Abstract
We report four new patients with a submicroscopic deletion in 15q24 manifesting developmental delay, short stature, hypotonia, digital abnormalities, joint laxity, genital abnormalities, and characteristic facial features. These clinical features are shared with six recently reported patients with a 15q24 microdeletion, supporting the notion that this is a recognizable syndrome. We describe a case of an ~2.6 Mb microduplication involving a portion of the minimal deletion critical region in a 15-year-old male with short stature, mild mental retardation, attention deficit hyperactivity disorder, Asperger syndrome, decreased joint mobility, digital abnormalities, and characteristic facial features. Some of these features are shared with a recently reported case with a 15q24 microduplication involving the minimal deletion critical region. We also report two siblings and their mother with duplication adjacent and distal to this region exhibiting mild developmental delay, hypotonia, tapering fingers, characteristic facial features, and prominent ears. The deletion and duplication breakpoints were mapped by array comparative genomic hybridization and the genomic structure in 15q24 was analyzed further. Surprisingly, in addition to the previously recognized three low-copy repeat clusters (BP1, BP2, and BP3), we identified two other paralogous low-copy repeat clusters that likely mediated the formation of alternative sized 15q24 genomic rearrangements via non-allelic homologous recombination.
Introduction
The recent expanded use of high resolution genome analysis by array comparative genomic hybridization (array CGH) has led to the identification of several novel microdeletion and microduplication syndromes (Slavotinek 2008; Stankiewicz and Beaudet 2007). These rearrangements can be recurrent, resulting from non-allelic homologous recombination (NAHR) between homologous low-copy repeats (LCRs), and lead to loss or gain of a dosage-sensitive gene or genes (Stankiewicz and Lupski 2002) like 15q13.3 deletion (Sharp et al. 2008), 15q24 deletion (Sharp et al. 2007), 17p11.2 deletion (Smith–Magenis syndrome) and duplication (Potocki–Lupski syndrome) (Bi et al. 2003; Potocki et al. 2007), and 17q21.31 deletion and duplication (Kirchhoff et al. 2007; Koolen et al. 2006; Sharp et al. 2006; Shaw-Smith et al. 2006). Recently, a number of non-recurrent rearrangements have been shown also to result from genomic architectural features that may stimulate their formation through the proposed fork stalling and template switching (FoSTeS)/microhomology-mediated break-induced replication (MMBIR) mechanism (Lee et al. 2007), e.g., MECP2 duplication (Carvalho et al. 2009), deletions and duplications in 17p13.3 (Bi et al. 2009; Nagamani et al. 2009), and deletions and duplications in 17p11.2 (Zhang et al. 2009; for review see Gu et al. 2008).
With the exception of the common recurrent 15q11.2q12 deletions which, depending on parental origin, result in either Prader–Willi syndrome or Angelman syndrome, and deletions and duplications in 15q13.3 (Sharp et al. 2008; Ben-Shachar et al. 2009), rearrangements of chromosome 15q are relatively rare (Cushman et al. 2005); those involving the 15q24 region were described in patients with growth deficiency, psychomotor retardation, and birth defects (Bettelheim et al. 1998; Clark 1984; Formiga et al. 1988; Spruijt et al. 2004). Cushman et al. (2005) reported three patients with a 15q24 deletion and reviewed the previously reported cases with cytogenetically visible deletions involving the 15q22q24 region. They concluded that the majority of these cases shared developmental delay, growth deficiency, hypotonia, skeletal abnormalities, urogenital system defects, and similar facial features, including epicanthal folds, ear abnormalities, and arched palate, and it was suggested that these cases represent a new cytogenetic deletion syndrome.
Sharp et al. (2007) identified overlapping submicroscopic 15q24 deletions in four patients with developmental delay, growth deficiency, digital abnormalities, hypospadias, loose connective tissue, and characteristic facial features, including high anterior hair line, broad medial eyebrows, hypertelorism, down-slanting palpebral fissures, long smooth philtrum, and full lower lip. The deletion breakpoints mapped to homologous LCR clusters, designated as BP1, BP2, and BP3, with a minimal deletion critical region of ~1.7 Mb spanning the genomic interval from BP1 to BP2. Subsequently, Klopocki et al. (2008) reported another patient who shared common clinical features with the previously reported patients and had a 15q24 microdeletion with breakpoints mapping to BP1 and BP3. Recently, Van Esch et al. (2009) described an additional patient with a 15q24 microdeletion, who presented with features common to the previously reported patients, including developmental delay, loose connective tissue, digital and genital anomalies, and distinct facial features; in addition, this patient has a congenital diaphragmatic hernia. The proximal deletion breakpoint mapped to an LCR cluster located proximal to BP1, whereas the distal breakpoint coincided with BP2.
Six cytogenetically visible interstitial duplications involving 15q24 have been reported in patients with growth deficiency, psychomotor retardation, birth defects, and characteristic facial features, including facial asymmetry, ear malformations, micrognathia and palatal clefting (Browne et al. 2000; Dhaliwal et al. 1990; Han et al. 1999; Roggenbuck et al. 2004). Pectus excavatum was found to be associated with a duplication in 15q23–q26 (Brewer et al. 1999). Recently, an ~1.7 Mb microduplication in 15q24, extending from BP1 to BP2, has been described in a 2-year-old boy with global developmental delay, characteristic facial features, digital and genital abnormalities. This duplication is apparently reciprocal to the minimal deletion critical region for the 15q24 deletion syndrome and was inherited from his healthy father (Kiholm Lund et al. 2008).
We report four new patients with an overlapping 15q24 microdeletion (cases 1–4) and further delineate the 15q24 deletion syndrome. In addition, we describe four patients with 15q24 microduplications: one patient (case 5) with duplication involving part of the minimal deletion critical region, and two siblings (cases 6 and 7) and their mother, who have duplication adjacent and distal to the minimal deletion critical region. We also redefine the genomic structure of this chromosomal region guided by breakpoint mapping of the above patients that revealed additional LCR clusters.
Subjects and methods
DNA samples
Patients 1–5 were referred to the Medical Genetics Laboratories of Baylor College of Medicine for clinical array-CGH analysis. Patients 6 and 7 were referred to the Cytogenetic Laboratory of Cincinnati Children’s Hospital Medical Center for SNP array analysis. DNA samples were obtained from the probands and their family members after informed consents approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine or Cincinnati Children’s Hospital Medical Center. DNA was extracted from whole blood using the Puregene DNA extraction kit (Gentra, Minneapolis, MN, USA) according to the manufacturer’s instructions.
Array CGH
The four 15q24 deletion cases 1–4 and the duplication case 5 were identified through screening the database of Baylor College of Medicine Medical Genetics Laboratories of array CGH studies performed in over 9,000 patients using oligonucleotide Chromosomal Microarray Analysis (CMA Versions 6 and 7 OLIGO) (Cheung et al. 2005; Ou et al. 2008). Of these patients, approximately 4,700 patients were tested using V6 OLIGO (44 K array) and over 4,000 patients using V7 OLIGO (105 K array).
The oligonucleotide based chromosomal microarray version 6 (CMA V6 OLIGO) consists of 44 K targeted oligonucleotides emulating genomic regions contained within BAC clones arranged in the BAC V6 CMA. It contains an average of 28–30 oligos per genomic region interrogated by single BAC clones with a minimum of approximately 10–15 oligos whenever possible (Ou et al. 2008). This array was used for patients 2, 3, and 4.
The oligonucleotide based chromosomal microarray version 7 (CMA V7 OLIGO) utilizes array-based comparative genomic hybridization with approximately 105,000 oligos covering the whole genome at an average resolution of 30 kb with increased coverage at known disease loci. Included in CMA are probes for all the known microdeletion/microduplication syndromes (over 270 genetic syndromes), 41 unique subtelomeric regions, all 43 unique pericentromeric regions and the mitochondrial genome. In addition to these targeted regions, the entire genome (between disease regions) is covered with an average resolution of 30 kb, excluding repetitive sequences through a combination of bioinformatics, computation, and empirical studies. The array also includes six regions of known polymorphic variants: the Amylase (AMY1A) gene cluster in lp21.2, the UDP glucuronosyltransferase 2 (UGT2B17) gene in 4q13.2, the chemokine CC motif ligand 3 and 4 (CCL3, CCL4) genes in 17q12, the defensin beta 107B (DEFB107B) gene family in 8p23.1, the opsin 1 medium-wave-sensitive 2 (OPN1MW2) gene in Xq28, and exon 2 of the Apolipoprotien (LPA) gene in 6q26 to serve as internal controls. This array was performed in patients 1 and 5. Patient 2 was also analyzed with CMA V.7 OLIGO to better delineate the breakpoints (Fig. 1a, b).
