Summary
Purpose
Intracerebral vascular malformations including cavernous angiomas (CAs) and arteriovenous malformations (AVMs) are an important cause of chronic pharmacoresistant epilepsies. Little is known about the pathogenetic basis of epilepsy in patients with vascular malformations. Intracerebral deposits of iron-containing blood products have been generally regarded as responsible for the strong epileptogenic potential of CAs. Here, we have analyzed whether blood–brain barrier (BBB) dysfunction and subsequent astrocytic albumin uptake, recently described as critical trigger of focal epilepsy, represent pathogenetic factors in vascular lesion–associated epileptogenesis.
Methods
We examined the correlation between hemosiderin deposits, albumin accumulation, and several clinical characteristics in a series of 80 drug-refractory epilepsy patients with CAs or AVMs who underwent surgical resection. Analysis of clinical parameters included gender, age of seizure onset, epilepsy frequency, duration of epilepsy before surgery, and postoperative seizure outcome classification according to Engel class scale. Hemosiderin deposits in the adjacent brain tissue of the vascular lesion were semiquantitatively analyzed. Fluorescent double-immunohistochemistry using GFAP/albumin costaining was performed to study albumin extravasation.
Key Findings
Our results suggest that a shorter duration of preoperative epilepsy is correlated with significantly better postsurgical outcome (p < 0.05), whereas no additional clinical or neuropathologic parameter correlated significantly with the postsurgical seizure situation. Intriguingly, we observed strong albumin immunoreactivity within the vascular lesion and in perilesional astrocytes (57.65 ± 4.05%), but not in different control groups.
Significance
Our present data on albumin uptake in brain tissue adjacent to AVMs and CAs suggests BBB dysfunction and accumulation of albumin within astrocytes as a new pathologic feature potentially associated with the epileptogenic mechanism for vascular lesions and provides novel therapy perspectives for antiepileptogenesis in affected patients.
Keywords: Epilepsy, Vascular malformation, Cavernoma, Albumin, Hemosiderin
Focal chronic seizures are frequent in patients with vascular malformations of the brain (Kraemer & Awad, 1994), and often require epilepsy surgery as a consequence of pharmacoresistance. Cavernous angiomas (CAs, also called cavernous hemangiomas, cavernous malformations, or cavernomas) and arteriovenous malformations (AVMs) represent the most frequent vascular lesions, and approximately 40–80% of patients with intracerebral CAs have seizures (Del Curling et al., 1991; Moran et al., 1999; Moriarity et al., 1999; Awad & Jabbour, 2006; Baumann et al., 2007). From a histopathologic standpoint, CAs are characterized by dilated, endothelium-lined blood vessels without arterial features. Generally, the sinusoidal blood cavities lie back-to-back and there is no substantial brain tissue interposed between the vessels. However, a rim of gliotic parenchyma with varying amounts of hemosiderin deposits containing large amounts of iron is frequently present in the surrounding tissue due to blood–brain barrier (BBB) dysfunction. Magnetic resonance images typically show a “popcorn”-like pattern with mixed high- and low-signal intensities in the core and a dark rim of hemosiderin (Fig. 1, Aa–Bc). Ultrastructural studies revealed dysfunction of BBB components with poorly formed tight junctions and abnormalities in the basal lamina (Wong et al., 2000; Clatterbuck et al., 2001).
Figure 1.
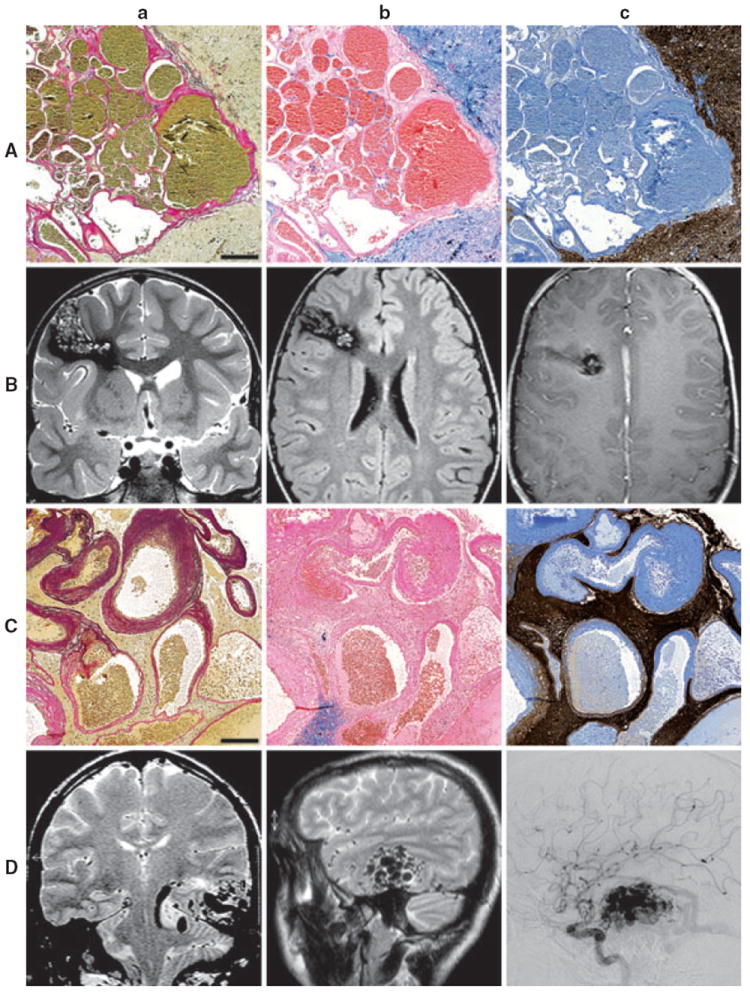
Representative histopathologic and neuroradiologic findings in patients with cavernous angiomas and arteriovenous malformations. Cavernous angiomas (CAs) are composed of thin, endothelium-lined vascular channels without muscular layers (Aa–c, scale bar = 200 μm, patient ID No. 61). Arterial vessels are absent (Aa: elastica-van Gieson staining). Usually, the sinusoidal blood cavities lie back-to-back and no brain tissue is present between the blood vessels. Prussian blue staining shows abundant intraparenchymal hemosiderin deposits in the vicinity of the lesion (Ab). GFAP-immunohistochemistry reveals strong reactive astrogliosis (Ac). Coronal T2-weighted fast spin echo (Ba), axial fluid-attenuated inversion recovery (FLAIR) fast spin echo (Bb) and axial contrast-enhanced T1-weighted spin echo images show a CA in the right inferior frontal gyrus. The oblong CA matrix is surrounded by hemosiderinladen brain parenchyma. Arteriovenous malformations (AVMs) are characterized by abnormal blood vessels with variable size and configuration (Ca–c, scale bar = 200 μm, patient ID No. 8). Different amounts of thick-walled arterial elements with parts of the tunica media and frequently dispersed fractions of the lamina elastica interna can be observed near thin, amuscular venous walls and transitory shunt vessels (Ca: elastica-van Gieson staining). Hemosiderin deposits are generally less abundant than in CAs (Cb: Prussian blue staining). Intervening brain tissue is a further feature of AVMs (Cc: GFAP-immunohistochemistry). On radiological evaluation, non–space-occupying lesions with tubular or rete-like “flow void” structures may be visible (Da–b, coronal and sagittal T2-weighted MRI). Digital subtraction angiography (DSA) shows feeding arteries (Dc).
