Abstract
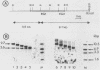
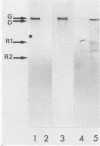
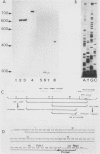
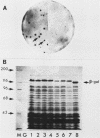
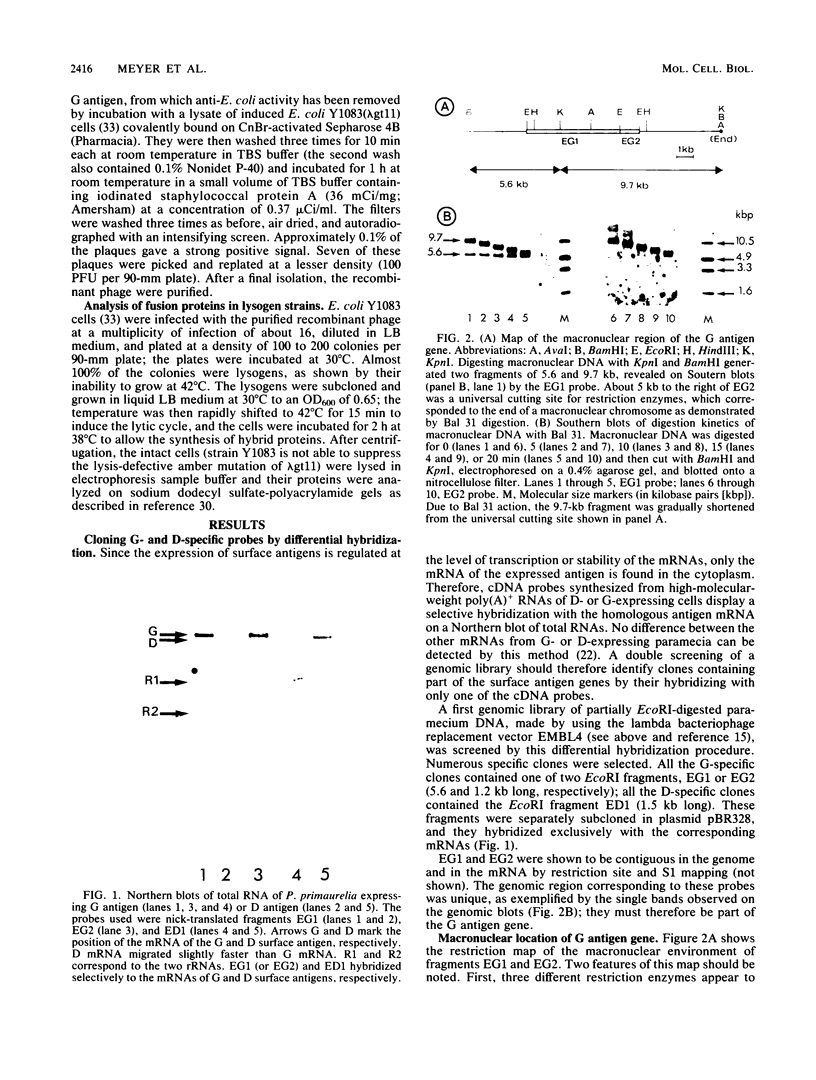
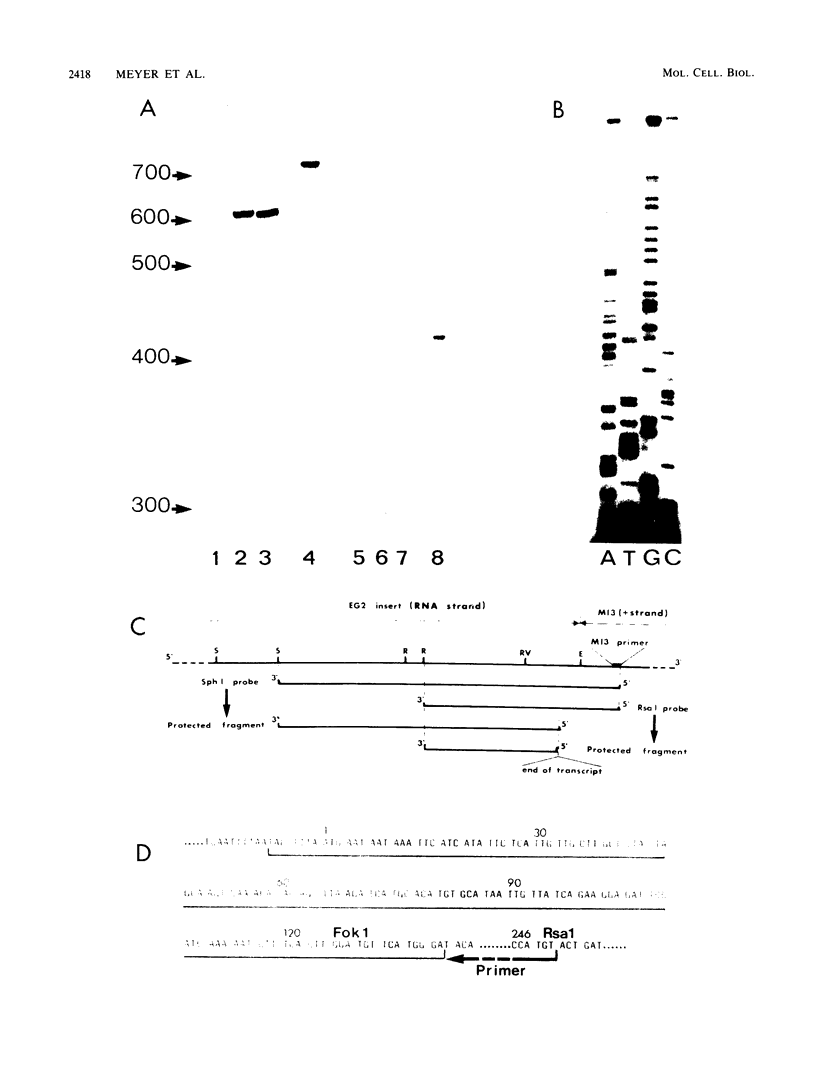
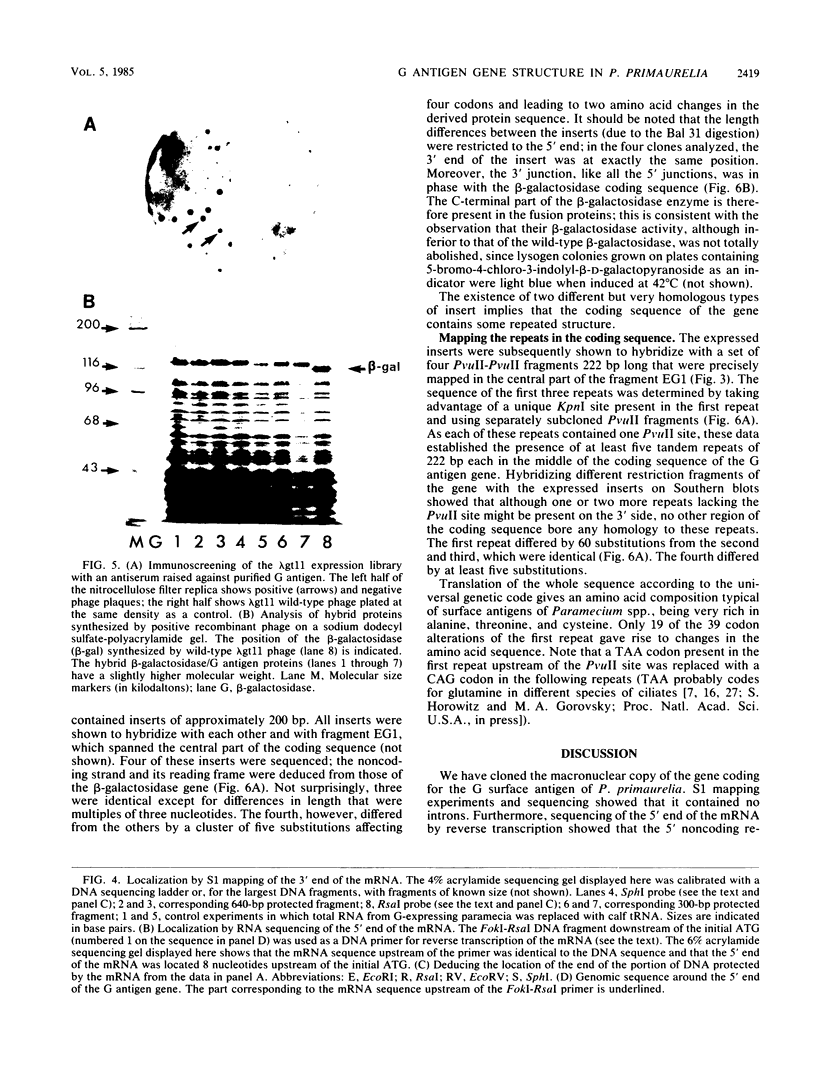
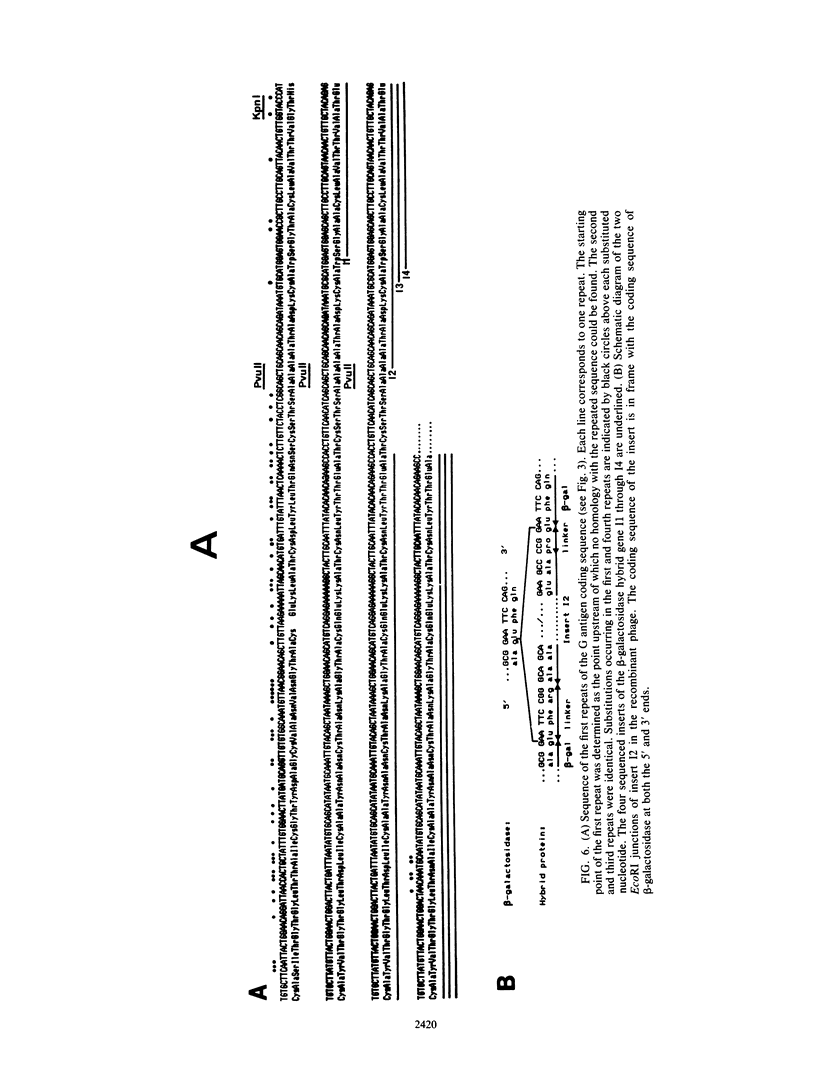
The gene encoding the G surface antigen of Paramecium primaurelia was cloned from a macronuclear DNA library by a screening procedure involving differential hybridization with cDNA probes synthesized from polyadenylated RNAs of cells expressing one of two alternate antigens. S1 mapping experiments and sequencing of the cloned DNA and the mRNA showed that the cloned gene corresponded to the high-molecular-weight mRNA that had been indirectly identified as that of the G surface antigen. Because the genetic code of Paramecium spp. is different from the "universal" code, this mRNA cannot be correctly translated in vitro; direct proof that it encoded the antigenic determinants of this protein was therefore obtained through expression of fragments of the coding sequence in Escherichia coli by using the expression vector lambda gt11. Studies on the structure of this gene revealed that the central part of the coding sequence contained at least five tandem repeats of 222 base pairs, encoding immunogenic domains of the protein. We also showed that, like other surface antigen genes of trypanosomes and paramecia, this gene lay next to a chromosome end and that no rearrangement of its immediate genomic environment was associated with its expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blackburn E. H. The molecular structure of centromeres and telomeres. Annu Rev Biochem. 1984;53:163–194. doi: 10.1146/annurev.bi.53.070184.001115. [DOI] [PubMed] [Google Scholar]
