Summary
The MRN (MRE11-RAD50-NBS1) complex has been implicated in many aspects of the DNA damage response. It has key roles in sensing and processing DNA double-strand breaks, as well as in activation of ATM (ataxia-telangiectasia mutated). We reveal a new function for MRN in ATR (ATM and RAD3-related) activation by using defined ATR-activating DNA structures in Xenopus egg extracts. Strikingly, we demonstrate that MRN is required to recruit TOPBP1 to an ATR-activating structure that contains a single-stranded DNA (ssDNA) and a double-stranded DNA (dsDNA) junction, and that this recruitment is necessary for phosphorylation of Chk1. We also show that the 911 (RAD9-RAD1-HUS1) complex is not required for TOPBP1 recruitment, but is essential for TOPBP1 function. Thus, whereas MRN is required for TOPBP1 recruitment at a ss/dsDNA junction, 911 is required for TOPBP1 “activation”. These findings provide new molecular insight into how ATR is activated.
Introduction
The DNA damage checkpoint plays an important role in maintaining genome stability by detecting DNA damage and coordinating a response that results in repair of the damage, cell cycle arrest, and in the case of severe damage, apoptosis or senescence. Two key players in this process are the ATM (ataxia-telangiectasia mutated) and ATR (ATM and RAD3-related) kinases that, once activated, phosphorylate a range of downstream targets (Ciccia and Elledge, 2010). A critical difference between these kinases lies in the type of DNA damage to which they respond. Whereas ATM is mainly activated by DNA double-stranded breaks (DSBs), ATR is activated by a structure that consists of single-stranded DNA (ssDNA) and a junction between ssDNA and double-stranded DNA (dsDNA) (Michael, 2000; Byun et al., 2005; MacDougall et al., 2007). ATR-activating structures are thought to be present at stalled replication forks, during nucleotide excision repair and also at telomere ends (Cimprich and Cortez, 2008). In addition, processing of DSBs by nucleases generates an ATR-activating structure (Jazayeri et al., 2005; Myers and Cortez, 2006).
Once formed, ATR-activating structures act as a scaffold to recruit ATR and other proteins that facilitate its activation. ssDNA is bound by replication protein A (RPA), a ssDNA binding protein that binds ATRIP (ATR-interacting protein), and in turn recruits ATR (Zou and Elledge, 2003). At a junction of ssDNA and dsDNA, a heterotrimeric clamp consisting of RAD9-RAD1-HUS1 (911) is loaded by the RAD17/RFC2–5 complex (Navadgi-Patil and Burgers, 2009). The 911 complex has been proposed to recruit TOPBP1, a BRCT repeat protein that stimulates ATR’s kinase activity through a region known as the ATR activating domain (AAD) and through binding of ATRIP (Kumagai et al., 2006; Mordes et al., 2008).
Several observations link the 911 complex to TOPBP1 and ATR activation. First, TOPBP1 has been shown to interact directly with the constitutively phosphorylated C-terminal tail of RAD9 (Rappas et al., 2011). Indeed, this tail has been shown to be essential for checkpoint signaling, since a mutant RAD9 that cannot be phosphorylated does not support ATR activation (Furuya, 2004; Delacroix et al., 2007; Lee et al., 2007). Furthermore, ATR signaling defects that result from the loss of RAD17 can be rescued by expressing a form of TOPBP1 that is artificially recruited to chromatin (Delacroix et al., 2007). Interestingly, however, mutants of the 911 complex that do not bind TOPBP1 still permit the accumulation of TOPBP1 on chromatin following aphidicolin treatment (Lee and Dunphy, 2010). This finding indicates that our understanding of TOPBP1 recruitment is incomplete and raises the possibility that other proteins may be involved in this process.
The MRN complex has been implicated in many aspects of the DNA damage response, including DSB recognition, DSB processing and regulation of ATM signaling (Stracker and Petrini, 2011). Mutations in MRE11, NBS1 and RAD50 result in ataxia-telangiectasia-like disease (ATLD), Nijmegen breakage syndrome (NBS) and NBS-like disorder, respectively. In agreement with roles for MRN and ATM in the same pathway, patients with these diseases share clinical features with patients that have ataxia-telangiectasia, a disorder caused by a mutation in ATM. Interestingly, patients with mutations in MRN complex components also share clinical features with patients that have mutations in ATR (Seckel syndrome), such as microcephaly (Stracker and Petrini, 2011). Furthermore, similar to the ATR null mouse, MRE11, RAD50 or NBS1 null mice are inviable, whereas the ATM null mouse is viable (Cimprich and Cortez, 2008; Stracker and Petrini, 2011). These findings raise the possibility that the MRN complex acts in the ATR pathway.
