively a secondary consequence of the damage within the cochlea since the effect of gentamicin toxicity in the cochlear nucleus may occur simultaneously or prior to toxicity in the cochlea. Roehm et al. (2007) reported that gentamicin immunolabeling was present in ipsilateral dorsal cochlear nucleus after transtympanic administration of gentamicin in chinchilla, providing further evidence for potentially direct toxic effects of gentamicin in the cochlear nucleus. However, the precise distribution of gentamicin in the cochlear nucleus after transtympanic administration of gentamicin remains unknown. In addition, it is not known whether the vestibular nuclear complex takes up gentamicin as does the cochlear nucleus.
In the present study, we used immunofluorescence to localize gentamicin in the guinea pig cochlear nucleus, superior olivary nucleus (SOC), vestibular nuclear complex, as well as vestibular efferent neurons (EVe), since they innervate auditory and vestibular organs respectively. Immunolabeling for beta-III tubulin was used as a specific marker for neurons.
2. Materials and methods
2.1. Animals
Twenty-four adult male or female albino guinea pigs (weighing 250–350 g) were used. Guinea pigs were randomly divided into 6 groups (4/group), including 5 groups treated with gentamicin that were sacrificed at 1, 3, 7, 14 or 30 days later, and 1 group treated with saline as the control group. All experimental protocols were performed in accordance with the guidelines of Ethical Board of Eye Ear Nose and Throat Hospital, Fudan University, and approved by Committee on Care and Use of Animals (Shanghai).
2.2. Treatment and surgical procedures
The animals were anesthetized with intramuscular ketamine (40 mg/kg,) and xylazine (10 mg/kg), and placed on a warming blanket during anesthesia. The external auditory canal was sterilized with a povidone–iodine solution. Only animals without visible otitis media were used. Gentamicin (product #: G3632, Sigma, Saint Louis, USA.) at a concentration of 30 mg/ml dissolved in 0.9% saline was injected into the tympanic cavity after puncturing the tympanic membrane using a 100-μl microsyringe. The treatment solution was injected until the middle ear space was visibly full (100–120 μl). The animals’ heads were held stationary with the treated ear and nose turned toward the ceiling for 1 h to ensure the injected solution remained in contact with the round window. For the control group, sterile normal saline was administered in the same manner. The animals were sacrificed at 1, 3, 7, 14 and 30 days after drug administration, respectively.
2.3. Tissue preparation
At the designated time point, animals were deeply anesthetized with ketamine 40 mg/kg and xylazine 10 mg/kg and then exsanguinated by transcardial perfusion with 300 ml 37 °C physiological saline, followed by 250 ml of fixative (4% paraformaldehyde in 0.1 M phosphate buffer, pH = 7.4). After removal from the skull, the brainstem containing the vestibular nuclear complex, vestibular efferent neurons, cochlear nucleus and superior olivary complex was post-fixed overnight at 4 °C in the same solution. Tissues were then immersed in 20% sucrose solution for 24 h at 4 °C, followed by 30% sucrose solution. Afterwards, tissues were rapidly frozen with dry ice (solid carbon dioxide, CO2, −80 °C), and cut into 30 μm coronal sections using a cryostat (Leica, Germany). Sections were transferred to a cryoprotectant consisting of sucrose (300 g/l) and ethylene glycol (300 ml/l) dissolved in 0.1 M phosphate buffer saline, and stored −20 °C.
2.4. Immunohistochemistry
After washing in PBS (0.01 M phosphate buffer saline, pH = 7.2–7.4) for 10 min for three times, sections were blocked for non-specific immunoreactivity (10% goat serum in 0.2% Triton X-100) for 1.5 h at room temperature (20–25 °C). Sections from all groups were incubated with mouse anti-gentamicin monoclonal antibodies (1:250; QED Biosciences, San Diego, CA) and rabbit anti-beta-III tubulin antibodies (1:500; Abcam), in blocking solution for 48 h at 4 °C, and secondarily-labeled with Alexa Fluor-555-conjugated goat anti-mouse antibodies (1:200 in PBS containing 5% goat serum and 0.1% Triton X-100, Invitrogen) and Alexa Fluor-488-conjugated goat anti-rabbit antibodies for 1 h at 37 °C. Subsequently, sections were incubated with 4,6-diamidino-2-phenylindole (DAPI, 1:500 in PBS) for 20 min at room temperature to label nuclei (Invitrogen). Between all labeling steps, 10 min (3×) rinses in PBS were performed. Sections were then mounted under coverslips on glass slides in a 1:1 mixture of 0.1 M PB and glycerol. Negative control sections were processed without the primary antibody. Adjacent sections were stained with 1% toluidine blue solution for 3 minutes, rinsed with flowing tap water, degraded through 70%, 95% and 2 changes of 100% alcohol, cleared in xylene (2 changes, 3 min each) and mounted with resinene.
2.5. Confocal microscopy
Fluorescent signals were detected by sequential excitation at 490 nm and emission at 520 nm (Alexa Fluor-488), 555 nm and 570 nm (Alexa Fluor 546), 358 nm and 461 nm (DAPI) by confocal laser scanning microscopy (TCS SP5, Leica, Germany).
2.6. Stereology
The number of neurons with gentamicin immunolabeling in the lateral vestibular efferent neurons (L-EVe) was counted using the optical fractionator. Briefly, neurons were counted as having gentamicin immunoreactivity if they showed: (a) gentamicin immunoreactivity within the cell body, and (b) part of the neuronal nucleus was visible.
2.7. Statistical analysis
Measurements of gentamicin-positive cells were statistically analyzed using SPSS 16.0 (SPSS, Inc., Chicago, IL). One-way analysis of variance (ANOVA) was performed and followed by the least-significant difference (LSD test) to test the differences between groups of different survival time points. Comparison of cell counts between the regions of interest on either side of the midline for each group was performed using the paired-sample t test. p Value < 0.05 was determined to be statistically significant.