- Borst P., Cross G. A. Molecular basis for trypanosome antigenic variation. Cell. 1982 Jun;29(2):291–303. doi: 10.1016/0092-8674(82)90146-5. [DOI] [PubMed] [Google Scholar]
- Campbell D. A., Thornton D. A., Boothroyd J. C. Apparent discontinuous transcription of Trypanosoma brucei variant surface antigen genes. 1984 Sep 27-Oct 3Nature. 311(5984):350–355. doi: 10.1038/311350a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capdeville Y. Regulation of surface antigen expression in Paramecium primaurelia. II. Role of the surface antigen itself. J Cell Physiol. 1979 Jun;99(3):383–393. doi: 10.1002/jcp.1040990313. [DOI] [PubMed] [Google Scholar]
- Caron F., Meyer E. Does Paramecium primaurelia use a different genetic code in its macronucleus? Nature. 1985 Mar 14;314(6007):185–188. doi: 10.1038/314185a0. [DOI] [PubMed] [Google Scholar]
- Coppel R. L., Cowman A. F., Lingelbach K. R., Brown G. V., Saint R. B., Kemp D. J., Anders R. F. Isolate-specific S-antigen of Plasmodium falciparum contains a repeated sequence of eleven amino acids. Nature. 1983 Dec 22;306(5945):751–756. doi: 10.1038/306751a0. [DOI] [PubMed] [Google Scholar]
- Dame J. B., Williams J. L., McCutchan T. F., Weber J. L., Wirtz R. A., Hockmeyer W. T., Maloy W. L., Haynes J. D., Schneider I., Roberts D. Structure of the gene encoding the immunodominant surface antigen on the sporozoite of the human malaria parasite Plasmodium falciparum. Science. 1984 Aug 10;225(4662):593–599. doi: 10.1126/science.6204383. [DOI] [PubMed] [Google Scholar]