Consistent with a role for MRN outside of DSB signaling and repair, mutation of MRN complex components in budding yeast results in sensitivity to replication stress and defects in the replication checkpoint (D’amours and Jackson, 2001). Furthermore, MRE11 forms replication foci in S-phase (Maser et al., 2001), and in Xenopus extracts the absence of MRE11 results in DSB formation during S-phase (Costanzo et al., 2001). However, there are conflicting data to support a role for MRN in ATR checkpoint signaling. One NBS1 patient cell line was shown to be defective in ATR signaling, while an ATLD cell line was not (Stiff et al., 2005). Furthermore, depletion of either MRE11 or NBS1 through various means was shown to reduce checkpoint activation in one study (Olson et al., 2007a) but not in three others (Difilippantonio et al., 2005; Myers and Cortez, 2006; Wang et al., 2011). Because MRN has a role in DSB resection (Paull, 2010), a process that converts an ATM-activating structure into an ATR-activating structure, it has been proposed that the effects of MRN on ATR may be due to DSB processing, and a direct role for MRN in ATR activation has not been definitively shown.
In Xenopus egg extracts, defined DNA structures can be used to elicit DNA damage responses. We previously described model DNA structures consisting of circular ssDNA and a junction of ssDNA and dsDNA that specifically activate the ATR pathway in Xenopus nuclear extracts (MacDougall et al., 2007). Here, we extended these studies by isolating those ATR-activating structures and identifying bound proteins. Surprisingly, we found that MRE11, RAD50 and NBS1 are enriched on ATR-activating structures. We also demonstrate that the MRN complex is directly required for ATR activation and is required to recruit the ATR activator TOPBP1.
Results
Identification of proteins bound to a specific ATR-activating structure
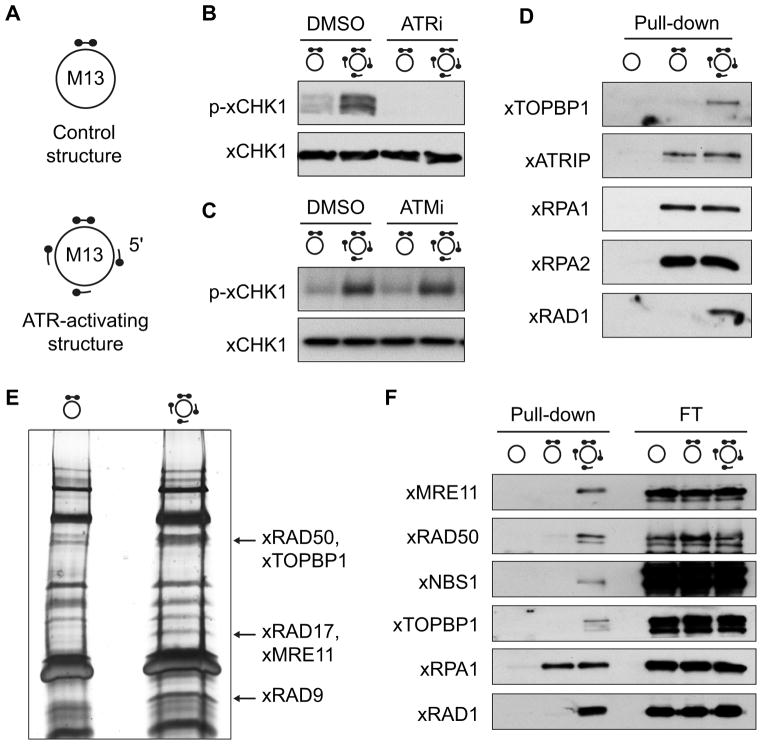
We previously developed well-defined DNA structures that specifically activate ATR in Xenopus nucleoplasmic extract (NPE), the soluble nuclear fraction of in vitro replicated Xenopus sperm chromatin (MacDougall et al., 2007). We wanted to build on this approach to identify proteins that specifically recognize these ATR-activating structures and to define their role in ATR-mediated checkpoint signaling. To this end, we annealed three oligonucleotides of 80 bases to the circular ssDNA M13 phage genome of approximately 7 kilobases. To prevent primer elongation in extracts, the 3′ end of the oligonucleotide was labeled with biotin and streptavidin (MacDougall et al., 2007). An annealed oligonucleotide of 50 bases that was labeled on both ends with a longer biotin linker provided a handle for isolation of both the control structure, which lacked the 80 base-pair oligonucleotides, and the ATR-checkpoint activating structure (Figure 1A). As expected from previous work (MacDougall et al., 2007), only the structure containing the free 5′ ends activated ATR in NPE as read-out by phosphorylation of the ATR target xCHK1 at serine 345 (Figure 1B). Furthermore, xCHK1 phosphorylation was abrogated by inhibiting ATR, but not ATM, with specific small molecule inhibitors, and activation of ATM through phosphorylation at serine 1989 (analogous to human ATM serine 1981) was not observed (Figure 1B, C and Figure S1).