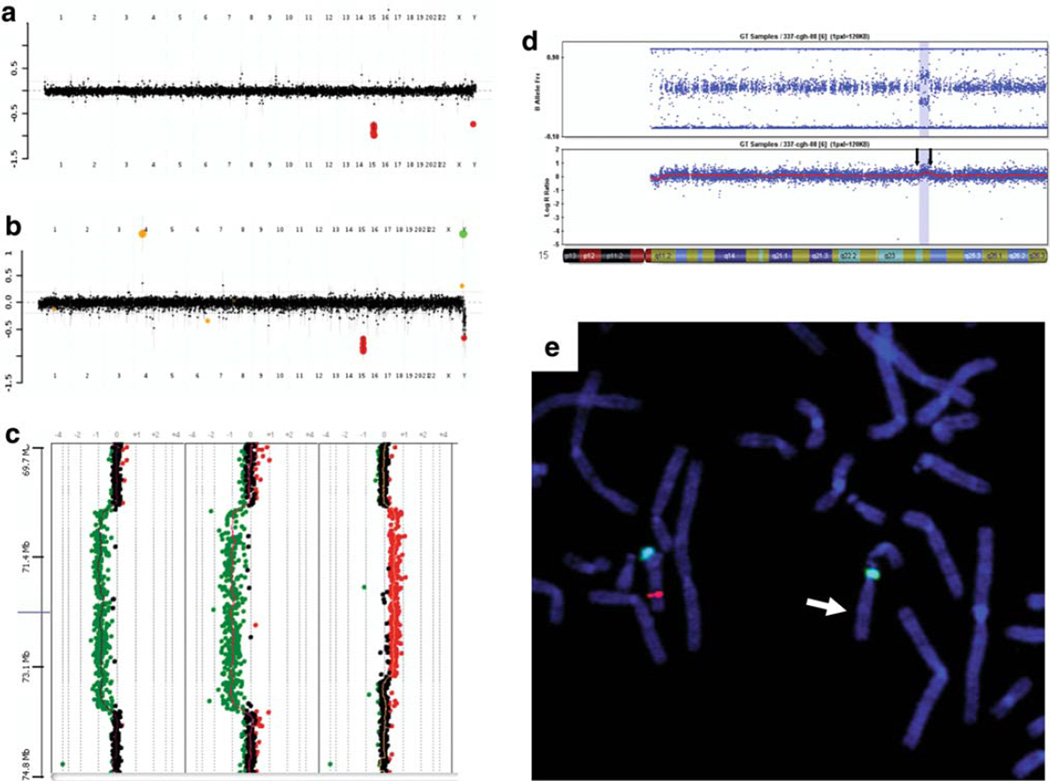
Fig. 1.
a, b The oligonucleotide based chromosomal microarray version 7 (CMA V7 OLIGO) profiles for patients 1 and 2. The averaged combined data of hybridizations performed using reference DNA. Oligonucleotides presented with red color and displaced down, indicating a loss of chromosome 15 material in the patient versus the reference DNA. c Results of Agilent 244 K array-based oligonucleotide CGH performed on DNA samples for patients 3 showing the deleted region 15q24 (70.750–73.856 Mb), patient 4 showing the deleted region (70.780–73.856 Mb), and patient 5 showing the duplicated region 15q24 (70.708–73.332 Mb). A deviation of the dots to the left of the central axis indicates a loss in copy number (represented with green dots), and a deviation to the right of the central axis indicates a gain in copy number (represented by red dots). d Chromosome 15q24 duplication in patients 6 and 7 using the Illumina DNA Beadarray (SNP) 370 K chip (region indicated by arrows and shaded). The upper plot shows the B allele frequency and the lower plot shows the Log R ratio (DNA dosage). SNP region is showing duplication between rs9672824 and rs4886534. Linear position on chromosome 15:73858160–75969880, with the total size of 2.11 Mb, and including 208 markers. e Results of the FISH analysis in patient 4. The two green fluorescence signals represent chromosome 15 centromeric FISH probe, and the single red signal represents probe specific to the deleted region (indicated by arrow), confirming the 15q24 deletion
The rearrangements in three patients (cases 3–5) were further investigated using a high-resolution Agilent 244 K Whole Human Genome Oligo Microarray (Agilent Technologies, Inc., Santa Clara, CA), which contains 238,459 probes, representing a compiled view of the human genome at a 8.9 kb overall median probe spacing (7.4 kb in Refseq genes) (http://www.chem.agilent.com) (Fig. 1c). The procedures for DNA digestion, labeling, and hybridization were performed according to the manufacturer’s instructions with some modifications (Probst et al. 2007).
The slides were scanned into image files using a GenePix Model 4000B microarray scanner (Molecular Devices, Sunnyvale, CA, USA) or an Agilent G2565 laser scanner. Microarray image files of oligo arrays were quantified using Agilent Feature extraction software (v9.0), and text file outputs from the quantitation analysis were imported to our in-house analysis package for copy number analysis, as described (Ou et al. 2008).
SNP microarray
SNP microarray studies were performed in patients 6 and 7 using the commercially available Illumina 370 K DNA Beadchip. DNA was processed according to manufacturer’s specifications and chips processed according to the protocol.
Cytogenetic and FISH analyses
Confirmatory chromosome and FISH analyses with BAC clones were performed on peripheral blood lymphocytes using standard procedures after detection of copy number changes observed in the CMA as described by Shchelochkov et al. (2008).
Bioinformatics and in silico sequence analysis
Genomic sequence based on the oligonucleotide coordinates from the array CGH experiment was downloaded from UCSC genome browser (Build 36, UCSC genome browser, March 2006). Interspersed repeat sequences were analyzed by RepeatMasker (http://www.repeatmasker.org). Regional assemblies for known LCR15q24s (BP1, BP2, and BP3) and a search for additional LCRs were assembled using NCBI BLAST 2 and the Sequencher software (Gene Codes). We eliminated small insertions/deletions from the calculation of the DNA sequence identity and took only base pair changes into account.
Results
Clinical findings associated with 15q24 deletion and duplication
We identified overlapping 15q24 microdeletions involving the minimal critical region and ranging in size from 3.10 to 3.95 Mb, in four unrelated patients (cases 1–4). These individuals share many features, including developmental delay, short stature, hypotonia, digital anomalies, joint laxity, genital abnormalities, and characteristic facial features, including a long smooth philtrum and ear malformations (Table 1; Fig. 2a, b). The deletions are de novo in patients 1, 3, and 4. The mother of patient 2 was reported to be healthy, but limited data are available about the father, and both parents were not tested.
Table 1.