3. Results
3.1. Identification of nuclei
Cross-sectioned brain slices stained with toluidine blue illustrate the relative locations of EVe, vestibular nuclear complex, SOC and cochlear nucleus in Fig. 1 (facilitate the interpretation of Figs. 5 and 9). The descending pathways of SOC that project to the cochlea were marked in white dashed lines (Fig. 1), based on (i) the relative locations of SOC nuclei, and (ii) previous studies by Møller (2006). The dorsal group of vestibular efferent neurons (lateral EVe, L-EVe) is located in close proximity to the genu (g7), the root of facial nerve and the subependymal granular layer of the fourth ventricle (4V). Neurons of M-EVe are scattered across the lateral tegmental field. Three subgroups of the vestibular nuclear complex, including the superior (SV), lateral (LV) and medial vestibular nuclei (MV) are present in the same section and occupy a large area lateral to the 4th ventricle (Fig. 1). Toluidine blue staining of the SOC also demonstrated the location of the lateral superior olive (LSO) and medial superior olive (MSO), respectively. The cochlear nuclei including the dorsal (DCN) and the ventral (VCN) nucleus are situated at the lateral border of each section on either side of the midline (Fig. 1).
Fig. 1.
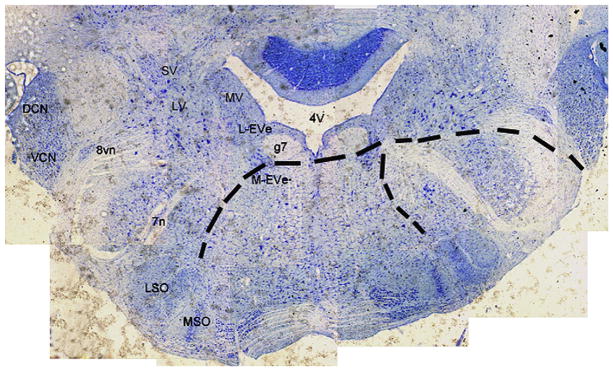
Cross-section of the brainstem stained with toluidine blue, showing the location of the vestibular efferent neurons, vestibular nuclear complex, cochlear nucleus and superior olivary complex in the same slice. The dashed lines represent the descending pathways of SOC to the cochlea. DCN, dorsal cochlear nucleus; VCN, ventral cochlear nucleus; SV, superior vestibular nucleus; LV, lateral vestibular nucleus; MV, medial vestibular nucleus; 8vn, vestibular root of the 8th nerve; 7n, facial nerve; L-EVe, lateral vestibular efferent neurons; M-EVe, medial vestibular efferent neurons; g7, genu of the facial nerve; 4V, 4th ventricle; MSO, medial superior olive; LSO, lateral superior olive; and SOC, superior olivary complex.
Fig. 5.
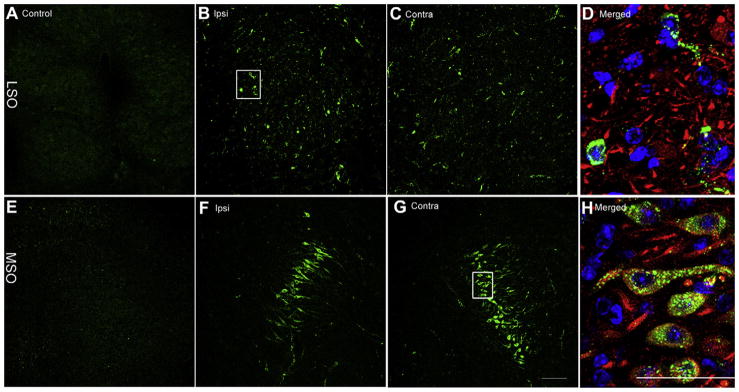
Bilateral distribution of gentamicin immunolabeling in the MSO and LSO 7 days following gentamicin administration. Negligible gentamicin immunolabeling is present in LSO (A) and MSO (E) from the saline control group. Scattered positive gentamicin immunloabeling was observed in the ipsilateral (B) and contralateral (C) LSO, with a focused band of labeled neurons in the ipsilateral (F) and contralateral (G) MSO. (D) and (H) Triple-labeled high-resolution images (of the boxed area in B and G, respectively) revealed that gentamicin immunolabeling (green) is mainly distributed within the cytoplasm and axons of neurons. Blue, DAPI; red, beta-III tubulin immunolabeling. Scale bar = 50 μm in A, B, C, E, F, and G, and 100 μm in D and H. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 9.
Gentamicin immunolabeling along the descending efferent pathways from the SOC 7 days after transtympanic administration. Adjoining images taken from the same section were spliced together to demonstrate the route of nerve fibers with gentamicin immunolabeling. White dashed lines indicate the route of gentamicin immunolabeled nerve fibers (green) following the typical route of the descending efferent pathways from the contralateral SOC, along the surface of the floor of fourth ventricle, to the root of the vestibular nerve. Red dashed lines indicate the route of gentamicin immunolabeled nerve fibers (green) along the descending pathway from the ipsilateral SOC to the root of vestibular nerve. Each lower panel magnifies the corresponding image in the respective upper panel. Red, beta-III tubulin immunolabeling. Scale bar is 300 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
3.2. Distribution of gentamicin in the central vestibular system
No gentamicin immunolabeling was detected in the Scarpa’s ganglion neurons between 3 and 14 days after transtympanic gentamicin injection. Neither were any subgroups of the vestibular nuclear complex labeled (data not shown), indicating that little gentamicin penetrated into vestibular afferent neurons post-transtympanic injection.
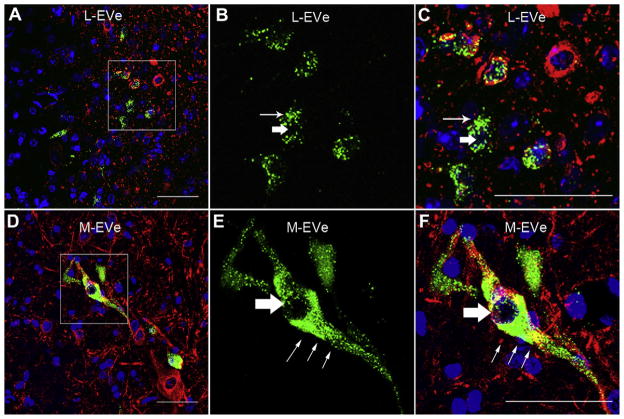
In contrast, both subgroups of the EVe neurons, the L-EVe and M-EVe, were immunolabeled for gentamicin 3 days after transtympanic injection of gentamicin (Fig. 2). Fewer gentamicin immunolabeled neurons were observed in the M-EVe than in the L-EVe (Fig. 2A and D), and neurons were not always immunolabeled in the M-EVe on either side of the midline in a single section. Particulate gentamicin immunolabeling was located in the cytoplasm and axons of EVe neurons (Fig. 2, B and E) but was not detected within the nucleus (Fig. 2, B and E). Gentamicin immunolabeled neurons in the L-EVe were smaller than those in the M-EVe, and typically consisted of fusiform, circular or polygonal shapes (Fig. 2C and F). In contrast, large bipolar gentamicin immunolabeled neurons were typically observed in the M-EVe (Fig. 2D). The intensities of gentamicin immunolabeling between the ipsilateral and contralateral L-EVe were almost equivalent (Fig. 3). In control groups incubated without primary antibodies, or treated with saline and subsequently processed for gentamicin immunofluorescence, no specific labeling was demonstrated (Fig. 3).