Figure 1. MRN binds an ATR-activating structure.
A M13-based structures used in this study consist of 7 kb M13 phage circular ssDNA and annealed oligonucleotides. Both structures contain a double biotinylated oligonucleotide of 50 bases. The ATR-activating structure has in addition three oligonucleotides of 80 bases biotinylated on the 3′ end and with a free 5′ end. Biotinylated primer ends are blocked with streptavidin as represented by dots. B Model structures induce ATR-dependent phosphorylation of Chk1 in Xenopus nucleoplasmic extracts (NPE) as detected by western blot analysis. ATRi = ATR inhibitor (ATR-45) C Similar to experiment 1B but effect of an ATM inhibitor (ATMi = KU-55933) was assessed. D Western blot analysis of a pull-down showing specific binding to the ATR-activating structure. o = M13 only. E Silver-stained gel showing proteins isolated from NPE with the structures shown. Arrows indicate the bands isolated for mass spectrometry analysis and the proteins subsequently identified in those bands. F Western blot analysis of proteins associated with control and ATR activating structures isolated from NPE confirms enriched binding of the xMRN complex to the ATR activating structure. 5% of flow-through (FT) is shown. See also Figure S1.
Next, we tested the ability of these structures to isolate proteins expected to specifically recognize the ATR-activating structure. Both the control structure and the ATR-activating structure were bound to streptavidin beads, incubated in NPE and isolated. Equal recovery of both structures was confirmed by equal binding of the RPA subunits xRPA1 and xRPA2 (Figure 1D). We also observed equal binding of the ATR binding partner xATRIP, consistent with its ability to recognize RPA-coated ssDNA (Zou and Elledge, 2003). Importantly, specific binding of both xRAD1 and xTOPBP1 to the ATR-activating structure was observed (Figure 1D). This is in line with previously published data showing loading of the 911 complex onto ss/dsDNA junctions (Navadgi-Patil and Burgers, 2009). Together, these results show that we have developed a bona fide system to isolate proteins that bind specifically to an ATR-activating structure.
The MRN complex binds specifically to the ATR-activating structure and is required for ATR-dependent checkpoint activation
To identify additional proteins binding to the ATR-activating structure in an unbiased way, we performed a large-scale pull-down experiment and ran the isolated proteins on a gel that was then silver stained. Three bands that were enriched in pull-downs containing the ATR-activating structure and three bands in the control region were excised, and peptides isolated from those bands were identified by mass spectrometry. Three of the proteins enriched on the checkpoint-activating structure were xRAD17, xRAD9 and xTOPBP1, consistent with our findings and their key roles in ATR checkpoint activation. Interestingly, xRAD50 and xMRE11 were also enriched on this structure (Figure 1E), a finding that was confirmed for all three subunits of the MRN complex by western blotting (Figure 1F).
The specific association of MRN with the ATR-activating structure suggested that the MRN complex could play a role in ATR activation. To test this hypothesis, we depleted xMRE11 from NPE and examined the phosphorylation of xChk1 induced by the ATR-activating structure. As expected, immunodepletion of xMRE11 co-depleted xRAD50 and xNBS1 (Figure 2A). Importantly, immunodepletion of the xMRN complex completely abrogated the phosphorylation of xChk1. In addition, we observed reduced phosphorylation of the ATR target xRPA2 at threonine 36, the site analogous to human RPA2 serine 33 phosphorylation. Importantly, checkpoint activation could be restored by addition of recombinant human MRN (Figure 2B). Furthermore, depletion of MRN prevented maximal activation of ATR in Xenopus extracts replicating chromatin treated with the DNA polymerase inhibitor aphidicolin without affecting DNA replication (Figure 2C and data not shown). Lastly, we observed a defect in ATR activation after knockdown of NBS1 in human cells, albeit only in response to treatment with a low dose of hydroxyurea (HU), and inhibition of ATM did not affect this activation (Figure S2). Taken together, these observations strongly suggest that MRN plays a direct role in ATR checkpoint activation.