Epilepsia © ILAE
AVMs are composed of structurally altered veins, arteries, and transitory “shunt”-vessels surrounded by intervening brain tissue. Microscopically, the vascular walls show various pathologies such as collagenous replacement of muscles and interruption of the lamina elastic interna. Tightly packed flow void structures can be seen on MR imaging (MRI) (Fig. 1, Ca–Dc).
Although seizures are the most frequent initial symptoms in patients with supratentorially and cortically located CAs, little is known about the basic mechanisms of epileptogenicity. Dysfunction of BBB and intracerebral deposits of iron-containing blood products may be responsible for the strong epileptogenic potential of CAs (Willmore et al., 1978; Singh & Pathak, 1990; Kraemer & Awad, 1994). This is in line with reports that neurosurgical removal of the hemosiderin deposits next to the vascular lesions achieves better seizure results than the restricted vascular lesionectomy (Baumann et al., 2006). Nevertheless, restricted lesionectomy has been shown to reduce seizures in some cases (Ferroli et al., 2006). Results regarding the association between a long history of epilepsy and clinical outcome vary (Moran et al., 1999; Baumann et al., 2007; Stavrou et al., 2008). Hammen and collaborators suggested a more extensive resection in patients with long disease duration (Hammen et al., 2007). Because an intrinsic epileptogenicity of CAs is unlikely, cellular and/or structural alterations in adjacent brain tissue probably play an important role in the epileptogenic potential of vascular lesions.
Recent data emphasized a contribution of BBB opening to the progression of mesial temporal lobe epilepsy and neocortical epilepsy (Ivens et al., 2007; van Vliet et al., 2007). Particularly, albumin uptake into astrocytes has been discussed as a key pathogenic factor (Ivens et al., 2007; Ralay Ranaivo et al., 2010). Here we retrospectively analyzed a series of 80 patients with vascular malformations who underwent surgery for drug-refractory epilepsy between 1988 and 2009. We addressed correlations between iron deposits, albumin accumulation, and several clinical parameters.
Materials and Methods
Patients
From the database of 1,721 patients with resective epilepsy surgery after having comprehensive presurgical evaluation for drug-refractory focal epilepsy at Bonn Epilepsy Center between 1988 and 2009, we identified 100 patients with a diagnosis of CA or AVM. Surgical removal of the vascular malformation was clinically indicated to achieve seizure control in every case. All procedures were conducted in accordance with the Declaration of Helsinki and approved by the ethics committee of the University of Bonn Medical Center. Written informed consent was obtained from all patients. Requirements for inclusion in this study were epilepsy surgery for drug-refractory epilepsy as defined by the International League Against Epilepsy (ILAE) (Kwan et al., 2010) as well as radiologic and histologic diagnosis of a vascular brain lesion. These inclusion criteria were fulfilled by 80 patients (CAs, n = 64; AVMs, n = 16). Twenty patients who present either a dual pathology, a discrepancy between radiologic and histologic diagnosis, or did not undergo presurgical MRI were excluded. Particularly, cases with limited biopsy specimens, which did not allow definite diagnosis of either CA or AVM were not included to the study. As part of our standard practice, all patients in the present study underwent neuro-psychological tests, presurgical 1.5-Tesla (1988–2004) or 3-Tesla (2005–2009) (Philips Medical Systems, Best, The Netherlands) brain MRI and noninvasive video–electroencephalography (EEG) monitoring using conventional scalp EEG recordings (10–20 system) (Kral et al., 2002). Additional intracranial recordings (grid, strip, and depth electrodes) were performed in 25 patients. Clinical characteristics used for this study include gender, age of seizure onset, duration of epilepsy before surgery, seizure frequency, and postoperative seizure outcome classification according to Engel scale (Engel et al., 1993). The follow-up period was defined as 2 weeks after surgery until the last available follow-up. Follow-up had to be >2 years to be considered seizure free. Ascertainment of seizure outcome was performed at standardized postoperative checkups within the Bonn Neuro-Center. In addition, telephone interviews were used to finalize data.