The deleted regions and the clinical manifestations in patients with the 15q24 deletion
| Case 1 | Case 2 | Case 3 | Case 4 | Van Esch et al. (2009) | Klopocki et al. (2008) |
Sharp et al. (2007) IMR349 |
Sharp et al. (2007) C45/06 | Sharp et al. (2007) ID204 | Sharp et al. (2007) IMR371 | |
|---|---|---|---|---|---|---|---|---|---|---|
| del 15q24 (length) | 72.252– 75.937 Mb (3.686 Mb) |
72.130– 76.080 Mb (3.950 Mb) |
70.750– 73.856 Mb (3.106 Mb) |
70.708– 73.856 Mb (3.148 Mb) |
70.6– 73.7 Mb (3.1 Mb) |
72.2– 75.9 Mb (3.7 Mb) |
72.15– 76.01 Mb (3.86 Mb) |
72.15– 76.01 Mb (3.86 Mb) |
72.15– 73.85 Mb (1.7 Mb) |
70.40– 74.21 Mb (3.72 Mb) |
| Inheritance | De novo | Unknown | De novo | De novo | De novo | De novo | De novo | De novo | Unknown | De novo |
| Age and gender | 33 months | 5 months | 14 years | 9 years | 33 years | 10 years | 14 years | 14 years | 33 years | 15 years |
| Male | Female | Male | Male | Male | Male | Male | Male | Male | Male | |
| Growth | Short stature | Short stature | Normal growth | Obesity | Obese Macrocephaly | Obesity | Short stature Microcephaly IUGR |
Short stature Microcephaly IUGR |
Short stature Microcephaly IUGR |
Normal growth |
| Development | Mild delay | MR | Delayed | Moderate MR | Severe MR | Mild MR | Mild MR | Mild MR | Mild MR | Mild MR |
| Face | High anterior hair line | Frontal bossing | Normal appearance | Asymmetry | High anterior hair line |
High anterior hair line |
High anterior hair line |
High anterior hair line |
High anterior hair line |
High anterior hair line |
| Broad forehead | Brachycephaly | Round face | Long face | Long narrow face | Asymmetry | Asymmetry | Asymmetry | |||
| Small maxilla large mandible |
Happy facial expression |
Happy facial expression |
Happy facial expression |
|||||||
| Long narrow face | ||||||||||
| Eye | Sparse eye brows | Normal appearance | Broad medial eyebrows |
Broad medial eyebrows |
Broad medial eyebrows |
Broad medial eyebrows |
Broad medial eyebrows |
Broad medial eyebrows |
||
| Hypertelorism | Hypertelorism | Hypertelorism | Hypertelorism | Hypertelorism | Hypertelorism | |||||
| Down-slanting palpebral fissures |
Down-slanting palpebral fissures |
Down-slanting palpebral fissures |
Down-slanting palpebral fissures |
Down-slanting palpebral fissures |
Down-slanting palpebral fissures |
|||||
| Epicanthus | Epicanthus | Epicanthus | Strabismus | Strabismus | Strabismus | Strabismus Epicanthus | ||||
| Nose | Depressed nasal bridge | Broad upturned nasal tip | Normal appearance | Normal appearance | Broad nasal base | Hypoplastic nostrils | Broad nasal base | Broad nasal base | Small nose | Broad nasal base |
| Flaring alae nasi | Flaring alae nasi | Hypoplastic alae nasi | Flaring alae nasi | |||||||
| Mouth | Long smooth philtrum |
Small mouth | Small mouth | Smooth philtrum | Long smooth philtrum |
Long smooth philtrum |
Long philtrum | Long smooth philtrum |
Long smooth philtrum |
Long philtrum |
| Full lower lip | High palate | Full lower lip | Full lower lip | Full lower lip | Full lower lip | |||||
| Bifid uvula | ||||||||||
| Ear | Ear lobe pit | Normal appearance | Thick, small ears | Cup-shaped protruding ears |
Large ears | NA | Ear abnormalities | Ear abnormalities | Small everted ears | Hearing loss |
| Ear lobe pit | Hearing loss | |||||||||
| Nervous system | Hypotonia | Myelomeningocele Hydrocephalus |
Hypotonia | Hypotonia | Hypotonia | Hypotonia | Wide basal cisterna on brain MRI |
Hypotonia | NA | NA |
| Dysplastic corpus callosum on brain MRI |
NA | NA | ||||||||
| Seizure | ||||||||||
| Genital | Normal | Normal | Hypospadias | Microphallus | Microphallus Cryptorchidism | Microphallus | Normal | Hypospadias | Hypospadias | Hypospadias |
| Skeletal | No abnormalities | Clubfeet | Joint laxity | Joint laxity | Scoliosis | Joint laxity | Joint laxity | Scoliosis | Joint laxity | Joint laxity |
| Pectus carinatum | Pes planus | Clubfeet | Lumbar lordosis Genua valga |
Scoliosis | Narrow chest Pes cavus |
|||||
| Digital anomalies | Small hands | Overriding second toes | Long first toes and short second toes |
Brachymesopalangy II and V | Long slender fingers with distal tapering |
Broad thumb | Long slender fingers | Proximally implanted thumbs |
Small hands | Hypoplastic right thumb |
| Clinodactyly | Clinodactyly | Brachydactyly | Proximally implanted thumbs |
Brachydactyly | Contractures of fingers |
|||||
| Clinodactyly | ||||||||||
| Respiratory | No symptoms | No symptoms | Recurrent ear infections |
Recurrent ear infections |
High pitched voice |
Nasal speech | Recurrent ear infections |
Low tone voice | Recurrent upper airway infections |
Recurrent chest infections |
| Velo-pharyngeal insufficiency |
Hoarse voice | Asthma | ||||||||
| Soft nasal speech | Clinodactyly | |||||||||
| Other | Acute lymphoblastic leukemia |
Tetralogy of Fallot | Café au lait spots | Aggressiveness | Hyperactivity Aggressiveness | Inguinal and umbilical hernia | Attention deficit hyperactivity disorder |
Edema of extremities | Feeding difficulties as child |
Diaphragmatic hernia |
| Feeding difficulties | Feeding difficulties | Cafe-au-lait spots | Diaphragmatic hernia | Skin laxity | Autistic features | GH deficiency | Inguinal hernia | |||
| Hepatomegaly | Acanthosis nigricans | Inguinal hernia | Splenomegaly | GH deficiency | Hypogonado-tropic hypogonadism |
|||||
| Delayed puberty Bowel atresia |
Bowel atresia |
NA not reported, MR mental retardation, IUGR intrauterine growth restriction
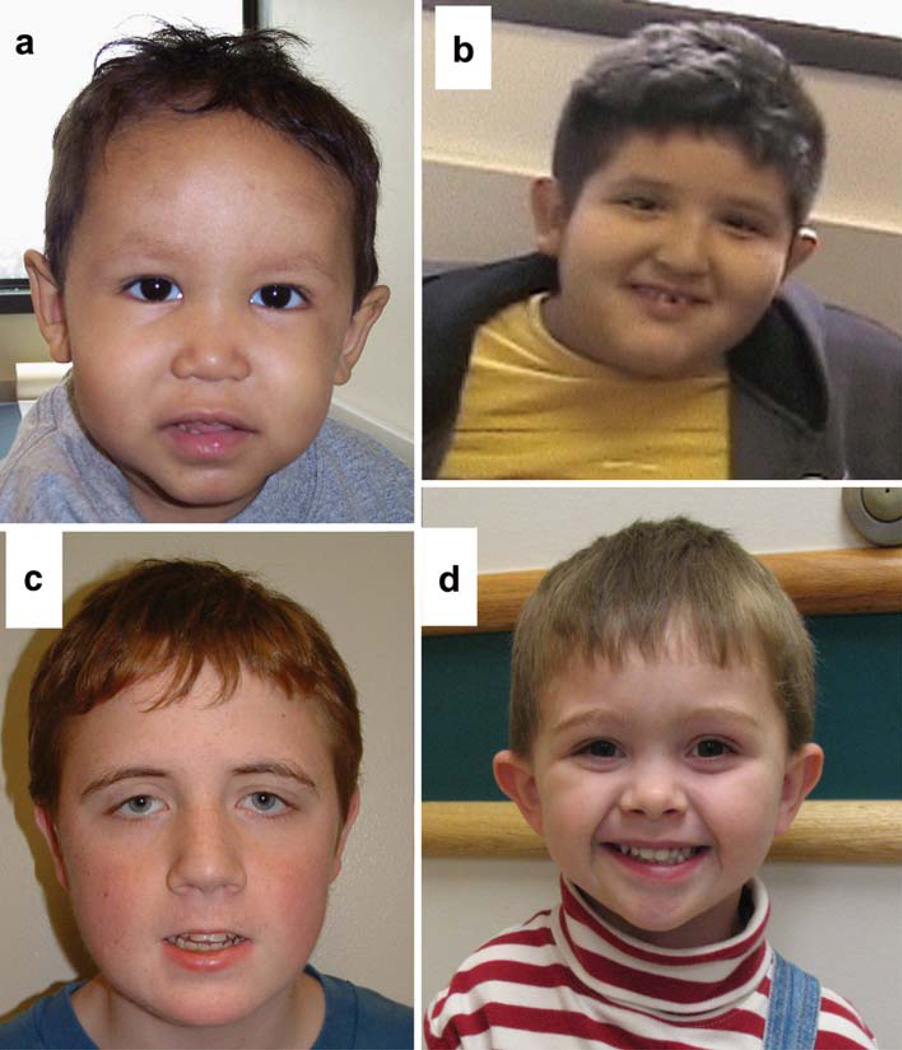
Fig. 2.
a Patient 1: Facial features, including high anterior hair line, broad forehead, sparse eye brows, epicanthal fold, depressed nasal bridge, long smooth philtrum, and full lower lip. b Patient 4: Facial features, including round face, facial asymmetry with left-sided smaller, cup-shaped protruding ears, and smooth philtrum. c Patient 5: Facial features, including long face, epicanthal folds, down-slanting palpebral fissures, ptosis, full puffy hooded eyelids, high nasal bridge, smooth philtrum, thin upper lip, and full lower lip. d Patient 6: Facial features, including rounded face, hypertelorism, flattened nasal bridge, and prominent ears
We also identified four patients with a 15q24 microduplication, one patient (case 5) has a duplication involving a portion of the minimal deletion critical region, whereas two siblings (case 6–7) and their mother have a duplication adjacent and distal to the minimal deletion critical region. Patient 5 is a 15-year-old male with short stature, mild mental retardation (IQ 71), behavioral problems, decreased range of motion of elbows, wrist and fingers, increased tone, and characteristic facial features (Table 2; Fig. 2c). Serum amino acids and urine organic acid analysis, karyotype, fragile X syndrome DNA testing, brain MRI, and EEG were all normal. Chromosomal microarray analysis revealed an ~ 2.62 Mb duplication in 15q24 (70.708–73.332 Mb, build 36). The mother was reported to have depression. She was tested by FISH for the duplication with normal results. The father was reported to have schizophrenia; he was not available for either examination or testing, but the mother reported that the patient’s facial features do not resemble his father’s facies.