Fig. 2.
Gentamicin immunolabeling in the L-EVe and M-EVe 7 days following transtympanic administration. (A) Gentamicin immunolabeling (green) in the ipsilateral L-EVe 3 days after transtympanic injection. Blue represents DAPI labeling of ds-DNA in the nuclei of cells and red represents specific beta-III tubulin immunolabeling in neurons. (B) Magnified image of the boxed area in A for gentamicin immunofluorescence only. Labeling is intense and punctate within the cytoplasm (thin arrow in B and C) but not in nucleus (thick arrow in B and C) of neurons. (C) Magnified image of the boxed area in (A) for all three fluorophores. (D) Ipsilateral M-EVe 3 days after transtympanic gentamicin treatment. One bipolar neuron showed intense gentamicin immunolabeling within the cytoplasm and axons but not within the nucleus. (E) High-resolution image of the boxed area in (D) for gentamicin immunofluorescence only. Gentamicin immunolabeling is intense and granular in cytoplasm (thin arrow in E and F) and is absent in the neuronal nucleus (thick arrow in E and F). (F) Magnified image of the boxed area in D for all three fluorophores. Fewer and larger diameter gentamicin immunolabled neurons are present in the M-EVe than in the L-EVe. The fluorescence intensity of gentamicin immunolabeling was similar between M-EVe and L-EVe. Scale bar = 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
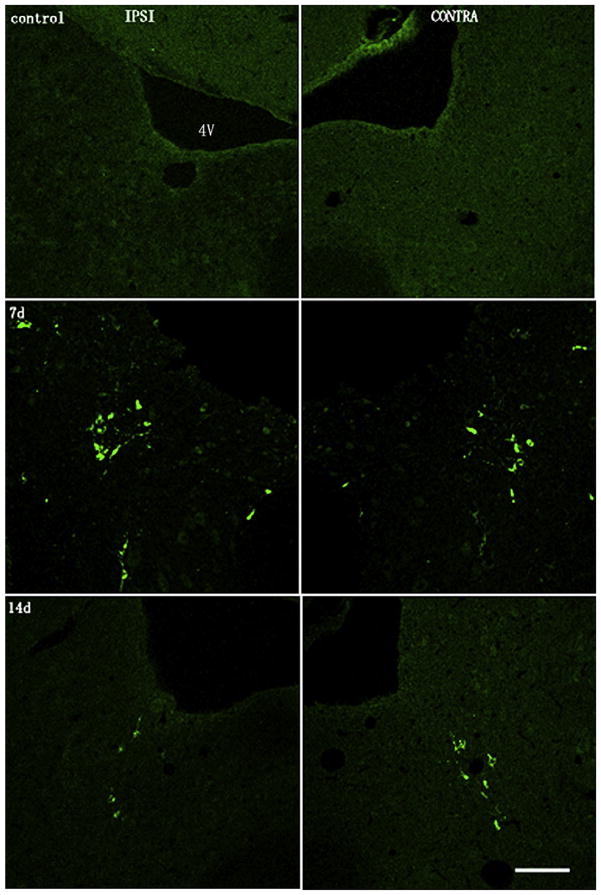
Fig. 3.
Distribution of gentamicin in ipsilateral and contralateral L-EVe at different timepoints following unilateral transtympanic injection. Control: L-EVes showed negligible gentamicin immunolabeling after treatment with saline only. 7d: Ipsilateral and contralateral L-EVes are immunolabeled for gentamicin (green) 7 days post-transtympanic administration. Gentamicin is mainly immunostained within the cytoplasm of neurons. 14d: 14 days post-transtympanic administration of gentamicin, L-EVes show qualitatively decreased intensity of gentamicin immunolabeling compared with 7d. Scale bar = 50 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
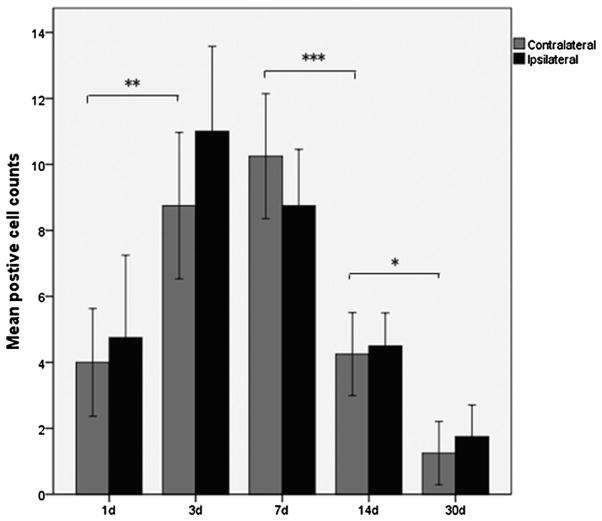
Gentamicin immunolabeling was detected at the earliest time-point, 24 h post-injection (Fig. 4). The number of gentamicin immunolabeled neurons in the L-EVe increased between 1 and 3 days, and remained at this high level at 7 days, before decreasing at 14 days post-injection. At 30 days post-injection, few gentamicin-positive neurons were observed in the L-EVe (Fig. 4). Statistical comparisons of the number of gentamicin immunolabeled neurons in L-EVe at different time points, using one-way ANOVA (F = 21.632, p < 0.001) and LSD test, revealed a significant increase of labeled neurons at 3 days compared with the 24 h timepoint (p < 0.001), no significance difference between 3 and 7 days (p = 0.204), a significant decrease between 7 and 14 days (p < 0.001), and a further significant decrease between 14 and 30 days (p = 0.014; Fig. 4). However, there was no statistical difference in numbers of gentamicin immunolabeled neurons between the ipsilateral and contralateral L-EVe within each group using the paired-samples t test (p > 0.05).
