Abstract
Study Design
A retrospective study.
Purpose
The aims of this study were to investigate the diagnostic value of 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) in PET/computed tomography (CT) in the evaluation of spinal metastatic lesions.
Overview of Literature
Recent studies described limitations regarding how many lesions with abnormal 18F-FDG PET findings in the bone show corresponding morphologic abnormalities.
Methods
The subjects for this retrospective study were 227 patients with primary malignant tumors, who were suspected of having spinal metastases. They underwent combined whole-body 18F-FDG PET/CT scanning for evaluation of known neoplasms in the whole spine. 99mTc-methylene diphosphonate bone scan was performed within 2 weeks following PET/CT examinations. The final diagnosis of spinal metastasis was established by histopathological examination regarding bone biopsy or magnetic resonance imaging (MRI) findings, and follow-up MRI, CT and 18F-FDG PET for extensively wide lesions with subsequent progression.
Results
From a total of 504 spinal lesions in 227 patients, 224 lesions showed discordant image findings. For 122 metastatic lesions with confirmed diagnosis, the sensitivity/specificity of bone scan and FDG PET were 84%/21% and 89%/76%, respectively. In 102 true-positive metastatic lesions, the bone scan depicted predominantly osteosclerotic changes in 36% and osteolytic changes in 19%. In 109 true-positive lesions of FDG PET, osteolytic changes were depicted predominantly in 38% while osteosclerotic changes were portrayed in 15%.
Conclusions
18F-FDG PET in PET/CT could be used as a substitute for bone scan in the evaluation of spinal metastasis, especially for patients with spinal osteolytic lesions on CT.
Keywords: 18F-fluorodeoxyglucose positron emission tomography, Technetium Tc 99m (Sn)methylenediphosphonate, Positron emission tomography and computed tomography, Spine, Metastasis
Introduction
Very few factors can change the strategy for cancer treatment, including spine surgery, in such a radical way as the presence or absence of bone metastases assessed during the initial stage or during follow up. The vertebral column is the region of the skeleton most frequently affected by metastasis. The tumors that most commonly metastasize to the vertebrae are carcinomas of the breast in women and carcinomas of the lung and prostate in men; however, metastases are also frequently seen in patients with lymphoma and multiple myeloma [1,2]. It is well known that vertebral metastases represent hematogenous dissemination of the primary tumor, direct extension through the intervertebral foramina or invasion of the epidural space from adjacent vertebral segments. Metastatic lesions often cause compression of the adjacent neural structures such as the spinal cord, cauda equine and/or nerve roots and in the latter, they are often associated with radiculopathy [1]. In order to increase the chances of a favorable outcome, the most important issue remains avoiding the development of any permanent neurological or functional deficit through early diagnosis and treatment [3]. While in a selected group of patients a combined approach; including chemotherapy and surgery; may be plausible for the treatment of metastatic spinal tumors, radiation remains the primary choice [4].
The most commonly used imaging procedure for the assessment of bone metastases is 99mTc-methylene diphosphonate (MDP) bone scintigraphy, though it cannot detect accompanying soft-tissue abnormalities and lacks specificity, given the multiplicity of abnormal, but benign processes, that could affect bones, leading to false negative and positive results [5]. On the other hand, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) is more sensitive than bone scintigraphy in patients with lung cancer and lymphoma, and can detect early bone marrow involvement including spinal metastasis before the appearance of cortical changes on bone scintigraphy [6,7]. With the increased use of whole-body 18F-FDG PET for the staging and follow-up of malignant diseases, it is not uncommon to encounter vertebral metastases. Recent studies described limitations regarding how many lesions with abnormal 18F-FDG PET findings in bone show corresponding morphologic abnormalities, even if they are not suspected of representing definite bone metastasis [8]. Other studies have reported false-positive 18F-FDG PET results in patients suspected of bone metastases [9]. Due to these limitations, several clinical applications have become available for use with the recently released generation of combined PET/computed tomography (CT) scanners [10-12]; which can provide detailed anatomic information and abnormal findings beyond the capabilities of the PET scanner alone [13]. At present, detection of bone metastases on the PET is usually followed by the CT for assessment of the morphologic features regarding the lesions [14].