Figure 2. The xMRN complex is required for ATR checkpoint activation.
A Western blot analysis of mock- or Mre11-depleted NPE incubated with the structures shown. B Rescue of ATR signaling in xMRE11 depleted NPE by human recombinant MRN complex. Samples were prepared and analyzed as in A. C Western blot analysis of mock- or Mre11-depleted low speed extract supplemented with sperm chromatin and aphidicolin. See also Figure S2.
TOPBP1 binding to ATR-activating structures requires the MRN complex
In order to gain insight into the mechanism by which MRN affects ATR-dependent signaling, we performed a pull-down experiment with ATR-activating structures and monitored the effects of MRN depletion on the binding of known checkpoint proteins (Figure 3A). Depletion of xMRE11 had no effect on the association of xATRIP and xRAD1 with the checkpoint-activating structure. We also observed reduced phosphorylation of xRPA2 and xRAD1, another marker for ATR activation (Lupardus and Cimprich, 2006), indicating that ATR activation is reduced on the checkpoint-activating structures, as well as in the soluble extract, in the absence of xMRE11. Surprisingly, however, a marked decrease in the binding of xTOPBP1 was observed (Figure 3A), demonstrating that xMRN recruits xTOPBP1 to checkpoint-activating structures.
Figure 3. xMRN recruits xTOPBP1 to ATR-activating structures.
A Proteins isolated with the control structure or ATR-activating structure from mock- or xMRE11-depleted NPE were analyzed by western blotting. 5% of flow-through (FT) is shown. B Western blot analysis of proteins isolated with control or ATR-activating structures before and after addition of the xRAD9 C-terminal tail to NPE. C Western blot of NPE incubated with or without the xRAD9 C-terminal tail and with the structures shown. D Pull-down of control and ATR activating structures from NPE supplemented with recombinant protein of TOPBP1 BRCT 1–2, 3–6 or 6–8. See also Figure S3.
The above result also indicates that xTOPBP1 might bind checkpoint-activating structures in the absence of the 911 complex. To further test this hypothesis, we added to extracts a fragment of the xRAD9 C-terminal tail that disrupts loading of the 911 complex on the ATR-activating structure, as monitored by xHUS1 and xRAD1 binding. Importantly, only a slight change in xTOPBP1 binding was observed (Figure 3B). Nevertheless, ATR-dependent phosphorylation of xCHK1 was abolished in the presence of the xRAD9 C-terminal tail (Figure 3C) and upon depletion of xRAD1 (Figure S3A). Taken together, these results suggest that binding of the 911 complex to ATR-activating structures is not essential for xTOPBP1 recruitment, but is required for ATR-dependent checkpoint activation.
The interaction between the phosphorylated C-terminal tail of RAD9 and TOPBP1 is mediated by BRCT domains 1 and 2 in TOPBP1, and an interaction between MRN and TOPBP1 in response to double-strand breaks is mediated by the same TOPBP1 BRCT domains (Delacroix et al., 2007; Yoo et al., 2009). To determine which domains of TOPBP1 were required for its association with ATR checkpoint activating structures, we tested the ability of recombinant human forms of TOPBP1 containing BRCT domains 1–2, 3–6 and 6–8, to bind control and ATR activating structures in NPE. Interestingly, we observed no binding of the fragments containing BRCT domains 1–2 or 6–8 to these structures. Instead, we observed binding of BRCT 3–6, and this binding was increased at ATR activating structures (Figure 3D). These observations are consistent with the idea that the recruitment of TOPBP1 to ATR activating structures is independent of the 911 complex. Notably, immunoprecipitation experiments with TOPBP1 revealed an interaction between xTOPBP1 and all components of the xMRN complex (Figure S3B, C), suggesting that TOPBP1 could be recruited through a direct interaction with the MRN complex.