Fig. 4.
Mean number (±SEM) of gentamicin immunolabeled neurons in the L-EVe at different timepoints post-injection from the ipsilateral and contralateral L-EVe in the same section (N = 4) for each group. Differences in the number of gentamicin immunolabeled neurons were observed between 1 and 3 days (LSD: p < 0.01), 7 and 14 days (LSD: p < 0.001), as well as between 14 and 30 days (LSD: p < 0.05) were statistically significant, but not between 3 and 7 days (LSD: p = 0.204). Comparisons of the number of gentamicin immunolabeled neurons between the ipsilateral L-EVe (gray bars) and the contralateral (black bars) were not statistically different for each group (paired-samples t test, p > 0.05). (*p < 0.05; **p < 0.01; ***p < 0.001).
3.3. Distribution of gentamicin in the central auditory system
The SOC, including the LSO (Fig. 5B–D) and MSO (Fig. 5F–H), also exhibited bilateral gentamicin immunolabeling following unilateral transtympanic injection of gentamicin. More gentamicin immunolabeled neurons were observed in the ipsilateral LSO ((Fig. 5B) than in the contralateral LSO ((Fig. 5C) 7 days post-injection (subjective comparison by one of the authors unaware of treatment conditions). Higher resolution imaging revealed punctate gentamicin immunolabeling in the cytoplasm and along their processes, but not in the nucleus of neurons (Fig. 5D and H). Gentamicin was rarely detected outside the neuron, as in the EVe (Fig. 2).
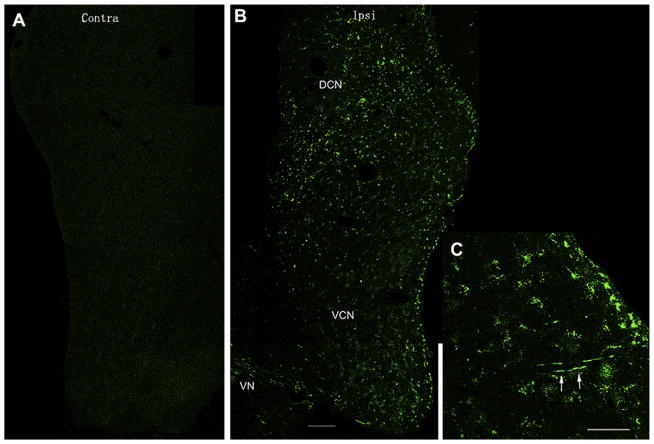
Gentamicin labeling was also identified in the cochlear nucleus. However, the pattern of gentamicin immunolabeling in the cochlear nucleus was different from the EVe and SOC. First, gentamicin immunolabeling occurred ipsilaterally in the cochlear nucleus (Fig. 6B), but bilaterally in the EVe and SOC. Second, gentamicin immunolabeling in the cochlear nucleus was identified outside and adjacent to the cell bodies of neurons (Fig. 7), which is possibly located within the nerve terminals from the spiral ganglion. Gentamicin immunolabeling was intense throughout the dorsal and ventral cochlear nucleus 7 days post-injection. Several axons within the dorsal cochlear nucleus were also immunolabeled with gentamicin, indicating that the cochlear nerve is a possible trafficking pathway for gentamicin entering the cochlear nucleus from the cochlear periphery (Figs. 6C, 7D and F). Long fibers with gentamicin immunolabeling were not frequently observed since most axons were cross-sectioned in these coronal sections.
Fig. 6.
Gentamicin immunolabeling in the ipsilateral cochlear nucleus at 1 day after transtympanic administration. (A) Gentamicin immunolabeling is absent in the contralateral cochlear nucleus (Contra/contralateral, DCN/dorsal cochlear nucleus). This figure is a composite of images from one brain section spliced together. (B) Gentamicin immunolabeling (green) was extensive in the ipsilateral DCN (Ipsi/ispilateral) and ventral cochlear nucleus (VCN). The intensity of anti-GT immunostaining in the DCN is stronger than that in the VCN. This figure is a composite of images from one brain section spliced together. (C) Nerve fibers within the DCN also displayed gentamicin immunolabeling (white arrows). Images are from the same tissue sample, different section. Scale bar = 100 μm. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
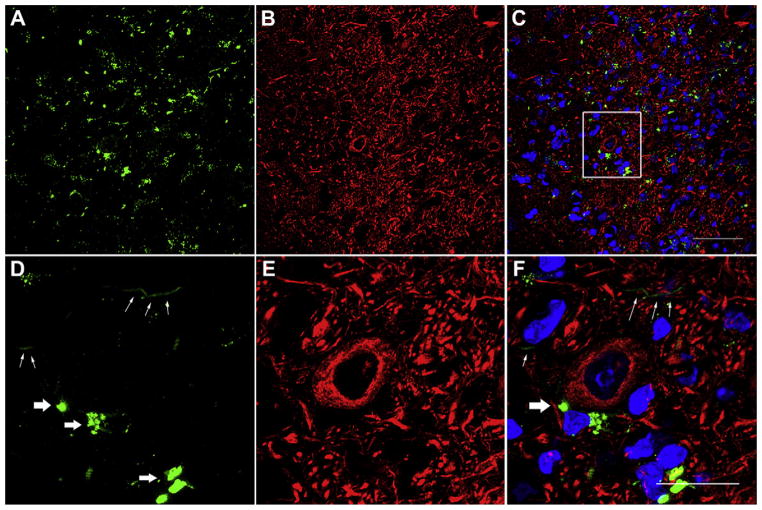
Fig. 7.