Neuropathology
Surgical specimens were fixed in formaldehyde overnight and embedded in paraffin. Macroscopic and histopathologic examinations were performed by experienced neuropathologists at the University of Bonn. Neuropathologic stainings of CAs (n = 64) and AVMs (n = 16) comprised at least hematoxylin and eosin (H&E), Prussian blue, and elastica-van Gieson. Furthermore, we carried out immunohistochemical reactions against glial fibrillary acidic protein (GFAP) as described before in detail (Campos et al., 2009). The pattern of hemosiderin deposits in the adjacent brain tissue of the vascular lesion allowed semiquantification (0, +, ++, +++) (Jellinger et al., 1990). Albumin extravasation was studied by fluorescent double-immunohistochemistry in CAs (n = 17) and AVMs (n = 10) using GFAP/albumin costainings. Samples were stratified from patients based on the amount of hemosiderin deposits (0 and +; +++) and postoperative outcome (seizure free; not seizure free). For this group of patients, seizure semiology and frequency were also available.
For the double-immunohistochemistry, we started from formalin fixed and paraffin-embedded sections, which were mounted on slides (Superfrost Plus, Braunschweig, Germany) and deparaffinated in xylene, rinsed in ethanol, and incubated in citrate buffer (10 mM, pH 6,0). Slides were then washed with phosphate-buffered saline (PBS) and stored for 2 h at 37°C in a humid chamber after being treated with a blocking solution containing fetal calf serum (FCS) 10%, normal goat serum (NGS) 1:100, and PBS. Slides were incubated overnight in anti-albumin antibody 1:4,000 (rabbit anti-human albumin; DakoCytomation, Glostrup, Denmark), monoclonal GFAP-antibody 1:400 (monoclonal mouse; Sigma-Aldrich, Steinheim, Germany), or CD34 Class II antibody 1:50 (monoclonal mouse; DakoCytomation), FCS 10%, and PBS at 4°C. Next, sections were incubated with Cy3 mouse 1:400 (Jackson ImmunoResearch, West Grove PA, U.S.A.) and FITC rabbit 1:400 (Jackson ImmunoResearch) secondary antibodies, 4′,6-diamidino-2-phenylindole 1:100 (DAPI; Sigma Aldrich, 5 mg/ml), FCS 10%, and PBS. Then, sections were stored in a dark place for 2 h, washed with PBS, and cover slipped with mounting medium for fluorescence (Vectashield, Burlingame, CA, U.S.A.). Microscope images of two representative perilesional regions were acquired for each patient using a Zeiss Axio Observer. A1 with 20×/0.8 NA objective and a Jenoptik ProgRes CFcool CCD camera (Jena, Germany) using the ImageJ plugin. Images were stored in their original formats, and final images for figures were prepared in Adobe (Adobe Systems, Munich, Germany) Photoshop: levels and brightness/contrast of images were minimally and evenly adjusted over the entire micrograph. In the GFAP/albumin costained images, the ratio of morphologic and immunohistochemical identified astrocytes with and without albumin expression was calculated in an area of 0.67 mm2.
Neocortical tissue (frontal lobe) from autopsies of patients with acute cardiac failure, but without neurologic disorders and without history of prolonged perimortem conditions served as controls for the double-immunohistochemistry (n = 3). As further control specimens, we analyzed GFAP/albumin costainings in frontal and temporolateral brain samples from three patients with a diagnosis of focal cortical dysplasia type IIa (according to Blumcke et al., 2011) and two patients with incidental detection of an AVM (frontal and parietal) but absence of epilepsy and severe hemorrhage.
Statistical analyses
Chi-square (χ2) test, Fisher’s two-sided exact test, and unpaired t-test were applied. (SPSS; SPSS Inc., Chicago, IL, U.S.A.). p < 0.05 was considered significant. Where appropriate, coexpression data and clinical parameters are given as mean ± standard error of the mean (SEM).
Results
Our analysis of the prospective database revealed a large cohort of 80 patients who underwent epilepsy surgical intervention due to drug-resistant epilepsy between 1988 and 2009, with concordant radiologic and neuropathologic diagnoses of either CA (n = 64) or AVM (n = 16). Representative histopathologic findings are shown in Fig. 1. Strong reactive astrogliosis with varying degrees of hypertrophy of cell bodies and processes was present in tissue neighboring the vascular malformations (Fig. S1). Analysis of perilesional cortical architecture in all cases with (1) sufficient neocortical tissue adjacent to the vascular lesion and (2) perpendicular orientation of the section to the pial surface (n = 54) showed presence of associated focal cortical dysplasia (FCD) type IIIc according to the new ILAE classification system in three patients (Blumcke et al., 2011). Seizures persisted postoperatively in one patient with AVM and FCD IIIc (Fig. 2), whereas the two remaining patients with CAs and FCD IIIc are seizure-free since surgery.
Figure 2.
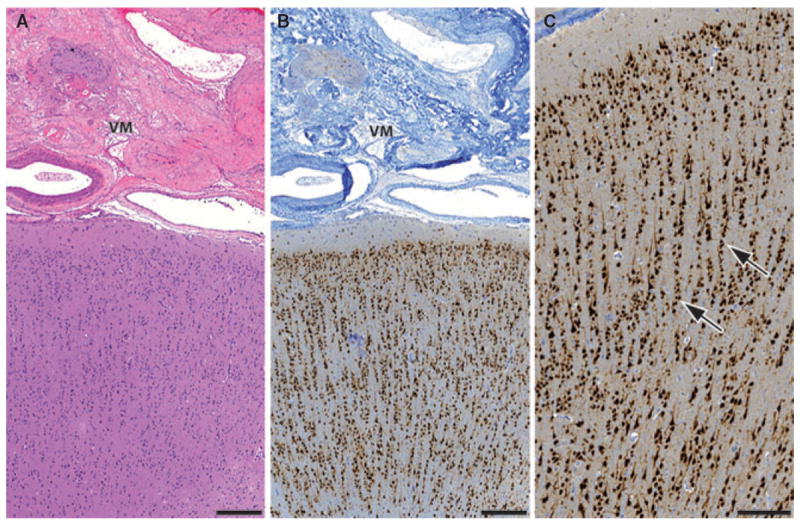
Histopathologic findings in vascular malformations associated with focal cortical dysplasia type IIIc. In three patients (ID No. 12, 34, and 48) vascular malformation was associated with cortical dyslamination. H&E staining shows an occipital arteriovenous malformation and adjacent cortex (A, patient ID No. 12, scale bar = 400 μm,). Neuronal nuclei immunohistochemistry reveals microcolumnar (radial) dyslamination in the neocortex below the vascular lesion (B, scale bar = 400 μm; C, scale bar = 200 μm). Abundant microcolumns with more than eight neurons aligned in a vertical direction can be observed (arrows). VM, vascular malformation.