Table 2.
The duplicated regions with the clinical manifestations in patients with the 15q24 duplication and the summary of the clinical features of the 10 patients with the 15q24 deletion
| 15q24 deletion | 15q24 duplications involving the minimal deletion critical region |
15q24 duplications not involving the minimal critical region |
|||
|---|---|---|---|---|---|
| Case 5 | Kiholm Lund et al. (2008) | Case 6 | Case 7 | ||
| Dup 15q24 (length) | 70.708–73.332 Mb (2.62 Mb) | 72.15–73.85 Mb (1.7 Mb) | 73.858–75.969 Mb (2.11 Mb) | 73.858–75.969 Mb (2.11 Mb) | |
| Inheritance | Unknown | Inherited from the father | Inherited from the mother | Inherited from the mother | |
| Age and gender | 15 years, male | 2 years, male | 3.5 years, male | 2 years, female | |
| Growth | |||||
| Short stature | 5/10 | Short stature | Normal growth | Normal growth | Normal growth |
| Microcephaly | 3/10 | – | – | – | – |
| IUGR | 3/10 | – | – | – | – |
| Obesity | 3/10 | – | – | – | – |
| Development delay | 10/10 | Mild MR | Delayed | Mildly delayed | Mildly delayed |
| Face | |||||
| High anterior hair line | 7/10 | – | Normal appearance | – | – |
| Facial asymmetry | 4/10 | – | – | – | – |
| Long face | 3/10 | Long face | – | Round face Flat occiput Plagiocephaly | Round face |
| Eye | |||||
| Broad medial eyebrows | 6/10 | – | – | – | – |
| Down-slanting palpebral fissures | 6/10 | Down-slanting palpebral fissures | Down-slanting palpebral fissures | – | – |
| Hypertelorism | 6/10 | – | Hypertelorism | Hypertelorism | Hypertelorism |
| Epicanthus | 4/10 | Epicanthus | Epicanthus | – | Epicanthus |
| Full puffy hooded eyelids | |||||
| Strabismus | 4/10 | – | Strabismus | – | – |
| Ptosis | Thick upper eyelid | ||||
| High arched eyebrows | |||||
| Nose | |||||
| Broad nasal base | 4/10 | High nasal base | Broad nasal bridge | Flat nasal bridge | Normal appearance |
| Mouth | |||||
| Long/smooth philtrum | 8/10 | Smooth philtrum | Smooth philtrum | Normal appearance | Normal appearance |
| Full lower lip | 5/10 | Full lower lip High arched palate Crowded teeth Retrognathia | Full lower lip Triangular mouth | ||
| Ear | |||||
| Malformation | 7/10 | Normal appearance | Low set posteriorly rotated ears | Prominent ears | Prominent ears |
| Ear tag | |||||
| Nervous system | |||||
| Hypotonia | 6/10 | Hypertonia | Hypertonia | Truncal hypotonia | Truncal hypotonia |
| Agenesis of corpus callosum on brain MRI | Lower extremities hypertonia | ||||
| Genital | 7/10 | Normal | Hypospadias | Normal | Normal |
| Skeletal | |||||
| Joint laxity | 6/10 | Decreased joint range of motion | Joint contractures | No abnormalities | No abnormalities |
| Digital | 10/10 | Broad thumbs | Overlapping fingers | Tapering fingers | Tapering fingers |
| Blunt finger tips | Overlapping fingers | ||||
| Hyperconvex nails | Hypoplastic nails | ||||
| Broad feet | Broad finger pads | ||||
| Other | Attention deficit hyperactivity disorder | Oligohydramnios | Recurrent sinusitis, bronchitis, and otitis | Gastro-esophageal disease | |
| Asperger syndrome | Low posterior hairline | ||||
Patient 6 is a 3.5-year-old boy with mild global developmental delay, truncal hypotonia, lower extremity hypertonia, and characteristic facial features (Table 2; Fig. 2d). Renal ultrasound, brain MRI, hearing evaluation, fragile X syndrome DNA testing, and karyotype all were normal. A SNP microarray revealed an ~2.11 Mb duplication of chromosome 15q24 (73858160–75969880, build 36). His 2-year-old sister (case 7) has developmental delay, truncal hypotonia, and characteristic facial features (Table 2). SNP microarray revealed the same ~2.11 Mb duplication in chromosome 15q24. Both children were adopted. The biological mother, who has been described as a slow learner and attended special education classes in school, was tested by FISH and found to have the same duplication; she was unavailable for physical examination, and limited data were available about her medical and mental history (Table 2). Both the sister (case 7) and the mother were also found to have gain of 15 clones at Xp22.31 that was interpreted as a benign copy number change.
Additional complexity to 15q24 genomic architecture
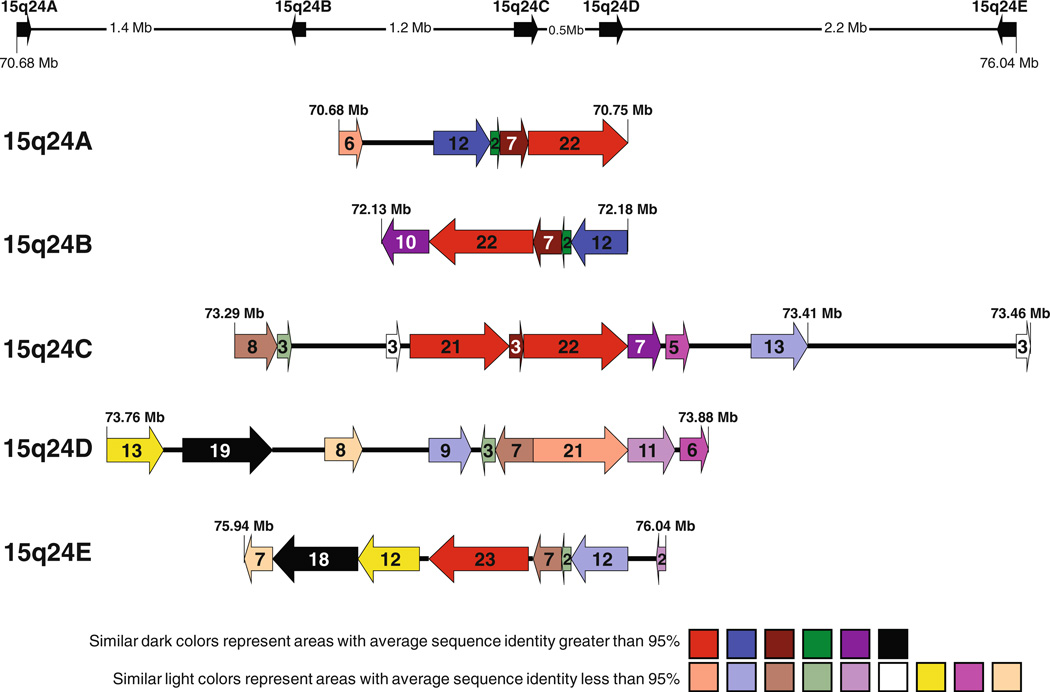
Using Blast2, we analyzed the DNA sequence in the 15q24 region between 70.600 and 76.600 Mb that harbors all the genomic rearrangements, and searched for alignments more than 300 bp in length and greater than 90% DNA sequence identity. Unexpectedly, in addition to the three previously identified LCR clusters (BP1, BP2, and BP3) (Sharp et al. 2007), we identified two more paralogous LCR clusters that we designated LCR15q24A, spanning ~65 kb, and located proximal to BP1, and LCR15q24C, spanning ~ 124 kb, and located between BP1 and BP2. For consistency, we elected to change the nomenclature for the previously identified LCR clusters as follows: LCR15q24B for BP1 (spanning ~54 kb), LCR15q24D for BP2 (spanning ~ 128 kb), and LCR15q24E for BP3 (spanning ~93 kb). We constructed a physical map for this region where LCR subunits with identity greater than 90% are represented with the similar-colored arrows with arrowheads indicating the LCR subunit orientations and arrows lengths, the subunit sizes. Most of these subunits have DNA sequence identity greater than 95% and are represented in darker colors (e.g. red), whereas some subunits have sequence identity between 90 and 94%, and those subunits are shown in lighter colors (e.g. light red) (Fig. 3; Table 3). Interestingly, the homologous subunits located in LCR15q24A and LCR15q24C are in direct orientation, whereas the subunits located in LCR15q24B (BP1) and LCR15q24E (BP3) are in the opposite orientation to the subunits located in LCR15q24A and LCR15q24C. In contrast, LCR15q24D (BP2) has a complex structure with most of the subunits being directly oriented with reference to LCR15q24A and LCR15q24C, whereas two small sub-units (~3 kb subunit in light green color and ~7 kb subunit in light brown color as shown in Fig. 3) are directly oriented to subunits in LCR15q24B and LCR15q24E.