Intense gentamicin immunolabeling (green) within the cochlear nucleus, but not within the cell bodies. (A)–(C)are higher magnification of DCN in Fig. 6, and (D), (E) and (F) show the boxed area in (C). White thick arrows in (D) and (F) indicate gentamicin immunolabeling outside neurons. White thin arrows in (F) show nerve fibers with gentamicin immunolabeling. Blue (DAPI), red (beta-III tubulin immunolabeling). Scale bar = 50 μm in A, B and C, and = 20 μm in D, E and F. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
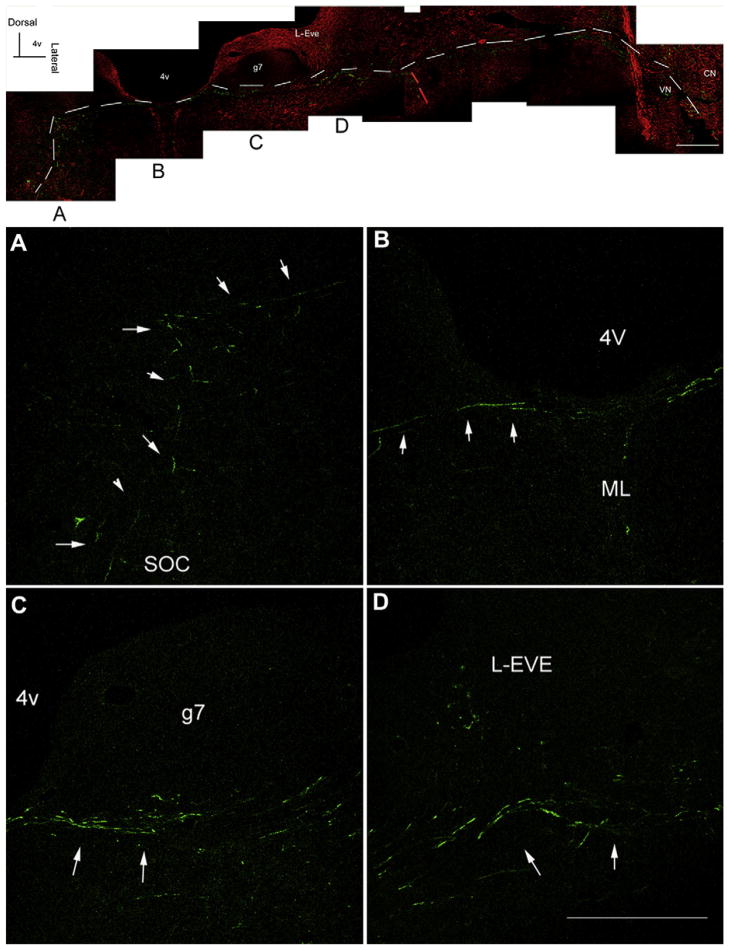
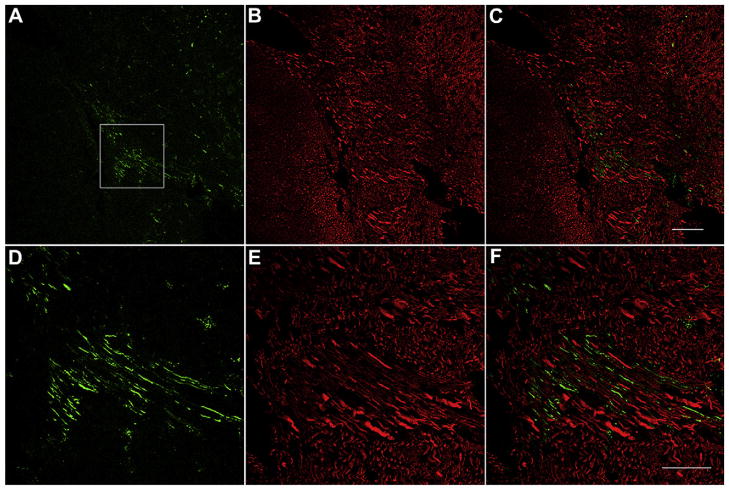
Significant gentamicin immunolabeling was also detected along the root of the 8th nerve within the brainstem (Fig. 8). This gentamicin fluorescence does not overlap with the beta-III tubulin immunolabeling, but lay parallel and adjacent to each other, suggesting that gentamicin may enter the brainstem via retrograde transport along these nerve fibers. One descending pathway of the SOC was continuously immunolabeled for gentamicin from the SOC to the root of the 8th nerve in the brainstem (Fig. 9). The pathway originates from the area of the SOC, travels close to the floor of the 4th ventricle, crosses the midline, and turns medially to the genu of the facial nerve, following the nerve fibers of the 8th nerve, and finally out of the brainstem toward the cochlear organs. The intensity of gentamicin immunolabeling along this descending pathway at 1 day after transtympanic administration is consistent.
Fig. 8.
Gentamicin immunolabeling (green) along the vestibular root of 8th nerve 7 days after transtympanic administration. (A)–(C) show the lower magnification and (D)–(F) illustrate the higher magnification of boxed area in (A). Red, beta-III tubulin immunlabeling. The gentamicin immunolabeling (green) along the nerve fibers does not overlap with the beta-III tubulin immunlabeling but lays parallel and adjacent to each other. Scale bar is 100 μm in A, B and C, and 50 μm in D, E, and F. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
This study explored the uptake and distribution of gentamicin in auditory and vestibular nuclei within the brainstem following transtympanic administration. The results demonstrated that transtympanically administered gentamicin is taken up by neurons in some but not all auditory and vestibular nuclei in the brainstem, in addition to uptake of gentamicin by the inner ear (Hirvonen et al., 2005; Dai et al., 2006; Roehm et al., 2007; Lyford-Pike et al., 2007). Gentamicin immunofluorescence was observed bilaterally in the EVe and SOC, and the ipsilateral cochlear nucleus, 24 h after unilateral injection. Neurons in the EVe retained gentamicin immunolabeling for at least 30 days following injection. In contrast, no gentamicin immunolabeling could be detected in vestibular nuclear complex. These findings provide new insight into effects of gentamicin on auditory and vestibular neural systems and their function following transtympanic injection. The direct effects of gentamicin toxicity may be associated with constant disequilibrium, skew deviation and hearing loss as complications following transtympanic administration of gentamicin. To our knowledge, this is the first published report of gentamicin uptake by central nuclei after transtympanic administration of gentamicin.
These data suggest that retrograde axonal transport of gentamicin is responsible for the observed distribution of gentamicin in the EVe and SOC, and anterograde transport of gentamicin to the ipsilateral cochlear nucleus. This drug trafficking pathway has potential to treat central auditory disorders associated with the superior olivary nucleus and vestibular efferent neurons such as migraine-anxiety related dizziness (Furman et al., 2005) and presbycusis (Zhu et al., 2007). Besides, dexamethasone and gentamicin are frequently applied transtympanically to manage patients with sudden hearing loss or to control vertigo attacks in patients with intractable Meniere’s disease in clinical practice. Our present results show that gentamicin can be transported to the central part of the auditory and vestibular systems. This raises the question of whether other drugs could be transported into the central nervous system via the same mechanism(s).