The present study was designed to retrospectively investigate the diagnostic values of 18F-FDG PET in the evaluation of spinal metastases and to compare 18F-FDG PET in PET/CT with the conventional 99mTc-MDP bone scan, and to assess the added value of CT in PET/CT studies in lesion detection and localization of vertebral lesions, and to correlate between the morphological changes detected on CT and the positive findings of 18F-FDG PET findings or bone scan in spinal metastatic lesions.
Materials and Methods
1. Study cohort
The subjects of this retrospective study were 227 patients (121 men, 106 women; age range, 35-82 years; mean, 59.9) with primary malignant tumors in the lung (n=91), breast (n=43), gastrointestinal tract (n=33) (esophageal, gastric and colonic cancers), kidney (n=21), prostate (n=15), uterus or ovaries (n=10), lymph nodes (n=8, malignant lymphoma), head and neck (n=3), and bone and soft tissues (n=3), who were suspected of having spinal metastases. They underwent combined whole-body PET/CT scanning for evaluation of known neoplasms in the whole spine between January 2005 and December 2008. 99mTc-MDP bone scan was performed within 2 weeks after PET/CT examinations. The final diagnosis of spinal metastasis was established by a histopathological examination of the bone biopsy or MRI findings, and a follow-up MRI, CT, and 18F-FDG PET of extensively wide lesions with subsequent progression.
2. 99mTc-MDP bone scans
Routine bone scans were obtained with a large field-of-view, dual-head gamma camera (E-CAM, Siemens Medical System, Hoffman Estates, IL, USA) with a low-energy high-resolution collimator. Anterior and posterior whole-body images were acquired 3 hours after an intravenous injection of approximately 740 MBq of 99mTc-MDP.
3. PET/CT scanning
18F-FDG PET/CT was performed using a combined PET/CT scanner (Discovery LS, General Electric Medical Systems, Waukesha, WI, USA). For the PET/CT scanners, 35 transaxial images were acquired simultaneously per field of view with an interslice spacing of 4.25 mm. The PET/CT scanner incorporates an integrated 4-slice multidetector CT scanner, which was used for attenuation correction. The CT scanning parameters were as follows: Auto mA (upper limit, 40 mA; noise index, 20), 140 kV, 5-mm section thickness, 15-mm table feed, and pitch of 4. After at least 4 hours of fasting, the patient received an intravenous injection of 185 MBq of 18F-FDG and image acquisition began 50 minutes after injection. A whole-body emission scan was performed from the head to the inguinal region with 2 minutes per bed position (7-8 bed positions). A transmission scan with CT was performed prior to the emission scan. CT images for attenuation correction were applied to the emission data and the attenuation-corrected emission images were reconstructed with an ordered-subset expectation maximization iterative reconstruction algorithm (2 iterations, 14 subsets).
4. Image analysis
Two experienced physicians (H.N., T.H.) identified the spinal lesions on bone scans as sites of increased MDP uptake relative to the surrounding normal bone activity. Whole-body FDG PET skeletal images were independently and visually analyzed by two experienced physicians (T.M., T.T.) on a high-resolution display to compare them with the corresponding 99mTc-MDP whole-body bone scan findings. A spinal lesion on 18F-FDG PET was defined as a focus of increased 18F-FDG uptake, above the intensity of the surrounding normal bone activity, excluding the physiologically increased 18F-FDG uptake areas of renal pelvis, urinary bladder, bowel, myocardium, and brain. In order to examine the differences between morphological changes on the CT with the true-positive lesions at each bone scan or FDG PET, a region of interest (ROI; size, 20×20 mm) was placed by one author (T.M.) over all suspected lesions, and the maximal single pixel value was determined for each lesion on the whole spine. The standardized uptake value (SUV) was calculated using the following formula: SUV=ROIRC/(ID/BW); where ROIRC is the radioactivity concentration within the region of interest (in becquerels per milliliter), ID is the injected dose of 18F-FDG (in becquerels), and BW is body weight in grams.
The CT images were evaluated by two physicians (D.S., S.W.) using CT planes that corresponded to the planes in which the lesion appeared on the FDG PET. The physicians were aware of the positive spinal lesions on the PET, and they used a workstation to display the CT scans with the bone and soft-tissue windows. The CT analysis determined the presence or absence of spinal metastasis and the type of morphologic changes (nonspecific, osteolytic, osteosclerotic). Nonspecific changes represented abnormal CT findings that were neither neoplastic nor degenerative changes [14]. Lesions exhibiting both osteolytic and osteosclerotic changes were considered to be either type based on the predominant change in that lesion.