Epilepsia © ILAE
By statistical analysis, we observed no differences in age at epilepsy onset, age at surgery, and disease duration in patients with either CA or AVM (Fig. 3). Sufficient clinical data for reliable classification of seizure outcome was available in 76 patients. The mean follow-up period was 4.3 years (range 2.0–16.7 years; Table S1). Engel class I outcome was reached in 70.3% of patients with CA and in 75.0% of patients with AVMs, (p > 0.5; Fisher’s exact test). Surgical procedures included resection of the vascular lesion and the adjacent hemosiderin rim (n = 15) and tailored lesionectomy with resection of bigger areas (n = 65). Outcome was similar in both groups (p > 0.05; Fisher’s exact test). Pure lesionectomy was not performed. No significantly worse outcome was present in the 25 patients with need of presurgical intracranial EEG recordings (60.0% vs. 76.4%, p > 0.05; Fisher’s exact test). CAs and AVMs associated with focal epilepsy were most often localized temporolateral/polar (48.8%) or temporomesial (27.5%). However, localization of vascular lesions was not significantly associated with seizure outcome. We observed larger amounts of hemosiderin deposits in CAs than in AVMs (p > 0.01; χ2 test), but detected no significant correlation with disease duration. Patients with epilepsy onset before the age of 30 years showed a tendency of higher hemosiderin amounts, but this trend did not reach statistical significance (p > 0.05, Fisher’s exact test). However, more extended amounts of hemosiderin were not associated with a worse outcome. In contrast, patients with epileptic seizures for >20 years were less likely to achieve seizure freedom with surgical intervention than patients with shorter disease duration (52.6% vs. 82.5%, p < 0.05; Fisher’s exact test). This applies for the entire cohort as well as for the representative subgroup with analysis of albumin/GFAP costainings. Patients with long disease duration were significantly younger at disease onset than patients with shorter disease duration (16.7 ± 1.7 vs. 26.3 ± 1.7 years, p < 0.01; unpaired t-test).
Figure 3.

Clinical parameters and hemosiderin deposits. Statistical analysis revealed no significant differences between epilepsy patients with either CA or AVM in gender, age at epilepsy onset, age at surgery (A), and outcome (B, p = 0.472, chi-square test). In contrast, higher amounts of hemosiderin deposits were present in patients with CA than in patients with AVM (C, p = 0.002, chi-square test). Localizations of the vascular lesions are shown in D. aFisher’s two-sided exact test. bUnpaired t-test. CA, cavernous angioma; AVM, arteriovenous malformation; f, female; m, male; nc, not classifiable.
Epilepsia © ILAE
Of interest, strong albumin immunoreactivity was not only observed within the vascular lesion but also in perilesional astrocytes in both CAs and AVMs (Fig. 4a). Fluorescent immunohistochemistry revealed costaining with GFAP/albumin in overall 57.65 ± 4.05% of perilesional astrocytes (CAs, 60.4 ± 4.7%, AVMs, 53.1 ± 7.6%; p > 0.05; unpaired t-test). We detected a varying pattern of albumin distribution with preferential membranous accumulation as well as diffuse cytoplasmatic immunoreactivity in samples from all patients with focal epilepsy and vascular malformations. No significant association between localization of the vascular malformation and astrocytic albumin uptake was noted. In contrast to our findings in epileptogenic vascular malformations, we observed no significant astrocytic albumin immunoreactivity in autopsy control brain tissue (4.0 ± 4.0%; p < 0.001 vs. CAs, p < 0.01 vs. AVMs; unpaired t-test). Also in an “epileptic nonvascular” control group (i.e., patients with FCD IIa) as well as in a “vascular nonepileptic” control group (i.e., patients with AVM but absence of epilepsy and severe hemorrhage) we observed only intravasal albumin immunoreactivity, but no relevant astrocytic albumin uptake (Fig. 4b–d). Additional CD34/albumin immunohistochemistry in tissue from patients with FCD IIa further confirmed the intravasal localization of albumin signals in these samples (Fig. S2).
Figure 4.