Targeting of the TOPBP1 ATR-activating domain to ssDNA bypasses the requirement for MRN and 911 in ATR signaling
To determine if the MRN-dependent recruitment of TOPBP1 to ATR-activating structures is required for phosphorylation of Chk1, we next asked whether absence of the MRN complex could be rescued by targeting the ATR-activating domain of xTOPBP1 to ATR on ssDNA. To this end, we fused the TOPBP1 AAD to two OB-fold domains of Xenopus RPA that are known to bind ssDNA (Figure S4A)(Fanning, 2006). The resulting fusion protein, which we named OB-AAD, bound to the ssDNA of M13 control and ATR-activating structures as expected (data not shown). Importantly, the addition of OB-AAD restored the phosphorylation of RPA and Chk1 in extracts depleted of MRN (Figure 4A). This observation argues that the defect in checkpoint activation observed in MRN-depleted extract is a result of reduced TOPBP1 recruitment. We also found that the phosphorylation of xCHK1 caused by addition of the RAD9 C-terminal tail could be rescued by addition of OB-AAD to extracts (Figure 4B), consistent with the idea that the RAD9 C-terminal tail disrupts ATR signaling by interfering with TOPBP1 function. Finally, we found that the checkpoint activation induced by OB-AAD after depletion of MRN or addition of the RAD9 C-terminal tail was dependent on the presence of ssDNA, but independent of the presence of a ss/dsDNA junction (Figure 4 and Figure S4B). Taken together, these findings demonstrate that a primary role for the ss/dsDNA junction in checkpoint activation is in the recruitment and activation of TOPBP1.
Figure 4. Targeting the TOPBP1 AAD to ssDNA rescues MRN depletion.
A Chk1 phosphorylation was monitored by western blot in mock- or MRE11-depleted NPE containing the indicated structure, and where indicated OB-AAD. Control (C) is water. B Experiment similar to A. Where indicated, xRAD9 C-terminal tail and OB-AAD were added to NPE. C Model for function MRN in ATR activation (see text for details). See also Figure S4.
Discussion
The MRN complex is a well established component of the DDR where it binds DNA DSBs and plays key roles in ATM activation and the regulation of DSB repair (Stracker and Petrini, 2011). We now demonstrate that the MRN complex also binds DNA structures consisting of a ssDNA and a ssDNA/dsDNA junction that specifically activate ATR in Xenopus extracts. Importantly, we find that the MRN complex has an essential role in the recruitment of TOPBP1 to these structures and that the MRN complex is required for ATR activation. On the basis of these results, we propose a new model for ATR checkpoint activation (Figure 4C). First, MRN binds to the 5′-end of a ss/dsDNA junction and recruits TOPBP1. At the same junction, the 911 complex is loaded by RAD17. In parallel, ATR is recruited to ssDNA by its binding partner ATRIP. Second, TOPBP1 binds the RAD9 C-terminal tail at the ss/dsDNA junction, a process that may be assisted by RAD17 (Lee and Dunphy, 2010). We hypothesize that the interaction between TOPBP1 and RAD9 results in exposure of the AAD of TOPBP1, and ATR activation at the ss/dsDNA junction. Phosphorylation of TOPBP1 by ATR may further enhance this interaction (Hashimoto et al., 2006; Yoo et al., 2007). Thus, we propose that the ss/dsDNA junction has at least two roles in TOPBP1 function: recruiting TOPBP1, by promoting association of the MRN complex, and “activating” TOPBP1, by facilitating an interaction with the 911 complex.
Our data show an interaction between TOPBP1 and members of the MRN complex and therefore direct recruitment of TOPBP1 by MRN is a likely possibility. An interaction between NBS1 and TOPBP1 has previously been observed in response to DSBs (Yoo et al., 2009; Morishima et al., 2007). However, the interaction of NBS1 and TOPBP1 in response to DSBs involves TOPBP1 BRCT 1–2, and we observe binding of TOPBP1 BRCT 3–6 to our ATR activating structures. It therefore appears that MRN recruits TOPBP1 through a distinct mechanism in the process of activating ATR at ss/dsDNA junctions.
The finding that MRN recruits TOPBP1 to ATR-activating structures may illuminate the mechanism by which ATR checkpoint signaling is amplified on ssDNA. It was previously not clear how the amount of ssDNA adjacent to a ss/dsDNA junction could affect the level of Chk1 phosphorylation (MacDougall et al., 2007). Our results raise the possibility that initial ATR activation at the ss/dsDNA junction, and local phosphorylation of proteins, enables MRN to bind RPA on ssDNA, while still binding to TOPBP1. This accumulation of TOPBP1 on ssDNA could facilitate activation of the ATR already bound to the adjacent ssDNA through ATRIP and poised for action. This hypothesis is in line with an observed interaction between MRE11 and RPA (Robison et al., 2004; Olson et al., 2007b; Xu et al., 2008) and the colocalization of MRE11 and RPA in response to replication stress (Robison et al., 2004). Interestingly, we find that the MRN complex is only required for ATR checkpoint activation in human cells if a low dose of HU or UV is used. This appears to account for the literature discrepancies (see introduction), and is in line with the findings of Olsen et al., 2007a. It is possible that high doses of HU do not require MRN-dependent ATR signal amplification or that ATR is activated through a distinct pathway at these doses. Importantly, MRN-dependent ATR signal amplification is likely to occur through a different mechanism than the chromatin-based mechanism reported to involve MDC1 and TOPBP1 (Wang et al., 2011), since these ssDNA-based structures should not be chromatinized in Xenopus extracts.