5. Statistical analysis
The Mann-Whitney U-test was used to compare differences between the percentages of patients being evaluated through "Bone scan" and "FDG PET" (Table 1). A probability value less than 0.05 denoted statistical significance. All statistical analyses were conducted using SPSS software (ver. 15.0, SPSS, Chicago, IL, USA).
Table 1.
Differences between morphological changes on CT with true-positive lesions on each bone scan or FDG PET
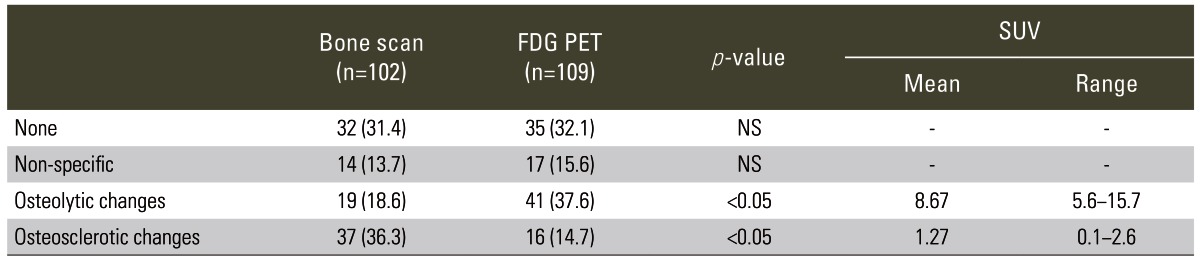
Values are presented as bone scan and FDG PET represent number of lesions.
Data in parentheses are percentages.
p<0.05; for "Bone scan" and "FDG PET" (by Mann-Whitney U-test).
CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography; SUV, standardized uptake value; NS, not significant.
Results
A total of 504 spinal lesions in 227 patients found on either bone scans or FDG PETs were evaluated. Positive FDG PET as well as positive bone scan findings were identified in 280 spinal lesions. In the remaining 224 lesions with discordant findings, 68 had positive bone scan images, but negative FDG PETs, while 156 had negative bone scans but positive FDG PETs (Table 2). In 156 spinal lesions (cervical 25, thoracic 90, and lumbar 41), including 22 examined histopathologically, the final diagnosis was 122 spinal metastatic lesions and 34 benign lesions. Of the 122 metastatic lesions, positive bone scan findings were recorded in 102 lesions and negative findings were recorded in 20 lesions. Positive FDG PET findings were recorded in 109 lesions and negative findings were recorded in 13 lesions. The sensitivity with regards to bone scans and FDG PETs were 84% and 89%, respectively. In contrast, for the 34 benign lesions, the positive and negative bone scan findings were recorded in 27 and 7 lesions, respectively, whereas positive and negative FDG PET findings were recorded in 8 and 26 lesions, respectively (the specificity for bone scans and FDG PETs were 21% and 76%, respectively) (Table 3).
Table 2.
Distribution of bone scan and FDG PET findings in spinal lesions examined in present study
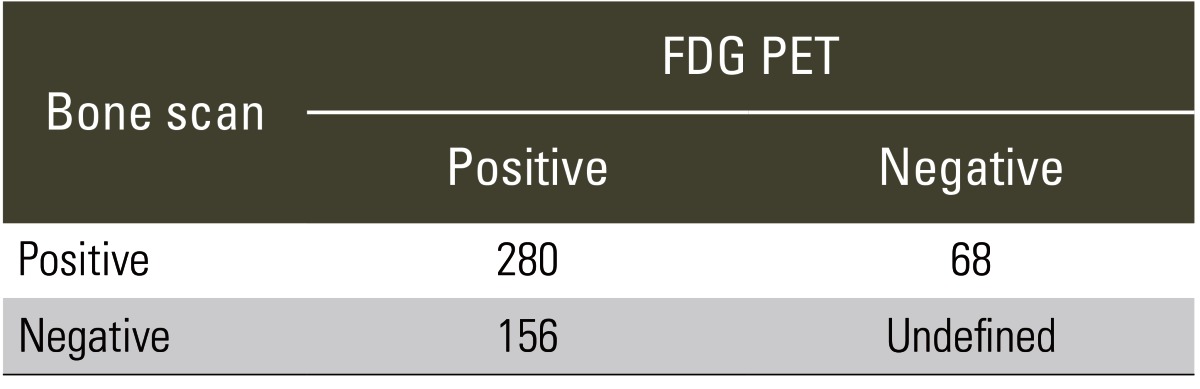
Values are presented as number of lesions.