Fig. 3.
Summary of the results of the computational analysis of the genomic architecture in 15q24. The five LCR clusters: ~65 kb LCR14q24A (70.685–70.750 Mb), ~54kb LCR14q24B (72.134–72.188 Mb), ~124 kb LCR14q24C (73.295–73.419 Mb), ~128kb LCR14q24D (73.760–73.888 Mb), and ~93 kb LCR15q24E (75.948–76.041 Mb). LCRs subunits with sequence identity greater than 90% are shown as arrows with similar colors. The subunits with sequence identity more than 95% are represented in darker colors (e.g. red), whereas, the subunits with sequence identity between 90 and 94% are shown in lighter colors (e.g. light red). Arrowheads indicate LCR subunits orientation. The numbers inside the arrow represent the length of the area in kb
Table 3.
The coordinates, length, and orientation of LCR subunits for the five LCR clusters, LCR15q24A, LCR15q24B, LCR15q24C, LCR15q24D, and LCR15q24E
| Start | End | Length (kb) |
Orientation | Subunit color | |
|---|---|---|---|---|---|
| LCR14q24A | 70,685,541 | 70,691,956 | 6,415 | + | Light red |
| 70,707,347 | 70,719,598 | 12,251 | + | Blue | |
| 70,719,670 | 70,721,200 | 1,530 | + | Green | |
| 70,721,201 | 70,728,580 | 7,379 | + | Brown | |
| 70,729,074 | 70,750,593 | 21,519 | + | Red | |
| LCR14q24B | 72,134,535 | 72,144,840 | 10,305 | − | Violet |
| 72145146 | 72,166,963 | 21,817 | − | Red | |
| 72,167,460 | 72,174,829 | 7,369 | − | Brown | |
| 72,174,830 | 72,176,359 | 1,529 | − | Green | |
| 72,176,431 | 72,188,813 | 12,382 | − | Blue | |
| LCR14q24C | 73,295,360 | 73,303,732 | 8,372 | + | Light brown |
| 73,304,189 | 73,307,393 | 3,204 | + | Light green | |
| 73,328,277 | 73,330,953 | 2,676 | + | White | |
| 73,332,931 | 73,354,116 | 21,185 | + | Red | |
| 73,354,117 | 73,356,715 | 2,598 | + | Brown | |
| 73,357,211 | 73,378,995 | 21,784 | + | Red | |
| 73,379,350 | 73,386,517 | 7,167 | + | Violet | |
| 73,387,968 | 73,393,463 | 5,495 | + | Pink | |
| 73,406,153 | 73,418,809 | 12,656 | + | Light blue | |
| 73,463,299 | 73,466,244 | 2,945 | + | White | |
| LCR14q24D | 73,760,220 | 73,772,788 | 12,568 | + | Yellow |
| 73,777,284 | 73,796,657 | 19,373 | + | Black | |
| 73,807,737 | 73,815,337 | 7,600 | + | Tan | |
| 73,828,848 | 73,838,186 | 9,338 | + | Light blue | |
| 73,839,617 | 73,842,915 | 3,298 | − | Light green | |
| 73,842,941 | 73,849,886 | 6,945 | − | Light brown | |
| 73,849,902 | 73,870,477 | 20,575 | + | Light red | |
| 73,870,478 | 73,881,040 | 10,562 | + | Light purple | |
| 73,882,218 | 73,888,387 | 6,169 | + | Pink | |
| LCR14q24E | 75,948,172 | 75,954,860 | 6,688 | − | Tan |
| 75,955,173 | 75,973,569 | 18,396 | − | Black | |
| 75,973,925 | 75,985,550 | 11,625 | − | Yellow | |
| 75,987,909 | 76,011,121 | 23,212 | − | Red | |
| 76,011,796 | 76,019,176 | 7,380 | − | Light brown | |
| 76,019,184 | 76,020,691 | 1,507 | − | Light green | |
| 76,020,763 | 76,033,111 | 12,348 | − | Light blue | |
| 76,039,907 | 76,041,935 | 2,028 | − | Light violet |
Rearrangement breakpoint analysis supports genomic complexity
The breakpoints for the deleted and the duplicated regions were determined using either array CGH or SNP arrays. The proximal and distal deletion breakpoints in patients 1 and 2 map to LCR15q24B (BP1) and LCR15q24E (BP3), respectively. The proximal breakpoints for the deletions in patients 3 and 4, and the duplication in patient 5, map to LCR15q24A. The distal breakpoints for deletions in patients 3 and 4 map to LCR15q24D (BP2); however, the distal breakpoint for duplication in patient 5 maps to the novel LCR15q24C. The proximal and distal breakpoints for duplications in patients 6 and 7 map to LCR15q24D (BP2) and LCR15q24E (BP3), respectively (Fig. 4). The findings of breakpoints mapping to LCRs support that NAHR is the mechanism for these alterations.
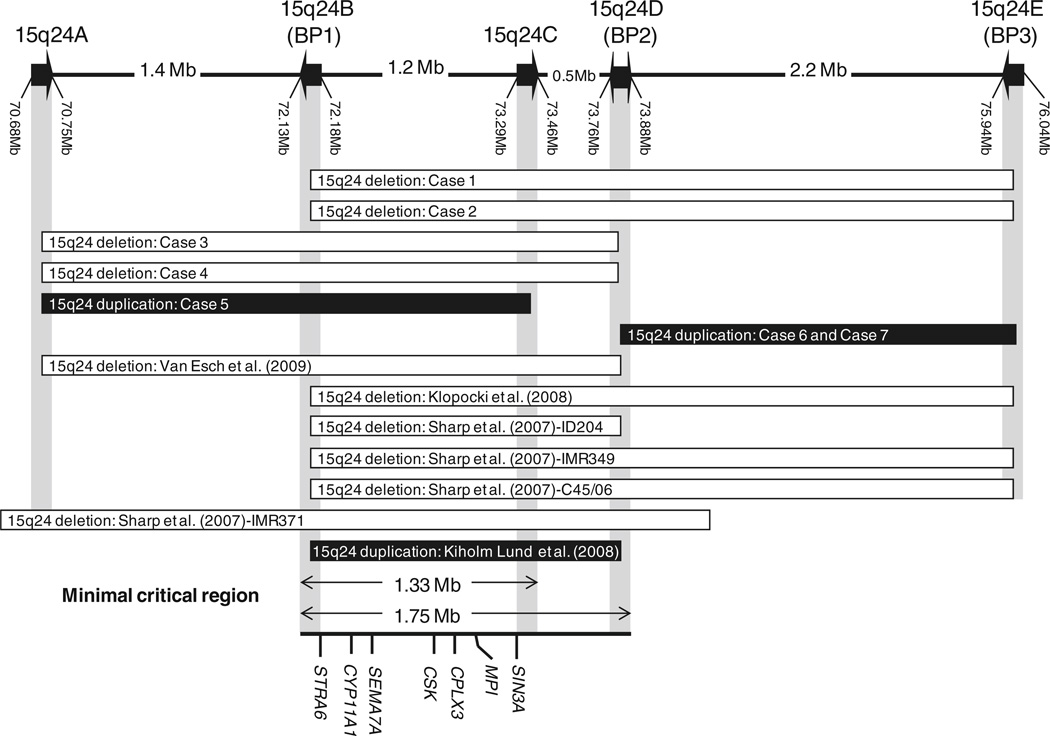
Fig. 4.