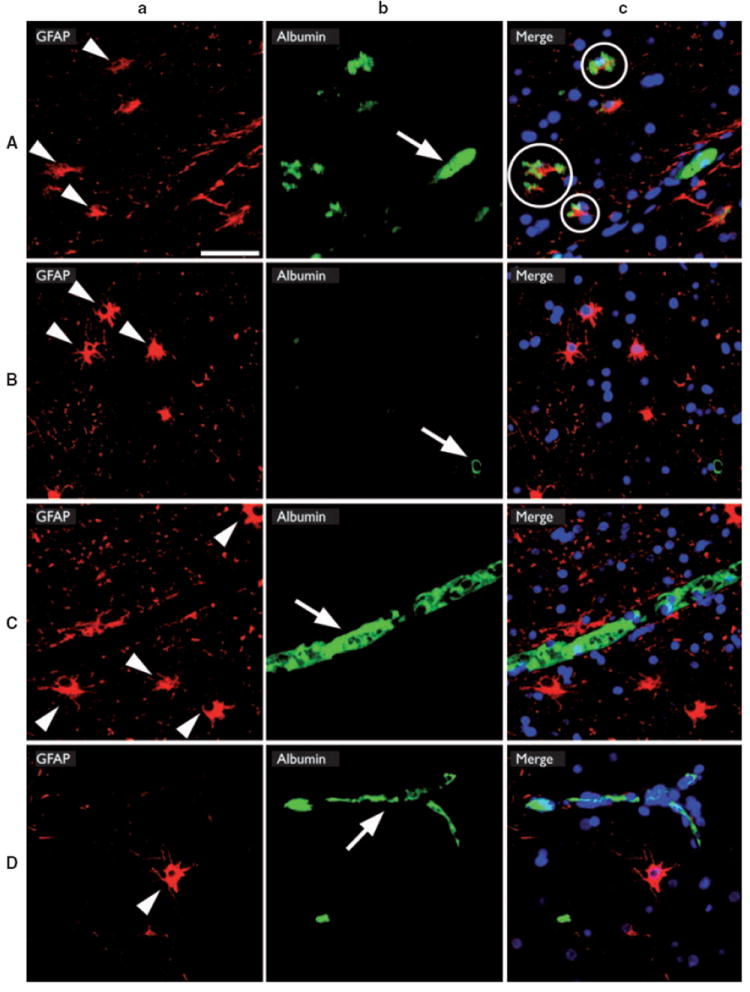
GFAP/albumin fluorescent double-immunohistochemistry in epilepsy patients with vascular malformations and different controls. Presence of GFAP-reactive astrocytes and albumin-reactive deposits was analyzed in biopsy specimens from patients with vascular malformations and drug-refractory epilepsy (A), patients with vascular malformations but absence of epilepsy (B), patients with epilepsy due to a focal cortical dysplasia type IIa (C), and in autopsy specimens from patients without cerebral pathology (D). Reactive astrocytes (arrowheads in column a) and intravasal albumin immunoreactivity (arrows in column b) were observed in all patients. In contrast, combined images (column c) revealed only in patients with vascular malformations and epilepsy colocalization of immunoreactivity (circles in Ac). Scale bar = 50 μm.
Epilepsia © ILAE
The neurons in the epileptic tissue showed a wide spectrum of reactive alterations, which were most often hypoxic changes. However, we did not observe a correlation between those reactive changes and the amount of perilesional albumin within the tissue. Particularly, no specific neuronal accumulation of albumin was present.
However, the presence of abundant albumin in brain parenchyma adjacent to CAs and AVMs was not associated with hemosiderin deposits, type of vascular lesion, seizure frequency, disease duration, postsurgical outcome, or any other analyzed parameter (Table 1).
Table 1.
Comprehensive overview on cliniconeuropathologic correlations
| CA versus AVMa | Gendera | Age at seizure onset >30 yearsa | Seizure frequency ≥6 versus ≤2 seizures/montha,b | Disease duration >20 yearsa | Outcome Engel III/IV versus Engel Ia | Hemosiderin abundant versus none/fewa | Albumin in astrocytesb,c | |
|---|---|---|---|---|---|---|---|---|
| CA versus AVMa | – | 1.000 | 0.367 | 1.000 | 1.000 | 0.496 | 0.046 | 0.396 |
| Gendera | – | 1.000 | 0.637 | 1.000 | 0.777 | 1.000 | 0.644 | |
| Age at seizure onset >30 yearsa | – | 1.000 | 0.026 | 0.582 | 0.087 | 0.177 | ||
| Seizure frequency ≥6 versus ≤2 seizures/montha,b | – | 0.577 | 0.294 | 1.000 | 0.300 | |||
| Disease duration >20 yearsa | – | 0.015 | 0.542 | 0.898 | ||||
| Outcome Engel III/IV versus Engel Ia | – | 0.377 | 0.964 | |||||
| Hemosiderin abundant versus none/fewa | – | 0.702 |
CA, cavernous angioma; AVM, arteriovenous malformation. Bold indicates p < 0.05.
Fisher’s two-sided exact test.
Detailed preoperative seizure frequency was obtained and analyzed in the representative patient group selected for albumin-immunohistochemistry (n = 27).
Unpaired t-test.
Discussion
Here, we analyzed cliniconeuropathologic correlations in a large series of vascular malformations of the brain. We observed an association between a long history of seizures and a worse clinical outcome as previously shown in other patients’ series (Moran et al., 1999; Stavrou et al., 2008). However, neither long disease duration nor early disease onset was associated with neuropathologic findings, potentially due to the limited size of analyzed subgroups. Of interest, the results from Baumann et al., (2007) indicate worse seizure outcome in patients with early epilepsy onset and in patients with surgery before an age of 30 years, but did not demonstrate an association with a long history of seizures. For obvious reasons, duration of epilepsy, age at onset, and age at surgery are at least indirectly correlated parameters. Patients from our cohort with long disease duration were significantly younger at disease onset than patients with shorter disease duration. Hence, the observation of worse seizure outcome in patients with vascular malformations and early seizure onset is in line with the findings in our cohort. In contrast, it appears not entirely clear why older age at surgery should be associated with a better outcome as reported by Baumann et al., (2007). Considering the increased morbidity in patients with drug-refractory epilepsy, our findings strongly argue for an early surgical intervention in patients with vascular lesions and drug-refractory epilepsy.
Of interest, we found no significant correlation between the need of invasive presurgical EEG measures and a worse epilepsy outcome in comparison to patients with noninvasive EEG recordings only. This may be different in cohorts with inclusion of MRI-negative patients with focal epilepsy (Tonini et al., 2004).
AVMs and CAs represent highly epileptogenic lesions. However, the detailed underlying mechanisms of seizure generation are not known. Electrophysiologic studies that analyze properties of neurons neighboring glial tumors or CAs in neocortical lesions showed greater probability of spontaneous synaptic events and increased excitability in response to synaptic stimulation in neurons adjacent to CAs compared with those surrounding glial tumors (Williamson et al., 2003). These results may suggest different epileptogenic mechanisms in vascular malformations and tumors.