FDG, fluorodeoxyglucose; PET, positron emission tomography.
Table 3.
Distribution of bone scan/FDG PET findings based on final diagnosis in all spinal lesions

Values are presented as number of lesions.
FDG, fluorodeoxyglucose; PET, positron emission tomography.
The morphological changes were determined for true-positive lesions on each bone scan (102 lesions) and FDG PET (109 lesions) (Table 1). No changes on CT were seen for bone scans in 32 lesions (31%), and on FDG PETs in 35 lesions (32%). Bone scans depicted predominantly sclerotic changes in 37 lesions (36%) and mainly osteolytic changes in 19 lesions (19%) (Fig. 1). On the other hand, FDG PETs depicted predominantly osteolytic changes in 41 lesions (38%) and mainly osteosclerotic changes in 16 lesions (15%) (Fig. 2).
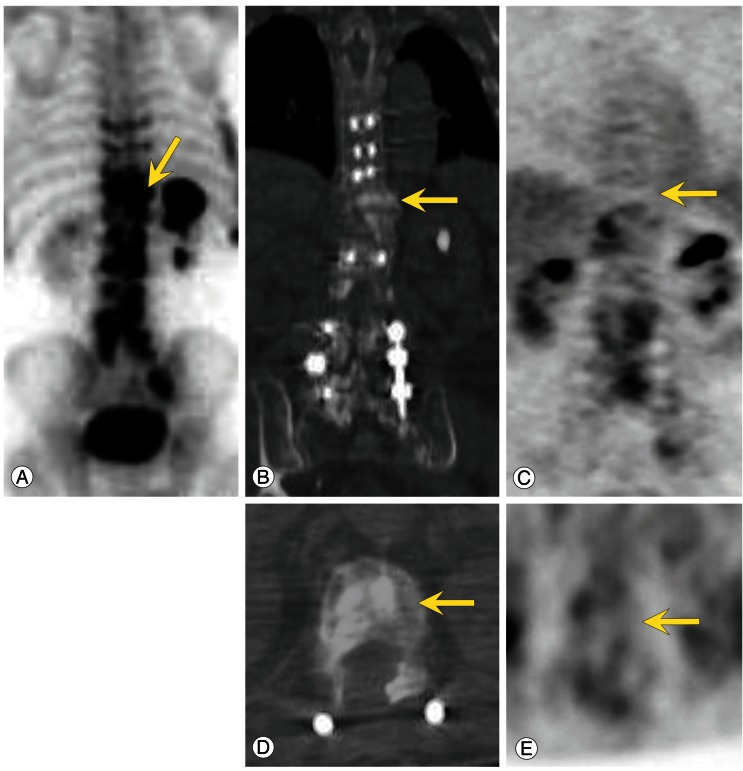
Fig. 1.
Bone scan (A), CT, (B, D) and FDG PET (C, E) images of a 77-year-old man with prostate carcinoma. Note the focal intense uptake of osteoblastic spine metastases (vertebral body of T11) on the bone scan only but not on the PET images (arrows). Axial and coronal CT images show ostesclerotic changes in the vertebral body (B, D) and no focal intense uptake on the PET images (C, E). CT, computed tomography; FDG, fluorodeoxyglucose; PET, positron emission tomography.