Schematic of the deleted/duplicated regions in chromosome 15q24: Case 1 (15q24del: 72.252–75.937 Mb), Case 2 (15q24del: 72.130–76.080 Mb), Case 3 (15q24del: 70.750–73.856 Mb), Case 4 (15q24del: 70.708–73.856 Mb), Case 5 (15q24dup: 70.708–73.332 Mb), and Cases 6 and 7 (15q24dup: 73.858–75.969 Mb), and the previously cases reported by Sharp et al. (2007), ID204 (15q24del: 72.15–73.85 Mb), IMR349 (15q24del: 72.15–76.01 Mb), C45/06 (15q24del: 72.15–76.01 Mb), and IMR371 (15q24del: 70.40–74.21 Mb), Klopocki et al. (2008), (15q24del: 72.2–75.9 Mb), Van Esch et al. (2009) (15q24del: 70.6–73.7 Mb), and Kiholm Lund et al. (2008) (15q24dup: 72.14–73.85 Mb). The LCRs are represented as arrows, LCR15q24A, LCR15q24B, LCR15q24C, LCR15q24D, and LCR15q24E. The minimal critical deletion region of ~1.75 Mb extends between LCR15q24B and LCR15q24D (72.134–73.888 kb) and the suggested minimal critical region for 15q24 duplication of ~1.33 Mb extending from LCR15q24B to LCR15q24C (72.134– 73.419 kb). Selected genes in this region are shown: STRA6, CYP11A1, SEMA7A, CSK, CPLX3, MPI, and SIN3A
Discussion
By comparing the clinical features in our four 15q24 microdeletion patients (cases 1–4) with the other six previously reported patients with a 15q24 microdeletion (Klopocki et al. 2008; Sharp et al. 2007; Van Esch et al. 2009), we observed many common features, supporting the notion that subjects harboring 15q24 deletion manifest a distinct syndrome (Table 1, Table 2). These features include developmental delay that varies from mild to severe, hypotonia, short stature, digital anomalies, joint laxity, genital anomalies, and characteristic facial features, including a high anterior hair line, facial asymmetry, ear malformations, broad medial eyebrows, down-slanted palpebral fissures, hypertelorism, epicanthal folds, strabismus, long smooth philtrum, full lower lip, and broad nasal base. The distal extremity malformations observed in all reported patients consist of thumb anomalies, small hands with brachydactyly, clinodactyly, and foot-ankle deformities. Less common features are microcephaly, IUGR, feeding difficulties, low tone or nasal speech, and obesity. Diaphragmatic hernia was observed in two patients (Sharp et al. 2007; Van Esch et al. 2009); tetralogy of Fallot and myelomeningocele were observed in one patient (case 2), and acute lymphoblastic leukemia was also found in one patient (case 1). All patients with a 15q24 microdeletion share the same ~ 1.75 Mb minimal deletion critical region located between LCR15q24B (BP1) and LCR15q24D (BP2) (Fig. 4).
Recently, a microduplication in 15q24 involving the minimal critical deletion region has been described with proximal and distal breakpoints mapping to LCR15q24B (BP1) and LCR15q24D (BP2), respectively (Kiholm Lund et al. 2008). We identified another case of a 15q24 microduplication involving a portion of the minimal deletion critical region, mapping from LCR15q24A to LCR15q24C (case 5) (Fig. 4). These two patients share some clinical features, including developmental delay, hypertonia, joints limitation, digital abnormalities, and certain facial features, including down-slanting palpebral fissures, epicanthus, full eyelids, smooth philtrum, and full lower lip. The previously reported patient with a 15q24 microduplication had hypospadias, agenesis of corpus callosum, low set ears, and normal growth parameters; however, our patient has normal genitalia, normal brain MRI, normally set ears, short stature, and behavioral problems (Table 2). These two patients with a 15q24 duplication involving the minimal critical deletion region share some overlapping features with patients with a 15q24 deletion, including developmental delay, short stature, digital anomalies, genital anomalies and some facial features (epicanthal folds, down-slanting palpebral fissures, hypertelorism, ear malformation, smooth philtrum, and full lower lip). Most patients with a 15q24 deletion have hypotonia and joint laxity, whereas the two patients with duplication exhibit increased tone and joints limitations. Patient 5 exhibits behavioral disorders that were not reported in patients with the 15q24 deletion syndrome (Table 2). The patient reported by Kiholm Lund et al. (2008) inherited the duplication from a healthy father; the father of patient 5 was unavailable for testing, so it is unclear whether the duplications are responsible for the observed phenotype. However, the similarity in the phenotype between these two patients and the overlapping features with patients with 15q24 deletion support the contention that this duplication could be responsible for the observed phenotype, suggesting that a 15q24 duplication may represent a clinical syndrome with minimal critical region of 1.33 Mb spanning between LCR15q24B (BP1) and LCR15q24C (Fig. 4). Nevertheless, more subjects with similar duplications need to be studied to support or refute this hypothesis.
We also report two siblings and their mother with a 15q24 microduplication distal and adjacent to the minimal deletion critical region with breakpoints mapping to LCR15q24D (BP2) and LCR15q24E (BP3) (cases 6 and 7) (Fig. 4). These siblings manifest a milder phenotype with mild developmental delay, hypotonia, tapering fingers, and characteristic facial features, including round face, hypertelorism, and prominent ears (Table 2). The overlapping features between these siblings and patients with a 15q24 deletion are less obvious and include only developmental delay, hypotonia, and hypertelorism. The two siblings inherited the duplication from their mother; thus it is also unclear whether this duplication is responsible for the phenotype. However, the fact that their mother has learning disabilities and the similarity in the phenotype between the siblings indicate that this duplication could be responsible for the observed phenotype; nevertheless, more subjects with similar duplication need to be studied.
The finding of 15q24 deletion and duplication breakpoints clustering in LCR regions supports the notion that NAHR is the most likely mechanism for these genomic rearrangements (Stankiewicz and Lupski 2002). LCR15q24B (BP1) and LCR15q24E (BP3) contain multiple homologous sub-units that are directly oriented and they harbor breakpoints for the deletions in patients 1 and 2. In addition, LCR15q24A and LCR15q24C contain multiple homologous subunits that are directly oriented and they encompass breakpoints of the duplication in patient 5. The proximal breakpoints for duplications in patients 6 and 7, and the distal breakpoints for deletions in patients 3 and 4 map to LCR15q24D (BP2) that has a complex structure with most subunits being directly oriented to LCR15q24A. The latter repeat harbors the proximal breakpoints for deletions in patients 3 and 4. LCR15q24D (BP2) has two small subunits (~3 kb subunit in light green color and ~ 7 kb subunit in light brown color) directly oriented to subunits in LCR15q24E (BP3), that harbors the distal breakpoints for duplication in patients 6 and 7. These two small subunits are also directly oriented to the subunits inLCR15q24B (BP1). We therefore believe that these two small subunits mediated the NAHR in patients 6 and 7 and in patient ID204 (Sharp et al. 2007), who has a deletion with breakpoints mapping to 15q24B (BP1) and 15q24D (BP2) (Fig. 3, Fig. 4).
The four 15q24 deletion patients (cases 1–4) share the same 1.7 Mb minimal deletion region extending from LCR15q24B (BP1) to LCR15q24D (BP2). The deleted regions extend more distally in patients 1 and 2, the cases IMR349 and C45/06 (Sharp et al. 2007), and the case reported by Klopocki et al. (2008); compared to patients 3 and 4, case IMR371 (Sharp et al. 2007), and the case reported by Van Esch et al. (2009) with deletions extend more proximally (Fig. 4). Although all these patients share many similar features, they also differ in several aspects, e.g., growth deficiency and obesity (Table 1), and thus it is difficult to find a specific genomotype-phenotype (Bi et al. 2009) correlation with this limited set of patients for each specific deletion.
The critical region contains the STRA6 gene that recently has been shown to be associated with syndromic microphthalmia, alveolar capillary dysplasia, and other malformations (Pasutto et al. 2007), the cholesterol side chain cleavage enzyme gene (CYP11A1) associated with lipoid congenital adrenal hyperplasia (Tajima et al. 2001), and the mannose phosphate isomerase gene (MPI) that is associated with congenital disorder of glycosylation type Ib (Niehues et al. 1998). All these disorders are autosomal recessive. It was suggested that the haploinsufficiency of cholesterol side chain cleavage enzyme might contribute to the genital abnormalities (Klopocki et al. 2008). There are two other candidate genes located in this region, which are related to the nervous system and their loss may contribute to the developmental delay. Semaphorin 7A (SEMA7A) enhances central and peripheral axon growth and is required for proper axon tract formation during embryonic development (Pasterkamp et al. 2007), and complexin 3 (CPLX3) is a positive regulator of neurotransmitter release in mouse hippocampal neurons (Reim et al. 2005) (Fig. 4).
Patient 1 developed acute lymphoblastic leukemia. The loss of two genes, SIN3A and CSK, may lead to an increased risk of developing neoplasm. SIN3A encodes a protein that participates in the formation of the histone deacetylase complex, which in turn interacts with tumor suppressor proteins like P53 and pRb (Fleischer et al. 2003). Cytoplasmic-src tyrosine kinase (CSK) down-regulates tyrosine kinase activity of the SRC oncoprotein and might function as an antioncogene (Armstrong et al. 1993) (Fig. 4). Larger numbers of patients with 15q24 deletion need to be studied before drawing any conclusions about neoplasm predisposition.
In conclusion, patients with a 15q24 deletion sharing the minimal deletion critical region between 15q24B (BP1) and 15q24D (BP2) have many common clinical features, supporting the notion that 15q24 deletion leads to a recognizable syndrome. Based on two patients with overlapping duplications, we better defined the phenotype of 15q24 duplication syndrome and narrowed its minimal critical region to ~1.3 Mb spanning between LCR15q24B (BP1) and the identified LCR15q24C. The breakpoints for the deleted and duplicated regions (except the case IMR371 (Sharp et al. 2007)) map to LCR clusters, indicating that NAHR is the likely mechanism of their formation.