- De Lange T., Borst P. Genomic environment of the expression-linked extra copies of genes for surface antigens of Trypanosoma brucei resembles the end of a chromosome. Nature. 1982 Sep 30;299(5882):451–453. doi: 10.1038/299451a0. [DOI] [PubMed] [Google Scholar]
- Enea V., Arnot D., Schmidt E. C., Cochrane A., Gwadz R., Nussenzweig R. S. Circumsporozoite gene of plasmodium cynomolgi (Gombak):cDNA cloning and expression of the repetitive circumsporozoite epitope. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7520–7524. doi: 10.1073/pnas.81.23.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein L. M., Forney J. D. Mendelian and non-mendelian mutations affecting surface antigen expression in Paramecium tetraurelia. Mol Cell Biol. 1984 Aug;4(8):1583–1590. doi: 10.1128/mcb.4.8.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrell P. J., Deininger P. L., Bankier A., Barrell B. Homologous upstream sequences near Epstein-Barr virus promoters. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1565–1569. doi: 10.1073/pnas.80.6.1565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forney J. D., Epstein L. M., Preer L. B., Rudman B. M., Widmayer D. J., Klein W. H., Preer J. R., Jr Structure and expression of genes for surface proteins in Paramecium. Mol Cell Biol. 1983 Mar;3(3):466–474. doi: 10.1128/mcb.3.3.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Helftenbein E. Nucleotide sequence of a macronuclear DNA molecule coding for alpha-tubulin from the ciliate Stylonychia lemnae. Special codon usage: TAA is not a translation termination codon. Nucleic Acids Res. 1985 Jan 25;13(2):415–433. doi: 10.1093/nar/13.