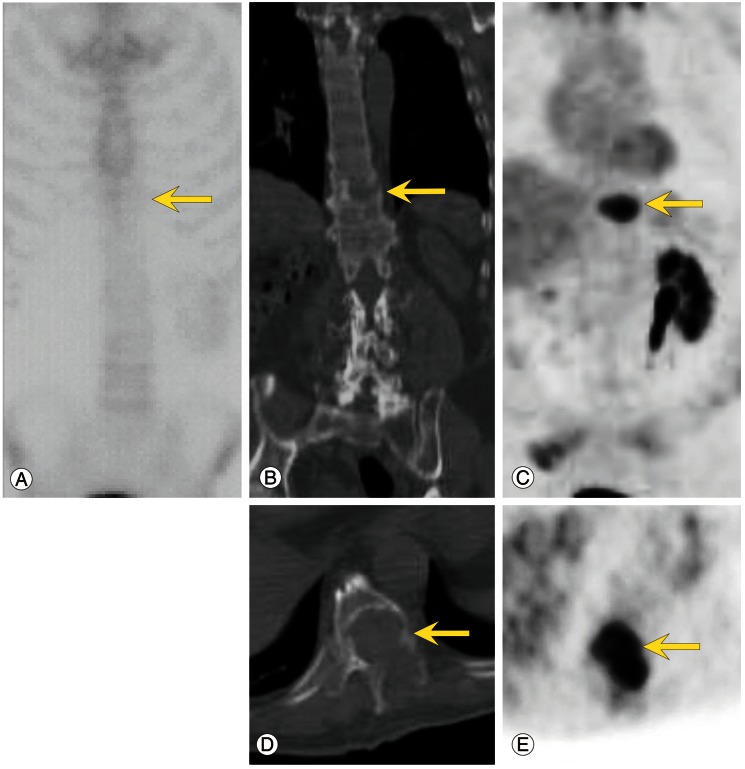
Fig. 2.
Bone scan (A), computed tomography (CT) (B, D) and fluorodeoxyglucose (FDG) positron emission tomography (PET) (C, E) images of a 69-year-old man with renal cell carcinoma. Note the focal intense uptake in osteolytic spine metastases (vertebral body of T10) on PET only but not on the bone scan (arrows). Axial and coronal CT images show osteolytic changes in the vertebral body (B, D) and focal intense uptake on the PET images (C, E).
Discussion
In the assessment of spine tumors, while evaluating the epidural extension or marrow involvement, the MRI remains as the gold standard [15-18]. However, when evaluating large numbers of screenings in suspected bone metastases cases, bone scintigraphy is the most common modality used due to its high sensitivity, availability, cost, and ease of surveying the entire skeleton. However, many patients with bone metastases do not show typical or specific patterns on these scans [5,19]. Several factors can influence the tracer uptake, such as degenerative joint disease and related benign bone diseases or osteocartilaginous abnormalities, such as previous skeletal trauma; all of which will finally limit the specificity of the bone scan. Since the conventional bone scan is sensitive enough to detect accelerated osteoblastic activities, which are nonspecific indicators of pathology; the image provided by a different modality such as tissue glucose utilization mapped with 18F-FDG, would be expected to provide a different view of bone pathology. Tumor cells release a myriad of biologically active substances that could actively interfere with glycolysis, following tumor invasion. Therefore, not only the measurement of mineral turnover, as conventionally achieved in bone scans, but also the assessment of glucose metabolic processes, could be of great significance for differentiating benign from malignant bone lesions [20]. The present study was undertaken in order to evaluate the usefulness of 18F-FDG PET in measuring glycolytic activity in bone marrow metastatic lesions in patients with suspected malignant spinal metastases, and to compare bone scans and FDG PETs in the detection of spinal metastases. Our results indicate that 18F-FDG PET is potentially useful for the detection of vertebral metastatic lesions based on the analysis of 122 spinal lesions.
Several studies compared bone scans to FDG PETs in the detection of different types of cancers [21,22]. FDG PETs were superior to bone scans in the detection of breast cancer in patients with known skeletal metastases; with the exception of a subgroup of patients with osteoblastic metastases [23], though the bone scan had been reported to be superior than PET in detecting osteoblastic metastatic lesions [22]. Such data emphasize the complementary nature of bone scans and FDG PETs in the evaluation of skeletal metastases in breast cancer patients, as reported previously [24]; as well as in the staging of bone metastasis in breast cancer, where each modality cannot substitute the other. It has been reported already that the FDG PET is less sensitive than the bone scan in prostate cancers [25]; where the FDG with a sensitivity of 65%, only identified 131 of 202 untreated metastases in a group of 22 patients [26]. Morris et al. [27] found that the bone scan was significantly more sensitive (94%) than the FDG (77%) in a series of 134 bone metastases. While the FDG appears to be quite useful in the detection of bone metastases from lung cancer, it is not so effective in prostate cancers due to the osteoblastic characteristics of the lesions [25]. A higher accuracy of PET in the detection of bone metastases, relative to the bone scan, was also reported [7]. Thus, it is clear that PETs and bone scans provide different diagnoses according to the type of the primary cancer, being an important limitation to our study, and implies the need for larger studies to examine each primary cancer.