Acknowledgments
We would like to thank our patients and their families. P.S. was supported in part by Grant R13-0005-04/2008 from the Polish Ministry of Science and Higher Education.
Contributor Information
Ayman W. El-Hattab, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA
Teresa A. Smolarek, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA
Martha E. Walker, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA
Elizabeth K. Schorry, Division of Human Genetics, Cincinnati Children’s Hospital Medical Center, Cincinnati, OH 45229, USA
LaDonna L. Immken, Clinical Genetics, Specially for Children, Austin, TX 78723, USA
Gayle Patel, Clinical Genetics, Specially for Children, Austin, TX 78723, USA.
Mary-Alice Abbott, Clinical Genetics, Baystate Medical Center, Springfield, MA 01199, USA.
Brendan C. Lanpher, Division of Genetics and Genomic Medicine, Vanderbilt University, Nashville, TN 37232, USA
Zhishuo Ou, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA.
Sung-Hae L. Kang, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA
Ankita Patel, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA.
Fernando Scaglia, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA.
James R. Lupski, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA
Sau Wai Cheung, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA.
Pawel Stankiewicz, Email: pawels@bcm.edu, Department of Molecular and Human Genetics, Baylor College of Medicine, One Baylor Plaza, Rm R809, Houston, TX 77030, USA; Department of Medical Genetics, Institute of Mother and Child, Warsaw, Poland.
References
- Armstrong E, Cannizzaro L, Bergman M, Huebner K, Alitalo K. The c-src tyrosine kinase (CSK) gene, a potential antioncogene, localizes to human chromosome region 15q23–q25. Cytogenet Cell Genet. 1993;60:119–120. doi: 10.1159/000133318. [DOI] [PubMed] [Google Scholar]
- Ben-Shachar S, Lanpher B, German JR, Potocki L, Sreenath Nagamani SC, Franco LM, Malphrus A, Bottenfield GW, Spence JE, Amato S, Rousseau JA, Moghaddam B, Skinner C, Skinner SA, Bernes S, Armstrong N, Shinawi M, Stankiewicz P, Patel A, Cheung S-W, Lupski JR, Beaudet Al, Sahoo T. Microdeletion 15q13.3: a locus with incomplete penetrance for autism, mental retardation, and psychiatric disorders. J Med Genet. 2009;46:382–388. doi: 10.1136/jmg.2008.064378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettelheim D, Hengstaschlager M, Drhonsky R, Eppel W, Bernaschek G. Two cases of prenatally diagnosed diaphragmatic hernia accompanied by the same undescribed chromosomal deletion (15q24 de novo) Clin Genet. 1998;53:319–320. doi: 10.1111/j.1399-0004.1998.tb02706.x. [DOI] [PubMed] [Google Scholar]
- Bi W, Park SS, Shaw CJ, Withers MA, Patel PI, Lupski JR. Reciprocal crossovers and a positional preference for strand exchange in recombination events resulting in deletion or duplication of chromosome 17p11.2. Am J Hum Genet. 2003;73:1302–1315. doi: 10.1086/379979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi W, Sapir T, Shchelochkov OA, Zhang F, Withers MA, Hunter JV, Levy T, Shinder V, Peiffer DA, Gunderson KL, Nezarati MM, Shotts VA, Amato SS, Savage SK, Harris DJ, Day-Salvatore DL, Horner M, Lu XY, Sahoo T, Yanagawa Y, Beaudet AL, Cheung SW, Martinez S, Lupski JR, Reiner O. Increased LIS1 expression affects human and mouse brain development. Nat Genet. 2009;41:168–177. doi: 10.1038/ng.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer C, Holloway S, Zawalnyski P, Schinzel A, FitzPatrick D. A chromosomal duplication map of malformations: regions of suspected haplo- and triplolethality—and tolerance of segmental aneuploidy—in humans. Am J Hum Genet. 1999;64:1702–1708. doi: 10.1086/302410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browne CE, Hatchwell E, Protopapas A, Ramos J. Duplication of medial 15q confirmed by FISH. J Med Genet. 2000;37:E10. doi: 10.1136/jmg.37.8.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho CM, Zhang F, Liu P, Patel A, Sahoo T, Bacino CA, Shaw C, Peacock S, Pursley A, Tavyev YJ, Ramocki MB, Nawara M, Obersztyn E, Vianna-Morgante AM, Stankiewicz P, Zoghbi HY, Cheung SW, Lupski JR. Complex rearrangements in patients with duplications of MECP2 can occur by Fork stalling and template switching. Hum Mol Genet. 2009;18:2188–2203. doi: 10.1093/hmg/ddp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung SW, Shaw CA, Yu W, Li J, Ou Z, Patel A, Yatsenko SA, Cooper ML, Furman P, Stankiewicz P, Lupski JR, Chinault AC, Beaudet AL. Development and validation of a CGH microarray for clinical cytogenetic diagnosis. Genet Med. 2005;7:422–432. doi: 10.1097/01.gim.0000170992.63691.32. [DOI] [PubMed] [Google Scholar]
- Clark RD. Letter to the editor: del(15)(q22q24) syndrome with Potter sequence. Am J Med Genet. 1984;19:703–705. doi: 10.1002/ajmg.1320190409. [DOI] [PubMed] [Google Scholar]
- Cushman LJ, Torres-Martinez W, Cherry AM, Manning MA, Abdul-Rahman O, Anderson CE, Punnett HH, Thurston VC, Sweeney D, Vance GH. A report of three patients with an interstitial deletion of chromosome 15q24. Am J Med Genet. 2005;137:65–71. doi: 10.1002/ajmg.a.30836. [DOI] [PubMed] [Google Scholar]
- Dhaliwal MK, Menos D, Checkley P, Lieber E. A case of an unbalanced interstitial translocation involving chromosome 4p and 15q in a newborn with multiple congenital anomalies. Am J Hum Genet. 1990;47(Suppl):A28. [Google Scholar]
- Fleischer TC, Yun UJ, Ayer DE. Identification and characterization of three new components of the mSin3A corepressor complex. Mol Cell Biol. 2003;23:3456–3467. doi: 10.1128/MCB.23.10.3456-3467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Formiga LF, Poenaru L, Couronne F, Flori E, Eibel JL, Deminatti MM, Savary JB, Lai JL, Gilgenkrantz S, Pierson M. Interstitial deletion of chromosome 15: two cases. Hum Genet. 1988;80:401–404. doi: 10.1007/BF00273663. [DOI] [PubMed] [Google Scholar]
- Gu W, Zhang F, Lupski JR. Mechanisms for human genomic rearrangements. Pathogenetics. 2008;1:4. doi: 10.1186/1755-8417-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JY, Kim KH, Lee HD, Moon SY, Shaffer LG. De novo direct duplication of 15q15 → q24 in a newborn boy with mild manifestations. Am J Med Genet. 1999;87:395–398. doi: 10.1002/(sici)1096-8628(19991222)87:5<395::aid-ajmg5>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- Kiholm Lund AB, Hove HD, Kirchhoff M. A 15q24 microduplication, reciprocal to the recently described 15q24 microdeletion, in a boy sharing clinical features with 15q24 microdeletion syndrome patients. Eur J Med Genet. 2008;51:520–526. doi: 10.1016/j.ejmg.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Kirchhoff M, Bisgaard AM, Duno M, Hansen FJ, Schwartz M. A 17q21.31 microduplication, reciprocal to the newly described 17q21.31 microdeletion, in a girl with severe psychomotor developmental delay and dysmorphic craniofacial features. Eur J Med Genet. 2007;50:256–263. doi: 10.1016/j.ejmg.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Klopocki E, Graul-Neumann LM, Grieben U, Tonnies H, Ropers HH, Horn D, Mundlos S, Ullmann R. A further case of the recurrent 15q24 microdeletion syndrome, detected by array CGH. Eur J Pediatr. 2008;167:903–908. doi: 10.1007/s00431-007-0616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koolen DA, Vissers LE, Pfundt R, de Leeuw N, Knight SJ, Regan R, Kooy RF, Reyniers E, Romano C, Fichera M, Schinzel A, Baumer A, Anderlid BM, Schoumans J, Knoers NV, van Kessel AG, Sistermans EA, Veltman JA, Brunner HG, de Vries BB. A new chromosome 17q21.31 microdeletion syndrome associated with a common inversion polymorphism. Nat Genet. 2006;38:999–1001. doi: 10.1038/ng1853. [DOI] [PubMed] [Google Scholar]