The role of the MRN complex in ATR activation has been ambiguous, due in large part to conflicting data and the inability to distinguish between direct and indirect effects of MRN’s activity on checkpoint activation. Using model structures that specifically activate ATR, we have demonstrated that MRN has a direct role in ATR activation through recruitment of TOPBP1. Together with all of the well-established roles of MRN in DSB recognition, ATM activation and signaling, MRN might now be considered a master regulator of the DNA damage response.
Experimental procedures
Chemicals and antibodies
The ATR inhibitor ATR-45 was synthesized by the Medicinal Chemistry shared resource (Ohio State University) based on (Charrier et al., 2011) and used at 20 μM concentration in extract. The ATM inhibitor KU-55933 (Calbiochem) was used at 200 μM. Both were added 5 min prior to addition of DNA structures. Xenopus antibodies used in this study have been previously described, and the human antibodies are commercially available. See supplementary data for details.
Xenopus extracts and immunodepletions
Xenopus nucleoplasmic extract (NPE) and Low Speed Extract (LSE) were prepared as described (Lebofsky et al., 2009). Immunodepletions were carried out with serum that was pre-coupled to protein A-Sepharose Fast-Flow beads (GE Healthcare). See supplementary data for details.
M13 structures, checkpoint activation and structure pull-down
M13 ssDNA was isolated using standard procedures. Oligonucleotides (IDT) were annealed to ssDNA and structures were purified from a 5% TBE acrylamide gel. Structures were incubated for 45 min at room temperature with 2.5 μg streptavidin (Sigma) per ng M13 structure. For checkpoint activation assays, 6–9 ng/μl of structure was used to induce checkpoint activation in NPE extract. Structures were incubated in NPE for 20 min at room temperature and reaction was stopped by adding Laemmli buffer.
For pulldown assays, structures were coupled to streptavidin dynabeads M-280 using the kilobaseBinder kit according to manufacturers instructions. Beads were washed thrice with TE buffer (Tris 10 mM pH 8, EDTA 20 mM pH 8), incubated with free streptavidin to block unbound biotin, and washed once with ELB buffer. Around 50–100 ng of M13 structure was used for each pulldown. Energy supplemented NPE was added for 10 min and beads were recovered on a magnet. Beads were taken up in ELB (+0.1% Triton and 50 mM KCl) and layered onto an ELB 0.25 M sucrose cushion and centrifuged for 30 sec in a horizontal centrifuge. Beads were washed once in ELB and resuspended in Laemmli buffer. See supplementary data for details.
Recombinant proteins
MRN, the BRCT fragments, the RAD9 fragment and OB-AAD were added to NPE in concentrations of 20 nM, 200 nM, 5 μM and 1 μM respectively. See supplementary data for details.
Supplementary Material
Highlights.