Hemosiderin deposits have been discussed repeatedly as potential epileptogenic triggers in patients with focal epilepsy due to vascular malformations; however, direct evidence for this notion is scarce. Signs of old or recent perilesional intracranial hemorrhages have been reported to be associated with a poorer outcome (Stefan & Hammen, 2004). Although our histopathologic examination revealed significantly higher amounts of hemosiderin deposits in patients with CAs than in patients with AVMs, no differences in either postoperative seizure outcome or preoperative seizure frequency was detectable. In contrast, we observed strikingly high levels of astrocytic albumin deposits in surgically removed brain parenchyma surrounding CAs and AVMs from patients with drug-refractory epilepsy. This observation suggests different pathophysiologic dispersion pathways for hemosiderin and albumin. However, the extent of albumin deposits was not statistically different between both vascular lesion groups. The absence of such specific astrocytic accumulation in “vascular nonepileptic” tissue and “epileptic nonvascular” tissue argues against a nonspecific surgical artifact or seizures itself as the underlying cause.
Although vascular damage has been described in epileptic brain areas (Cornford, 1999), recent data suggest that opening of the BBB may directly contribute to epileptogenesis, rather than simply result from seizures (Seiffert et al., 2004; Korn et al., 2005; Ivens et al., 2007; Marchi et al., 2007; Rigau et al., 2007). Direct application of albumin on the surface of the neocortex has been demonstrated to generate an epileptic focus in rats (Seiffert et al., 2004). In addition, hippocampal deposits of albumin were reported in patients with temporal lobe epilepsy, whereas no albumin was detected in autopsy controls (van Vliet et al., 2007). Using a rat model of temporal lobe epilepsy, van Vliet et al. further showed that transient dysfunction of the BBB following status epilepticus with parenchymal accumulation of serum proteins predicts and can potentially aggravate epileptic seizures. The intracellular accumulation of albumin may be protective as it may reduce the acute edema that follows BBB dysfunction and extravasation of proteins into the brain extracellular space. In addition, recent studies showed that albumin, through transforming growth factor β (TGF-β) signaling, underlies the transformation of astrocytes from a “resting” to a “reactive” state (Ivens et al., 2007; Cacheaux et al., 2009). Astrocytes critically contribute to BBB functions by formation of the glia limitans (Ballabh et al., 2004), and the transformation of astrocytes under BBB dysfunction, injury, or in epileptic tissue has been shown to induce robust changes in gene expression (David et al., 2009). From the functional point of view, these changes have been shown to involve reduced buffering of extracellular potassium and glutamate, thereby contributing to increased excitability within the neighboring neuronal network (David et al., 2009). It is thus tempting to speculate that dysfunction of the BBB in the pathologic vasculature in AVMs as well as in CAs is leading to the diffusion of albumin into the surrounding brain tissue. This diffusion of a large molecule within the brain is expected to lead to a significant drop of concentration within a few millimeters of brain tissue adjacent to the pathologic vessels (Thorne & Nicholson, 2006), leading to a rim of surrounding cortex exposed to serum proteins that activate astrocytes, leading to the development of an abnormally hyperexcitable focus. Indeed, magnetoencephalography demonstrated interictal spike activity in tissue bordering CAs (Stefan et al., 2004). Furthermore, intraoperative electrocorticography in patients with CA and pharmacoresistent epilepsy showed highly epileptiform discharge patterns in the vicinity of vascular lesions (Ferrier et al., 2007). Strong reactive astrogliosis is an often observed feature in brain tissue next to vascular malformations (McCormick, 1966; Fig. S1). Although our data do not differentiate between cause and effect, the quantity of albumin in astrocytes in the close vicinity of vascular malformations is striking in both AVMs and CAs. No direct correlation between hemosiderin deposits and albumin accumulation was observed, suggesting different pathophysiologic dispersion pathways. The absence of a direct link between the quantity of astrocytic albumin accumulation and epilepsy outcome may be caused by the limited number of analyzed samples in the specific subgroups or individual multifactorial aspects, which were clinically not documented or not analyzed in the current study.
Given the suboptimal outcome of postsurgical seizure control in a considerable number of patients with CA and patients with AVM, and the limited potential for more extended neurosurgical removal of the “hemosiderin rim” adjacent to the vascular lesions in functional brain regions, our present findings may open new perspectives for antiepileptic therapy. Pharmacologic interference with astrocytic functions, for example, with TGFβ signaling and/or potassium buffering capacity, may provide therapy options for patients that lack seizure relief after vascular malformation resection. Similarly, quantitative functional imaging of vessel permeability and/or astrocytic dysfunction may be indicative of a specific therapy, reveal the site of the epileptic lesion, and direct the pharmacological as well as surgical treatment.
Taken together, our present data suggest concentrated albumin storage by astrocytes in the perilesional brain parenchyma as a common neuropathologic feature of vascular malformations. Given the putative role of albumin-storing astrocytes in the generation of seizures, this converging pathogenetic aspect in vascular brain lesions that manifests with pharmacoresistant epilepsy, may point to a new target for antiepileptic strategies.
Supplementary Material
Acknowledgments
Our work is supported by Deutsche Forschungsgemeinschaft (SFB TR3, C6, B8, AJB; KForG “Innate Immunity” TP2, AJB), Bundesministerium für Bildung und Forschung (NGFNplus; AJB), European Union EPICURE (AJB), Euroepinomics Network of the European Science Foundation (AJB), Else-Kröner Fresenius Foundation (AJB), German Israeli Foundation (AJB) & the BONFOR program of the University of Bonn Medical Center (PN, AJB).