4.1. Uptake and axonal transport of gentamicin
Axonal transport is classified according to its direction: (i) anterograde refers to transport of substances from the cell body along the axon to distal locations, while (ii) retrograde refers to transport of substances along the axon toward the cell body (Alberts et al., 2002). Retrograde transport can traffic many substances including horseradish peroxidase (HRP), nerve growth factor, neurotoxic viruses, and significantly adriamycin (Oldfield and McLachlan, 1977; Sidenius and Jakobsen, 1981; van der Kooy et al., 1985; Bigotte and Olsson, 1982), and provides a means to traffic substances into the central nervous system. However, the mechanisms underlying the uptake of substances and their axonal transport remain controversial. Two mechanisms have been demonstrated for the uptake and retrograde transport of substances from the nerve terminals: (i) non-specific pinocytosis, and (ii) specific, receptor-mediated endocytosis (Anderson et al., 1981). Additionally, damage to nerve terminals can also facilitate the uptake of substances, such as HRP and neurotrophins (Oldfield and McLachlan, 1977; Curtis et al., 1998). Gentamicin can damage the nerve terminals of vestibular and cochlear end organs (Hennig and Cotanche, 1998; Hong et al., 2006), which may facilitate its uptake and subsequent transport to the brainstem.
Based on these previous studies, we hypothesized that gentamicin can be taken up by the nerve terminals surrounding hair cells and transported to the neuronal cell bodies. If this is the case, both afferent and efferent nuclei should present with gentamicin immunoreactivity. However, our results show specific gentamicin immunolabeling in bilateral EVe and SOC (Figs. 2 and 5), and ipsilaterally in the cochlear nucleus (Fig. 6). There was negligible immunoreactivity in the Scarpa’s ganglion and vestibular nuclear complex. This is discussed in more detail below.
4.2. Gentamicin immunoreactivity in EVe
The EVe of guinea pigs has two subgroups (Shumilina et al., 1986), the lateral and medial groups (Fig. 1). Axons of EVe project to vestibular hair cells bilaterally and terminate on the afferents of type I hair cells and cell bodies of type II. In the present study, intense gentamicin immunolabeling was observed bilaterally in both the EVe subgroups (Figs. 2A and 3) after a unilateral transtympanic injection of gentamicin onto the round window, suggesting that retrograde transport of gentamicin along vestibular efferent fibers is the most likely cause of gentamicin immunofluorescence observed in EVe neurons. Our observations contrast with those of Roehm et al. (2007) who reported no evidence of gentamicin immunoreactivity (using immunoperoxidase labeling techniques) in vestibular or cochlear efferent cell bodies or nerve fibers. This discrepancy may be due to the more sensitive detection of immunofluorescence signals compared to immunoperoxidase chromogenic deposition.
Intense gentamicin immunolabeling in the cytoplasm of cell bodies and axons of vestibular efferent neurons provides further evidence for the retrograde transport. The lack of gentamicin immunolabeling in the nucleus, suggesting that gentamicin cannot pass through the nuclear membrane, corresponds with the lack of nuclear labeling for either gentamicin or gentamicin conjugated to texas red (GTTR) in vestibular sensory hair cells (Lyford-Pike et al., 2007). However, the lack of immunolabeling in the nucleus may also be due to steric hindrance to the immunogenic site of gentamicin bound to nuclear RNA (Myrdal et al., 2005).
We also observed vestibular nerve fibers entering the brainstem with significant gentamicin immunoreactivity (Fig. 8), providing further evidence of gentamicin trafficking into the brainstem via retrograde trafficking along vestibular nerve fibers.
4.3. Gentamicin immunoreactivity in SOC
The SOC of guinea pigs consists of three main nuclei: LSO, MSO, and the medial nucleus of the trapezoid body (Reuss, 2000). The olivocochlear bundle, which projects from SOC to the cochlea has two parts. One part is uncrossed olivocochlear bandle, which travels medially to the genu of facial nerve, then along the root of ipsilateral vestibular nerve, and projects to the ipsilateral cochlea (Møller, 2006). The other part of the olivocochlear system is crossed olivocochlear bandle, which crosses the midline, medially to the genu of facial nerve, along the root of contralateral vestibular nerve, and projects to the contralateral cochlea (Møller, 2006). Neurons of the LSO mainly project to the ipsilateral cochlea and terminate on the afferent fibers of inner hair cells, whereas neurons of the MSO mainly project to the contralateral and terminate directly on the cell bodies of outer hair cells.
In the present study, the bilateral LSO and MSO nuclei, as well as the descending pathways of SOC have significant gentamicin immunofluorescence, suggesting that gentamicin enters the SOC by retrograde transport along the descending efferent pathways from the SOC (Figs. 5 and 9).
Combined with the results in EVe, we hypothesize that transtympanic administration has the potential to deliver drugs directly to the efferent neurons of auditory and vestibular nuclei in the brainstem. Further studies are needed to determine if other substances such as dexamethasone can be also transported retrogradely to the same nuclei after the transtympanic administration, and whether these drugs have a pharmacological effect on their activity and function.
4.4. Inner ear ganglia, cochlear nucleus and vestibular nucleus
The distribution of gentamicin in the spiral ganglion and cochlear nucleus following transtympanic administration has been previously reported (Roehm et al., 2007). However, its exact location within the cochlear nucleus was still unknown. In this study, we demonstrated that the entire cochlear nuclear complex displayed extensive gentamicin immunoreactivity, and that the intensity of immunoreactivity in the DCN was greater than in the VCN (Fig. 6B). Roehm et al. (2007) also reported that cell bodies in the ipsilateral dorsal cochlear nucleus bordering the cochlear aqueduct (CA) had a lateral to medial gradient of gentamicin immunoreactivity, and that the lining of the CA was also labeled with tritiated gentamicin, suggesting the CA could potentially transfer gentamicin to the contralateral cochlea. However, no such gradient was demonstrated in this study. High-resolution fluorescence imaging (Fig. 7D and F) revealed gentamicin immunoreactivity in the ipsilateral cochlear nucleus, but not within the cell bodies, which is different from the distribution in the EVe and SOC (Figs. 2 and 5, within the cell bodies). One possible reason is that efferent terminals originate from cell bodies within the brainstem nuclei that directly innervate the hair cells, while the afferent terminals originate from the primary afferent bipolar neurons in the spiral ganglion which innervate cochlear hair cells directly. This would suggest that gentamicin could be transported from the periphery by retrograde trafficking along the axon directly to the cell bodies of efferent neurons in brainstem from the periphery, while anterograde transport through cochlear afferent neurons and subsequent trans-synaptic trafficking into the cochlear nucleus is far less efficient. No gentamicin is demonstrated in the contralateral cochlear nucleus (Fig. 6A), since the afferents of cochlear hair cells mainly project to the ipsilateral cochlear nucleus.