MRN binds ATR-activating DNA structures consisting of ssDNA to dsDNA junctions
MRN recruits TOPBP1 to an ATR-activating structure
MRN is required for ATR activation
911 is required for TOPBP1 function but is not involved in TOPBP1 recruitment
Acknowledgments
We thank Drs. Jean Gautier, Howard D. Lindsay, David Cortez, Tanya Paull and Johannes C. Walter for generously providing reagents used in this study. We also thank Johannes C. Walter and Dominique B. Figueroa for technical assistance, members of the Cimprich lab for helpful discussions, and Dr. Rene Medema for support during the peer review process. This work was supported by a Dean’s Fellowship and KWF fellowship awarded to AMD and by NIH grant ES016486 to KAC.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Byun TS, Pacek M, Yee MC, Walter JC, Cimprich KA. Functional uncoupling of MCM helicase and DNA polymerase activities activates the ATR-dependent checkpoint. Genes & Development. 2005;19:1040–1052. doi: 10.1101/gad.1301205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charrier JD, Durrant SJ, Golec JMC, Kay DP, Knegtel RMA, Maccormick S, Mortimore M, O’Donnell ME, Pinder JL, Reaper PM, et al. Discovery of Potent and Selective Inhibitors of Ataxia Telangiectasia Mutated and Rad3 Related (ATR) Protein Kinase as Potential Anticancer Agents. J Med Chem. 2011;54:2320–2330. doi: 10.1021/jm101488z. [DOI] [PubMed] [Google Scholar]
- Ciccia A, Elledge SJ. The DNA Damage Response: Making It Safe to Play with Knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimprich KA, Cortez D. ATR: an essential regulator of genome integrity. Nat Rev Mol Cell Biol. 2008;9:616–627. doi: 10.1038/nrm2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costanzo V, Robertson K, Bibikova M, Kim E, Grieco D, Gottesman M, Carroll D, Gautier J. Mre11 protein complex prevents double-strand break accumulation during chromosomal DNA replication. Mol Cell. 2001;8:137–147. doi: 10.1016/s1097-2765(01)00294-5. [DOI] [PubMed] [Google Scholar]
- D’amours D, Jackson SP. The yeast Xrs2 complex functions in S phase checkpoint regulation. Genes & Development. 2001;15:2238–2249. doi: 10.1101/gad.208701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delacroix S, Wagner JM, Kobayashi M, Yamamoto KI, Karnitz LM. The Rad9-Hus1-Rad1 (9-1-1) clamp activates checkpoint signaling via TopBP1. Genes & Development. 2007;21:1472–1477. doi: 10.1101/gad.1547007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Difilippantonio S, Celeste A, Fernandez-Capetillo O, Chen HT, Martin BRS, Laethem FV, Yang YP, Petukhova GV, Eckhaus M, Feigenbaum L, et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nature Cell Biology. 2005;7:675–685. doi: 10.1038/ncb1270. [DOI] [PubMed] [Google Scholar]
- Fanning E. A dynamic model for replication protein A (RPA) function in DNA processing pathways. Nucleic Acids Research. 2006;34:4126–4137. doi: 10.1093/nar/gkl550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya K. Chk1 activation requires Rad9 S/TQ-site phosphorylation to promote association with C-terminal BRCT domains of Rad4TOPBP1. Genes & Development. 2004;18:1154–1164. doi: 10.1101/gad.291104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Tsujimura T, Sugino A, Takisawa H. The phosphorylated C-terminal domain of Xenopus Cut5 directly mediates ATR-dependent activation of Chk1. Genes Cells. 2006;11:993–1007. doi: 10.1111/j.1365-2443.2006.00998.x. [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GCM, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nature Cell Biology. 2005;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Kumagai A, Lee J, Yoo HY, Dunphy WG. TopBP1 activates the ATR-ATRIP complex. Cell. 2006;124:943–955. doi: 10.1016/j.cell.2005.12.041. [DOI] [PubMed] [Google Scholar]
- Lebofsky R, Takahashi T, Walter JC. DNA replication in nucleus-free Xenopus egg extracts. Methods Mol Biol. 2009;521:229–252. doi: 10.1007/978-1-60327-815-7_13. [DOI] [PubMed] [Google Scholar]
- Lee J, Dunphy WG. Rad17 plays a central role in establishment of the interaction between TopBP1 and the Rad9-Hus1-Rad1 complex at stalled replication forks. Mol Biol Cell. 2010;21:926–935. doi: 10.1091/mbc.E09-11-0958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kumagai A, Dunphy WG. The Rad9-Hus1-Rad1 checkpoint clamp regulates interaction of TopBP1 with ATR. J Biol Chem. 2007;282:28036–28044. doi: 10.1074/jbc.M704635200. [DOI] [PubMed] [Google Scholar]