Footnotes
Disclosures
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Please note: Wiley-Blackwell is not responsible for the content or functionality of any supporting information supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Awad I, Jabbour P. Cerebral cavernous malformations and epilepsy. Neurosurg Focus. 2006;21:e7. doi: 10.3171/foc.2006.21.1.8. [DOI] [PubMed] [Google Scholar]
- Ballabh P, Braun A, Nedergaard M. The blood–brain barrier: an overview: structure, regulation, and clinical implications. Neurobiol Dis. 2004;16:1–13. doi: 10.1016/j.nbd.2003.12.016. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Schuknecht B, Lo Russo G, Cossu M, Citterio A, Andermann F, Siegel AM. Seizure outcome after resection of cavernous malformations is better when surrounding hemosiderin-stained brain also is removed. Epilepsia. 2006;47:563–566. doi: 10.1111/j.1528-1167.2006.00468.x. [DOI] [PubMed] [Google Scholar]
- Baumann CR, Acciarri N, Bertalanffy H, Devinsky O, Elger CE, Lo Russo G, Cossu M, Sure U, Singh A, Stefan H, Hammen T, Georgiadis D, Baumgartner RW, Andermann F, Siegel AM. Seizure outcome after resection of supratentorial cavernous malformations: a study of 168 patients. Epilepsia. 2007;48:559–563. doi: 10.1111/j.1528-1167.2006.00941.x. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, Armstrong DD, Vinters HV, Palmini A, Jacques TS, Avanzini G, Barkovich AJ, Battaglia G, Becker A, Cepeda C, Cendes F, Colombo N, Crino P, Cross JH, Delalande O, Dubeau F, Duncan J, Guerrini R, Kahane P, Mathern G, Najm I, Ozkara C, Raybaud C, Represa A, Roper SN, Salamon N, Schulze-Bonhage A, Tassi L, Vezzani A, Spreafico R. The clinicopathologic spectrum of focal cortical dysplasias: a consensus classification proposed by an ad hoc Task Force of the ILAE Diagnostic Methods Commission. Epilepsia. 2011;52:158–174. doi: 10.1111/j.1528-1167.2010.02777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cacheaux LP, Ivens S, David Y, Lakhter AJ, Bar-Klein G, Shapira M, Heinemann U, Friedman A, Kaufer D. Transcriptome profiling reveals TGF-beta signaling involvement in epileptogenesis. J Neurosci. 2009;29:8927–8935. doi: 10.1523/JNEUROSCI.0430-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos AR, Clusmann H, von Lehe M, Niehusmann P, Becker AJ, Schramm J, Urbach H. Simple and complex dysembryoplastic neuroepithelial tumors (DNT) variants: clinical profile, MRI, and histopathology. Neuroradiology. 2009;51:433–443. doi: 10.1007/s00234-009-0511-1. [DOI] [PubMed] [Google Scholar]
- Clatterbuck RE, Eberhart CG, Crain BJ, Rigamonti D. Ultrastructural and immunocytochemical evidence that an incompetent blood–brain barrier is related to the pathophysiology of cavernous malformations. J Neurol Neurosurg Psychiatry. 2001;71:188–192. doi: 10.1136/jnnp.71.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornford EM. Epilepsy and the blood brain barrier: endothelial cell responses to seizures. Adv Neurol. 1999;79:845–862. [PubMed] [Google Scholar]
- David Y, Cacheaux LP, Ivens S, Lapilover E, Heinemann U, Kaufer D, Friedman A. Astrocytic dysfunction in epileptogenesis: consequence of altered potassium and glutamate homeostasis? J Neurosci. 2009;29:10588–10599. doi: 10.1523/JNEUROSCI.2323-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Curling O, Jr, Kelly DL, Jr, Elster AD, Craven TE. An analysis of the natural history of cavernous angiomas. J Neurosurg. 1991;75:702–708. doi: 10.3171/jns.1991.75.5.0702. [DOI] [PubMed] [Google Scholar]
- Engel J, Van Ness P, Rasmussen T, Ojemann L. Outcome with respect to seizures. In: Engel J, editor. Surgical treatment of the epilepsies. Raven Press; New York: 1993. pp. 609–621. [Google Scholar]
- Ferrier CH, Aronica E, Leijten FS, Spliet WG, Boer K, van Rijen PC, van Huffelen AC. Electrocorticography discharge patterns in patients with a cavernous hemangioma and pharmacoresistent epilepsy. J Neurosurg. 2007;107:495–503. doi: 10.3171/JNS-07/09/0495. [DOI] [PubMed] [Google Scholar]
- Ferroli P, Casazza M, Marras C, Mendola C, Franzini A, Broggi G. Cerebral cavernomas and seizures: a retrospective study on 163 patients who underwent pure lesionectomy. Neurol Sci. 2006;26:390–394. doi: 10.1007/s10072-006-0521-2. [DOI] [PubMed] [Google Scholar]
- Hammen T, Romstock J, Dorfler A, Kerling F, Buchfelder M, Stefan H. Prediction of postoperative outcome with special respect to removal of hemosiderin fringe: a study in patients with cavernous haemangiomas associated with symptomatic epilepsy. Seizure. 2007;16:248–253. doi: 10.1016/j.seizure.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Ivens S, Kaufer D, Flores LP, Bechmann I, Zumsteg D, Tomkins O, Seiffert E, Heinemann U, Friedman A. TGF-beta receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain. 2007;130:535–547. doi: 10.1093/brain/awl317. [DOI] [PubMed] [Google Scholar]
- Jellinger K, Paulus W, Grundke-Iqbal I, Riederer P, Youdim MB. Brain iron and ferritin in Parkinson’s and Alzheimer’s diseases. J Neural Transm Park Dis Dement Sect. 1990;2:327–340. doi: 10.1007/BF02252926. [DOI] [PubMed] [Google Scholar]