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES I. G. STUDIES ON THE CHARACTERIZATION AND STRUCTURE OF THE IMMOBILIZATION ANTIGENS OF PARAMECIUM AURELIA. Biochem J. 1965 Jul;96:17–23. doi: 10.1042/bj0960017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M., Scherf A., Mercereau O., Langsley G., Sibilli L., Dubois P., Pereira da Silva L., Müller-Hill B. Human antisera detect a Plasmodium falciparum genomic clone encoding a nonapeptide repeat. 1984 Sep 27-Oct 3Nature. 311(5984):382–385. doi: 10.1038/311382a0. [DOI] [PubMed] [Google Scholar]
- Kooter J. M., De Lange T., Borst P. Discontinuous synthesis of mRNA in trypanosomes. EMBO J. 1984 Oct;3(10):2387–2392. doi: 10.1002/j.1460-2075.1984.tb02144.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Meyer E., Caron F., Guiard B. Blocking of in vitro translation of Paramecium messenger RNAs is due to messenger RNA primary structure. Biochimie. 1984 May;66(5):403–412. doi: 10.1016/0300-9084(84)90024-5. [DOI] [PubMed] [Google Scholar]
- Ozaki L. S., Svec P., Nussenzweig R. S., Nussenzweig V., Godson G. N. Structure of the plasmodium knowlesi gene coding for the circumsporozoite protein. Cell. 1983 Oct;34(3):815–822. doi: 10.1016/0092-8674(83)90538-x. [DOI] [PubMed] [Google Scholar]
- Parsons M., Nelson R. G., Watkins K. P., Agabian N. Trypanosome mRNAs share a common 5' spliced leader sequence. Cell. 1984 Aug;38(1):309–316. doi: 10.1016/0092-8674(84)90552-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preer J. R., Jr, Preer L. B., Rudman B. M., Barnett A. J. Deviation from the universal code shown by the gene for surface protein 51A in Paramecium. Nature. 1985 Mar 14;314(6007):188–190. doi: 10.1038/314188a0. [DOI] [PubMed] [Google Scholar]