We had expected that vestibular afferent neurons would show gentamicin immunofluorescence similar to that in cochlear afferent neurons. Tritiated gentamicin was detected in the Scarpa’s ganglion in chinchilla after transtympanic administration (Roehm et al., 2007), however, in guinea pigs, negligible gentamicin immunoreactivity was observed (Imamura and Adams, 2003). Our data confirm this lack of gentamicin immunoreactivity in the Scarpa’s ganglion and vestibular nuclear complex between 24 h to 14 days in guinea pigs. This may be the result of different species or dose of gentamicin. Alternatively, the Scarpa’s ganglion, compared to the spiral ganglion, is farther from the site of drug delivery at the round and oval windows, which may account for the infrequent gentamicin immunoreactivity in the Scarpa’s ganglion. Furthermore, afferent neurons in Scarpa’s ganglion form calyces around vestibular hair cells, unlike the bouton synapses found on cochlear hair cells, which may restrict vestibular afferent fiber uptake of gentamicin.
4.5. Effects of gentamicin on auditory and vestibular nuclei
The impact of gentamicin on cochlear nucleus function after transtympanic administration may result from both the direct ototoxicity (Xu et al., 2009; Theopold, 1976) and the deafferentation (Moore et al., 1998). The distribution of gentamicin in the cochlear nucleus (Fig. 6) reported in this study provides evidence for the possibility of direct gentamicin-induced neurotoxicity. Little evidence was found reporting the effects of gentamicin on the SOC and EVe, while it has been reported that gentamicin can reversibly block the contralateral efferent suppression of ipsilateral cochlear activity following intramuscular injection of a high dose of gentamicin (150 mg/kg) in guinea pigs (Lima et al., 1998). This may be due to gentamicin blockade of ion channels in the post-synaptic membrane (Aran et al.,1999), or due to synaptic plasticity following toxicity. Our ongoing studies will further explore the morphological and electro-physiological impacts of gentamicin on brainstem neural networks.
Acknowledgments
This study was supported by Educational Ministry of China (No: NCET-06-0369); National Natural Science Foundation (No. 30772398, No. 81070785); Project on Advanced and Frontier Techniques for Shanghai Municipal Hospital (SHDC12010119); 973 Project (2011CB504504), and National Institute of Deafness and Other Communication Disorders (DC 04555, PSS).
Abbreviations
- ANOVA
analysis of variance
- CA
cochlear aqueduct
- DCN
dorsal cochlear nucleus
- EVe
vestibular efferent neurons
- g7
genu of facial nerve
- L-EVe
lateral vestibular efferent neurons
- LSO
lateral superior olivary nucleus
- LV
lateral vestibular nuclei
- M-EVe
medial vestibular efferent neurons
- MSO
medial superior olivary nucleus
- MV
medial vestibular nuclei
- SOC
superior olivary nucleus
- SV
superior vestibular nucleus
- VCN
ventral cochlear nucleus
References
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, Walter P. Molecular Biology of the Cell. 4. Garland Science; New York: 2002. [Google Scholar]
- Anderson PN, Mitchell J, Mayor D. On the mechanism of the uptake of horseradish peroxidase into the retrograde transport system of ligated post-ganglionic sympathetic nerves in vitro. J Anat. 1981;133:371–379. [PMC free article] [PubMed] [Google Scholar]
- Aran JM, Erre JP, Lima DCD, Debbarh I, Dulon D. Acute and chronic effects of aminoglycosides on cochlear hair cells. Ann N Y Acad Sci. 1999;884:60–68. doi: 10.1111/j.1749-6632.1999.tb08636.x. [DOI] [PubMed] [Google Scholar]
- Bigotte L, Olsson Y. Retrograde transport of doxorubicin (adriamycin) in peripheral nerves of mice. Neurosci Lett. 1982;32:217–221. doi: 10.1016/0304-3940(82)90296-8. [DOI] [PubMed] [Google Scholar]
- Bodmer D, Morong S, Stewart C, Alexander A, Chen JM, Nedzelski JM. Long-term vertigo control in patients after intratympanic gentamicin instillation for Meniere’s disease. Otol Neurotol. 2007;28:1140–1144. doi: 10.1097/mao.0b013e31815aea05. [DOI] [PubMed] [Google Scholar]
- Chia SH, Gamst AC, Anderson JP, Harris JP. Intratympanic gentamicin therapy for Meniere’s disease: a meta-analysis. Otol Neurotol. 2004;25:544–552. doi: 10.1097/00129492-200407000-00023. [DOI] [PubMed] [Google Scholar]
- Curtis R, Tonra JR, Stark JL, Adryan KM, Park JS, Cliffer KD, Lindsay RM, DiStefano PS. Neuronal injury increases retrograde axonal transport of the neurotrophins to spinal sensory neurons and motor neurons via multiple receptor mechanisms. Mol Cell Neurosci. 1998;12:105–118. doi: 10.1006/mcne.1998.0704. [DOI] [PubMed] [Google Scholar]
- Dai CF, Mangiardi D, Cotanche DA, Steyger PS. Uptake of fluorescent gentamicin by vertebrate sensory cells in vivo. Hear Res. 2006;213:64–78. doi: 10.1016/j.heares.2005.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman JM, Balaban CD, Jacob RG, Marcus DA. Migraine-anxiety related dizziness (MARD): a new disorder? J Neurol Neurosurg Psychiatr. 2005;76:1–8. doi: 10.1136/jnnp.2004.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennig AK, Cotanche DA. Regeneration of cochlear efferent nerve terminals after gentamycin damage. J Neurosci. 1998;18:3282–3296. doi: 10.1523/JNEUROSCI.18-09-03282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirvonen TP, Minor LB, Hullar TE, Carey JP. Effects of intratympanic gentamicin on vestibular afferents and hair cells in the chinchilla. J Neurophysiol. 2005;93:643–655. doi: 10.1152/jn.00160.2004. [DOI] [PubMed] [Google Scholar]