- Korn A, Golan H, Melamed I, Pascual-Marqui R, Friedman A. Focal cortical dysfunction and blood–brain barrier disruption in patients with Postconcussion syndrome. J Clin Neurophysiol. 2005;22:1–9. doi: 10.1097/01.wnp.0000150973.24324.a7. [DOI] [PubMed] [Google Scholar]
- Kraemer DL, Awad IA. Vascular malformations and epilepsy: clinical considerations and basic mechanisms. Epilepsia. 1994;35(Suppl. 6):S30–S43. doi: 10.1111/j.1528-1157.1994.tb05987.x. [DOI] [PubMed] [Google Scholar]
- Kral T, Clusmann H, Urbach J, Schramm J, Elger CE, Kurthen M, Grunwald T. Preoperative evaluation for epilepsy surgery (Bonn Algorithm) Zentralbl Neurochir. 2002;63:106–110. doi: 10.1055/s-2002-35826. [DOI] [PubMed] [Google Scholar]
- Kwan P, Arzimanoglou A, Berg AT, Brodie MJ, Allen Hauser W, Mathern G, Moshe SL, Perucca E, Wiebe S, French J. Definition of drug resistant epilepsy: consensus proposal by the ad hoc Task Force of the ILAE Commission on Therapeutic Strategies. Epilepsia. 2010;51:1069–1077. doi: 10.1111/j.1528-1167.2009.02397.x. [DOI] [PubMed] [Google Scholar]
- Marchi N, Angelov L, Masaryk T, Fazio V, Granata T, Hernandez N, Hallene K, Diglaw T, Franic L, Najm I, Janigro D. Seizure-promoting effect of blood–brain barrier disruption. Epilepsia. 2007;48:732–742. doi: 10.1111/j.1528-1167.2007.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick WF. The pathology of vascular (“arteriovenous”) malformations. J Neurosurg. 1966;24:807–816. doi: 10.3171/jns.1966.24.4.0807. [DOI] [PubMed] [Google Scholar]
- Moran NF, Fish DR, Kitchen N, Shorvon S, Kendall BE, Stevens JM. Supratentorial cavernous haemangiomas and epilepsy: a review of the literature and case series. J Neurol Neurosurg Psychiatry. 1999;66:561–568. doi: 10.1136/jnnp.66.5.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriarity JL, Clatterbuck RE, Rigamonti D. The natural history of cavernous malformations. Neurosurg Clin N Am. 1999;10:411–417. [PubMed] [Google Scholar]
- Ralay Ranaivo H, Patel F, Wainwright MS. Albumin activates the canonical TGF receptor-smad signaling pathway but this is not required for activation of astrocytes. Exp Neurol. 2010;226:310–319. doi: 10.1016/j.expneurol.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Rigau V, Morin M, Rousset MC, de Bock F, Lebrun A, Coubes P, Picot MC, Baldy-Moulinier M, Bockaert J, Crespel A, Lerner-Natoli M. Angiogenesis is associated with blood–brain barrier permeability in temporal lobe epilepsy. Brain. 2007;130:1942–1956. doi: 10.1093/brain/awm118. [DOI] [PubMed] [Google Scholar]
- Seiffert E, Dreier JP, Ivens S, Bechmann I, Tomkins O, Heinemann U, Friedman A. Lasting blood–brain barrier disruption induces epileptic focus in the rat somatosensory cortex. J Neurosci. 2004;24:7829–7836. doi: 10.1523/JNEUROSCI.1751-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Pathak DN. Lipid peroxidation and glutathione peroxidase, glutathione reductase, superoxide dismutase, catalase, and glucose-6-phosphate dehydrogenase activities in FeCl3-induced epileptogenic foci in the rat brain. Epilepsia. 1990;31:15–26. doi: 10.1111/j.1528-1157.1990.tb05354.x. [DOI] [PubMed] [Google Scholar]
- Stavrou I, Baumgartner C, Frischer JM, Trattnig S, Knosp E. Long-term seizure control after resection of supratentorial cavernomas: a retrospective single-center study in 53 patients. Neurosurgery. 2008;63:888–896. doi: 10.1227/01.NEU.0000327881.72964.6E. discussion 897. [DOI] [PubMed] [Google Scholar]
- Stefan H, Hammen T. Cavernous haemangiomas, epilepsy and treatment strategies. Acta Neurol Scand. 2004;110:393–397. doi: 10.1111/j.1600-0404.2004.00333.x. [DOI] [PubMed] [Google Scholar]
- Stefan H, Scheler G, Hummel C, Walter J, Romstock J, Buchfelder M, Blumcke I. Magnetoencephalography (MEG) predicts focal epileptogenicity in cavernomas. J Neurol Neurosurg Psychiatry. 2004;75:1309–1313. doi: 10.1136/jnnp.2003.021972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne RG, Nicholson C. In vivo diffusion analysis with quantum dots and dextrans predicts the width of brain extracellular space. Proc Natl Acad Sci U S A. 2006;103:5567–5572. doi: 10.1073/pnas.0509425103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, Tetto A, Vitelli E, Vitezic D, Wiebe S. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, da Costa Araujo S, Redeker S, van Schaik R, Aronica E, Gorter JA. Blood–brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain. 2007;130:521–534. doi: 10.1093/brain/awl318. [DOI] [PubMed] [Google Scholar]
- Williamson A, Patrylo PR, Lee S, Spencer DD. Physiology of human cortical neurons adjacent to cavernous malformations and tumors. Epilepsia. 2003;44:1413–1419. doi: 10.1046/j.1528-1157.2003.23603.x. [DOI] [PubMed] [Google Scholar]
- Willmore LJ, Sypert GW, Munson JV, Hurd RW. Chronic focal epileptiform discharges induced by injection of iron into rat and cat cortex. Science. 1978;200:1501–1503. doi: 10.1126/science.96527. [DOI] [PubMed] [Google Scholar]
- Wong JH, Awad IA, Kim JH. Ultrastructural pathological features of cerebrovascular malformations: a preliminary report. Neurosurgery. 2000;46:1454–1459. doi: 10.1097/00006123-200006000-00027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