- Lupardus PJ, Cimprich KA. Phosphorylation of Xenopus Rad1 and Hus1 defines a readout for ATR activation that is independent of Claspin and the Rad9 carboxy terminus. Mol Biol Cell. 2006;17:1559–1569. doi: 10.1091/mbc.E05-09-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDougall CA, Byun TS, Van C, Yee MC, Cimprich KA. The structural determinants of checkpoint activation. Genes & Development. 2007;21:898–903. doi: 10.1101/gad.1522607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Mirzoeva OK, Wells J, Olivares H, Williams BR, Zinkel RA, Farnham PJ, Petrini JHJ. Mre11 Complex and DNA Replication: Linkage to E2F and Sites of DNA Synthesis. Mol Cell Biol. 2001;21:6006–6016. doi: 10.1128/MCB.21.17.6006-6016.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael WM. Activation of the DNA Replication Checkpoint Through RNA Synthesis by Primase. Science. 2000;289:2133–2137. doi: 10.1126/science.289.5487.2133. [DOI] [PubMed] [Google Scholar]
- Mordes DA, Glick GG, Zhao R, Cortez D. TopBP1 activates ATR through ATRIP and a PIKK regulatory domain. Genes & Development. 2008;22:1478–1489. doi: 10.1101/gad.1666208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morishima KI, Sakamoto S, Kobayashi J, Izumi H, Suda T, Matsumoto Y, Tauchi H, Ide H, Komatsu K, Matsuura S. TopBP1 associates with NBS1 and is involved in homologous recombination repair. Biochem Biophys Res Commun. 2007;362:872–879. doi: 10.1016/j.bbrc.2007.08.086. [DOI] [PubMed] [Google Scholar]
- Myers JS, Cortez D. Rapid activation of ATR by ionizing radiation requires ATM and Mre11. J Biol Chem. 2006;281:9346–9350. doi: 10.1074/jbc.M513265200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navadgi-Patil VM, Burgers PM. A tale of two tails: Activation of DNA damage checkpoint kinase Mec1/ATR by the 9-1-1 clamp and by Dpb11/TopBP1. DNA Repair. 2009;8:996–1003. doi: 10.1016/j.dnarep.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E, Nievera CJ, Lee AYL, Chen L, Wu X. The Mre11-Rad50-Nbs1 complex acts both upstream and downstream of ataxia telangiectasia mutated and Rad3-related protein (ATR) to regulate the S-phase checkpoint following UV treatment. J Biol Chem. 2007a;282:22939–22952. doi: 10.1074/jbc.M702162200. [DOI] [PubMed] [Google Scholar]
- Olson E, Nievera CJ, Liu E, Lee AYL, Chen L, Wu X. The Mre11 complex mediates the S-phase checkpoint through an interaction with replication protein A. Mol Cell Biol. 2007b;27:6053–6067. doi: 10.1128/MCB.00532-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paull TT. Making the best of the loose ends: Mre11/Rad50 complexes and Sae2 promote DNA double-strand break resection. DNA Repair. 2010;9:1283–1291. doi: 10.1016/j.dnarep.2010.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappas M, Oliver AW, Pearl LH. Structure and function of the Rad9-binding region of the DNA-damage checkpoint adaptor TopBP1. Nucleic Acids Research. 2011;39:313–324. doi: 10.1093/nar/gkq743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robison JG, Elliott J, Dixon K, Oakley GG. Replication protein A and the Mre11.Rad50.Nbs1 complex co-localize and interact at sites of stalled replication forks. J Biol Chem. 2004;279:34802–34810. doi: 10.1074/jbc.M404750200. [DOI] [PubMed] [Google Scholar]
- Stiff T, Reis C, Alderton GK, Woodbine L, O’Driscoll M, Jeggo PA. Nbs1 is required for ATR-dependent phosphorylation events. Embo J. 2005;24:199–208. doi: 10.1038/sj.emboj.7600504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stracker TH, Petrini JHJ. The MRE11 complex: starting from the ends. Nature Rev Mol Cell Biol. 2011;12:90–103. doi: 10.1038/nrm3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Gong Z, Chen J. MDC1 collaborates with TopBP1 in DNA replication checkpoint control. J Cell Biol. 2011;193:267–273. doi: 10.1083/jcb.201010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Vaithiyalingam S, Glick GG, Mordes DA, Chazin WJ, Cortez D. The basic cleft of RPA70N binds multiple checkpoint proteins, including RAD9, to regulate ATR signaling. Mol Cell Biol. 2008;28:7345–7353. doi: 10.1128/MCB.01079-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. Ataxia-telangiectasia mutated (ATM)-dependent activation of ATR occurs through phosphorylation of TopBP1 by ATM. J Biol Chem. 2007;282:17501–17506. doi: 10.1074/jbc.M701770200. [DOI] [PubMed] [Google Scholar]
- Yoo HY, Kumagai A, Shevchenko A, Shevchenko A, Dunphy WG. The Mre11-Rad50-Nbs1 complex mediates activation of TopBP1 by ATM. Mol Biol Cell. 2009;20:2351–2360. doi: 10.1091/mbc.E08-12-1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou L, Elledge SJ. Sensing DNA Damage Through ATRIP Recognition of RPA- ssDNA Complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.