- Lee JA, Carvalho CM, Lupski JR. A DNA replication mechanism for generating nonrecurrent rearrangements associated with genomic disorders. Cell. 2007;28:1235–1247. doi: 10.1016/j.cell.2007.11.037. [DOI] [PubMed] [Google Scholar]
- Nagamani SC, Zhang F, Shchelochkov OA, Bi W, Ou Z, Scaglia F, Probst FJ, Shinawi M, Eng C, Hunter JV, Sparagana S, Lagoe E, Fong CT, Pearson M, Doco-Fenzy M, Landais E, Mozelle M, Chinault AC, Patel A, Bacino CA, Sahoo T, Kang SH, Cheung SW, Lupski JR, Stankiewicz P. Microdeletions including YWHAE in the Miller-Dieker syndrome region on chromosome 17p13.3 result in facial dysmorphisms, growth restriction, and cognitive impairment. J Med Genet. 2009 doi: 10.1136/jmg.2009.067637. (in press) [DOI] [PubMed] [Google Scholar]
- Niehues R, Hasilik M, Alton G, Körner C, Schiebe-Sukumar M, Koch HG, Zimmer KP, Wu R, Harms E, Reiter K, von Figura K, Freeze HH, Harms HK, Marquardt T. Carbohydrate-deficient glycoprotein syndrome type Ib: phosphomannose isomerase deficiency and mannose therapy. J Clin Invest. 1998;101:1414–1420. doi: 10.1172/JCI2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Z, Kang SH, Shaw CA, Carmack CE, White LD, Patel A, Beaudet AL, Cheung SW, Chinault AC, et al. Bacterial artificial chromosome-emulation oligonucleotide arrays for targeted clinical array-comparative genomic hybridization analyses. Genet Med. 2008;10:278–289. doi: 10.1097/GIM.0b013e31816b4420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasterkamp RJ, Peschon JJ, Spriggs MK, Kolodkin AL. Semaphorin 7A promotes axon outgrowth through integrins and MAPKs. Nature. 2007;424:398–105. doi: 10.1038/nature01790. [DOI] [PubMed] [Google Scholar]
- Pasutto F, Sticht H, Hammersen G, Gillessen-Kaesbach G, Fitzpatrick DR, Nürnberg G, Brasch F, Schirmer-Zimmermann H, Tolmie JL, Chitayat D, Houge G, Fernández-Martínez L, Keating S, Mortier G, Hennekam RC, von der Wense A, Slavotinek A, Meinecke P, Bitoun P, Becker C, Nürnberg P, Reis A, Rauch A. Mutations in STRA6 cause a broad spectrum of malformations including anophthalmia, congenital heart defects, diaphragmatic hernia, alveolar capillary dysplasia, lung hypoplasia, and mental retardation. Am J Hum Genet. 2007;80:550–560. doi: 10.1086/512203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potocki L, Bi W, Treadwell-Deering D, Carvalho CM, Eifert A, Friedman EM, Glaze D, Krull K, Lee JA, Lewis RA, Mendoza-Londono R, Robbins-Furman P, Shaw C, Shi X, Weissenberger G, Withers M, Yatsenko SA, Zackai EH, Stankiewicz P, Lupski JR. Characterization of Potocki-Lupski syndrome (dup(17)(pll.2pll.2)) and delineation of a dosage-sensitive critical interval that can convey an autism phenotype. Am J Hum Genet. 2007;80:633–649. doi: 10.1086/512864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst FJ, Roeder ER, Enciso VB, Ou Z, Cooper ML, Eng P, Li J, Gu Y, Stratton RF, Chinault AC, Shaw CA, Sutton VR, Cheung SW, Nelson DL. Chromosomal microarray analysis (CMA) detects a large X chromosome deletion including FMR1, FMR2, and IDS in a female patient with mental retardation. Am J Med Genet. 2007;143A:1358–1365. doi: 10.1002/ajmg.a.31781. [DOI] [PubMed] [Google Scholar]
- Reim K, Wegmeyer H, Brandstatter JH, Xue M, Rosenmund C, Drebach T, Hofmann K, Brose N. Structurally and functionally unique complexins at retinal ribbon synapses. J Cell Biol. 2005;69:669–680. doi: 10.1083/jcb.200502115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roggenbuck JA, Mendelsohn NJ, Tenenholz B, Ladda RL, Fink JM. Duplication of the distal long arm of chromosome 15: report of three new patients and review of the literature. Am J Med Genet. 2004;126A:398–402. doi: 10.1002/ajmg.a.20617. [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Hansen S, Selzer RR, Cheng Z, Regan R, Hurst JA, Stewart H, Price SM, Blair E, Hennekam RC, Fitzpatrick CA, Segraves R, Richmond TA, Guiver C, Albertson DG, Pinkel D, Eis PS, Schwartz S, Knight SJ, Eichler EE. Discovery of previously unidentified genomic disorders from the duplication architecture of the human genome. Nat Genet. 2006;38:1038–1042. doi: 10.1038/ng1862. [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Selzer RR, Veltman JA, Gimelli S, Gimelli G, Striano P, Coppola A, Regan R, Price SM, Knoers NV, Eis PS, Brunner HG, Hennekam RC, Knight SJ, de Vries BB, Zuffardi O, Eichler EE. Characterization of a recurrent 15q24 microdeletion syndrome. Hum Mol Genet. 2007;16:567–572. doi: 10.1093/hmg/ddm016. [DOI] [PubMed] [Google Scholar]
- Sharp AJ, Mefford HC, Li K, Baker C, Skinner C, Stevenson RE, Schroer RJ, Novara F, De Gregori M, Ciccone R, Broomer A, Casuga I, Wang Y, Xiao C, Barbacioru C, Gimelli G, Bernardina BD, Torniero C, Giorda R, Regan R, Murday V, Mansour S, Fichera M, Castiglia L, Failla P, Ventura M, Jiang Z, Cooper GM, Knight SJ, Romano C, Zuffardi O, Chen C, Schwartz CE, Eichler EE. A recurrent 15q13.3 microdeletion syndrome associated with mental retardation and seizures. Nat Genet. 2008;40:322–328. doi: 10.1038/ng.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw-Smith C, Pittman AM, Willatt L, Martin H, Rickman L, Gribble S, Curley R, Cumming S, Dunn C, Kalaitzopoulos D, Porter K, Prigmore E, Krepischi-Santos AC, Varela MC, Koiffmann CP, Lees AJ, Rosenberg C, Firth HV, de Silva R, Carter NP. Microdeletion encompassing MAPT at chromosome 17q21.3 is associated with developmental delay and learning disability. Nat Genet. 2006;38:1032–1037. doi: 10.1038/ng1858. [DOI] [PubMed] [Google Scholar]
- Shchelochkov OA, Cooper ML, Ou Z, Peacock S, Yatsenko SA, Brown CW, Fang P, Stankiewicz P, Cheung SW. Mosaicism for r(X) and der(X)del(X)(pll.23)dup(X) (pll.21pll.22) provides insight into the possible mechanism of rearrangement. Mol Cytogenet. 2008;1:16. doi: 10.1186/1755-8166-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavotinek AM. Novel microdeletion syndromes detected by chromosome microarrays. Hum Genet. 2008;124:1–17. doi: 10.1007/s00439-008-0513-9. [DOI] [PubMed] [Google Scholar]
- Spruijt L, Engelen JJM, Bruinen-Smeijsters LP, Albrechts JCM, Schrander J, Schrander-Stumpel CTRM. A patient with a de novo 15q24q26.1 interstitial deletion, developmental delay, mild dysmorphism and very blue irises. Am J Med Genet. 2004;129A:312–315. doi: 10.1002/ajmg.a.30185. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Beaudet AL. Use of array CGH in the evaluation of dysmorphology, malformations, developmental delay, and idiopathic mental retardation. Curr Opin Genet Dev. 2007;17:182–192. doi: 10.1016/j.gde.2007.04.009. [DOI] [PubMed] [Google Scholar]
- Stankiewicz P, Lupski JR. Genome architecture, rearrangements and genomic disorders. Trends Genet. 2002;18:74–82. doi: 10.1016/s0168-9525(02)02592-1. [DOI] [PubMed] [Google Scholar]
- Tajima T, Fujieda K, Kouda N, Nakae J, Miller WL. Heterozygous mutation in the cholesterol side chain cleavage enzyme (P450scc) gene in a patient with 46, XY sex reversal and adrenal insufficiency. J Clin Endocr Metab. 2001;86:3820–3825. doi: 10.1210/jcem.86.8.7748. [DOI] [PubMed] [Google Scholar]
- Van Esch H, Backx L, Pijkels E, Fryns JP. Congenital diaphragmatic hernia is part of the new 15q24 microdeletion syndrome. Eur J Med Genet. 2009;52:153–156. doi: 10.1016/j.ejmg.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Zhang F, Khajavi M, Connolly AM, Towne CF, Batish SD, Lupski JR. The DNA replication FoSTeS/MMBIR mechanism can generate human genomic, genic, and exonic complex rearrangements. Nat Genet. 2009 doi: 10.1038/ng.399. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]