- Hong SH, Park SK, Cho YS, Lee HS, Kim KR, Kim MG, Chung WH. Gentamicin induced nitric oxide-related oxidative damages on vestibular afferents in the guinea pig. Hear Res. 2006;211:46–53. doi: 10.1016/j.heares.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Imamura S, Adams JC. Distribution of gentamicin in the guinea pig inner ear after local or systemic application. J Assoc Res Otolaryngol. 2003;4:176–195. doi: 10.1007/s10162-002-2036-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima DCD, Erre JP, Aran JM. Aminoglycoside ototoxicity and the medial efferent system: I. comparison of acute and chronic gentamicin treatments. Audiology. 1998;37:151–161. doi: 10.3109/00206099809072969. [DOI] [PubMed] [Google Scholar]
- Lippe WR. Reduction and recovery of neuronal size in the cochlear nucleus of the chicken following aminoglycoside intoxication. Hear Res. 1991;51:193–202. doi: 10.1016/0378-5955(91)90036-9. [DOI] [PubMed] [Google Scholar]
- Lyford-Pike S, Vogelheim C, Chu E, Della SC, Carey JP. Gentamicin is primarily localized in vestibular type I hair cells after intratympanic administration. J Assoc Res Otolaryngol. 2007;8:497–508. doi: 10.1007/s10162-007-0093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Møller AR. Hearing: Anatomy, Physiology, and Disorders of the Auditory System. 2. Academic Press; 2006. pp. 89–92. [Google Scholar]
- Moore DR, Rogers NJ, O’Leary SJ. Loss of cochlear nucleus neurons following aminoglycoside antibiotics or cochlear removal. Ann Otol Rhinol Laryngol. 1998;107:337–343. doi: 10.1177/000348949810700413. [DOI] [PubMed] [Google Scholar]
- Myrdal SE, Johnson KC, Steyger PS. Cytoplasmic and intra-nuclear binding of gentamicin does not require endocytosis. Hear Res. 2005;204:156–169. doi: 10.1016/j.heares.2005.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng D, Fouladvand M, Lalwani AK. Skew deviation after intratympanic gentamicin therapy. Laryngoscope. 2011;121:492–494. doi: 10.1002/lary.21279. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, McLachlan EM. Uptake and retrograde transport of HRP by axons of intact and damaged peripheral nerve trunks. Neurosci Lett. 1977;6:135–141. doi: 10.1016/0304-3940(77)90008-8. [DOI] [PubMed] [Google Scholar]
- Postema RJ, Kingma CM, Wit HP, Albers FW, Van Der Laan BF. Intratympanic gentamicin therapy for control of vertigo in unilateral Meniere’s disease: a prospective, double-blind, randomized, placebo-controlled trial. Acta Otolaryngol. 2008;128:876–880. doi: 10.1080/00016480701762458. [DOI] [PubMed] [Google Scholar]
- Reuss S. Introduction to the superior olivary complex. Microsc Res Tech. 2000;51:303–306. doi: 10.1002/1097-0029(20001115)51:4<303::AID-JEMT1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Roehm P, Hoffer M, Balaban CD. Gentamicin uptake in the chinchilla inner ear. Hear Res. 2007;230:43–52. doi: 10.1016/j.heares.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Rosen JH, Thompson GC, Hill BB, Thompson AM. Neurodegenerative changes in the guinea pig brainstem after intratympanic injection of gentamicin. Brain Res. 1998;813:177–180. doi: 10.1016/s0006-8993(98)00972-x. [DOI] [PubMed] [Google Scholar]
- Schuknecht HF. Ablation therapy for the relief of Meniere’s disease. Laryngoscope. 1956;66:859–870. doi: 10.1288/00005537-195607000-00005. [DOI] [PubMed] [Google Scholar]
- Sidenius P, Jakobsen J. Retrograde axonal transport. A possible role in the development of neuropathy. Diabetologia. 1981;20:110–112. doi: 10.1007/BF00262011. [DOI] [PubMed] [Google Scholar]
- Shumilina VF, Preobrazhenskii NN, Maiskii VA. Study of vestibular efferent neurons of the guinea pig by the technic of retrograde axonal transport of horseradish peroxidase and with fluorochromes. Neirofiziologiia. 1986;18:738–747. [PubMed] [Google Scholar]
- Theopold HM. Animal experiments concerning the neurotoxicity of aminoglycosid antibiotics. Electron microscopic findings regarding dose-depending mitochondrial damage in the cochlear nucleus of the guinea pig (author’s transl) Laryngol Rhinol Otol (Stuttg) 1976;55:786–794. [PubMed] [Google Scholar]
- van der Kooy D, Zito KA, Roberts DC. Evidence on the retrograde neurotoxicity of doxorubicin. Neurosci Lett. 1985;53:215–219. doi: 10.1016/0304-3940(85)90188-0. [DOI] [PubMed] [Google Scholar]
- Xu M, Hu HT, Jin Z, Chen G, Wang WX, Fan YL, Anniko M, Duan M. Ototoxicity on cochlear nucleus neurons following systemic application of gentamicin. Acta Otolaryngol. 2009;129:745–748. doi: 10.1080/00016480802454716. [DOI] [PubMed] [Google Scholar]
- Zhai F, Liu JP, Dai CF, Wang Q, Steyger PS. Evidence-based modification of intratympanic gentamicin injections in patients with intractable vertigo. Otol Neurotol. 2010;31:642–648. doi: 10.1097/MAO.0b013e3181dbb30e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Vasilyeva ON, Kim S, Jacobson M, Romney J, Waterman MS, Tuttle D, Frisina RD. Auditory efferent feedback system deficits precede age-related hearing loss: contralateral suppression of otoacoustic emissions in mice. J Comp Neurol. 2007;503:593–604. doi: 10.1002/cne.21402. [DOI] [PubMed] [Google Scholar]