- Preer J. R., Preer L. B., Rudman B. M. mRNAs for the immobilization antigens of Paramecium. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6776–6778. doi: 10.1073/pnas.78.11.6776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell V., Aline R., Jr, Parsons M., Agabian N., Stuart K. Expression of a minichromosomal variant surface glycoprotein gene in Trypanosoma brucei. Nature. 1985 Feb 14;313(6003):595–597. doi: 10.1038/313595a0. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. The study of histone--histone associations by chemical cross-linking. Methods Cell Biol. 1978;18:429–440. [PubMed] [Google Scholar]
- Van der Ploeg L. H., Cornelissen A. W., Michels P. A., Borst P. Chromosome rearrangements in Trypanosoma brucei. Cell. 1984 Nov;39(1):213–221. doi: 10.1016/0092-8674(84)90207-1. [DOI] [PubMed] [Google Scholar]
- Yao M. C., Yao C. H. Repeated hexanucleotide C-C-C-C-A-A is present near free ends of macronuclear DNA of Tetrahymena. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7436–7439. doi: 10.1073/pnas.78.12.7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala F., Cochrane A. H., Nardin E. H., Nussenzweig R. S., Nussenzweig V. Circumsporozoite proteins of malaria parasites contain a single immunodominant region with two or more identical epitopes. J Exp Med. 1983 Jun 1;157(6):1947–1957. doi: 10.1084/jem.157.6.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]