In our research, among the lesions finally diagnosed as metastases using all the available resources; the CT characterized only 31% to 32% as probable or definite metastases whereas the remaining 68% to 69% of the lesions was undiagnosed. Furthermore, while morphologic changes were often identified using bone windows to display the CT images, only 50% of the lesions depicted minimal and more marked osteolytic or osteoblastic changes. These findings emphasize the limitations of the CT in confirming or excluding bone metastases detected on bone scans or PETs.
Our results also demonstrated differences in the detection of osteolytic versus osteoblastic lesions; where FDG-PET showed a higher sensitivity in the detection of osteolytic lesions, but lower sensitivity, relative to bone scans, in osteoblastic metastases. Whether the greater avidity for 18F-FDG in osteolytic metastases reflects a higher glycolytic rate in this type of lesion remains unknown; and since osteoblastic metastases are relatively acellular [28], the degree of 18F-FDG uptake may be influenced by the lower volume of viable tumor tissue within the lesion. It is also important to take into consideration the hypoxic environment typically noted in osteolytic lesions, compared with osteoblastic lesions, which is caused by poor blood supply in these lesions due to their aggressive growth. Such behavior may be an additional factor that could influence the diagnosis, since hypoxia is known to increase 18F-FDG uptake in some cell lines [29].
Conclusions
The present study showed a comparable diagnostic accuracy of spinal metastases by bone scan and FDG PET in PET/CT. Although the CT (as part of PET/CT) can provide detailed anatomic information, our data suggest that characterization of the spinal metastatic lesions is of limited value even when optimal CT window width and level were used. PET in PET/CT could be a substitute for bone scan regarding the evaluation of spinal metastasis, especially for patients with spinal osteolytic lesions identified on the CT. In contrast, osteoblastic metastases show lower metabolic activity and are frequently undetectable on the FDG PET. The biologic explanation for this observation remains to be elucidated.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Rodriguez M, Dinapoli RP. Spinal cord compression: with special reference to metastatic epidural tumors. Mayo Clin Proc. 1980;55:442–448. [PubMed] [Google Scholar]
- 2.Schiff D, O'Neill BP, Suman VJ. Spinal epidural metastasis as the initial manifestation of malignancy: clinical features and diagnostic approach. Neurology. 1997;49:452–456. doi: 10.1212/wnl.49.2.452. [DOI] [PubMed] [Google Scholar]
- 3.Bilsky MH, Lis E, Raizer J, Lee H, Boland P. The diagnosis and treatment of metastatic spinal tumor. Oncologist. 1999;4:459–469. [PubMed] [Google Scholar]
- 4.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 5.Jocobson AF. Bone scanning in metastatic disease. In: Collier DC, Fogelman I, Rosenthall L, editors. Skeletal nuclear medicine. St. Louis, MO: Mosby; 1996. pp. 87–123. [Google Scholar]
- 6.Moog F, Kotzerke J, Reske SN. FDG PET can replace bone scintigraphy in primary staging of malignant lymphoma. J Nucl Med. 1999;40:1407–1413. [PubMed] [Google Scholar]
- 7.Marom EM, McAdams HP, Erasmus JJ, et al. Staging non-small cell lung cancer with whole-body PET. Radiology. 1999;212:803–809. doi: 10.1148/radiology.212.3.r99se21803. [DOI] [PubMed] [Google Scholar]
- 8.Franzius C, Sciuk J, Daldrup-Link HE, Jurgens H, Schober O. FDG-PET for detection of osseous metastases from malignant primary bone tumours: comparison with bone scintigraphy. Eur J Nucl Med. 2000;27:1305–1311. doi: 10.1007/s002590000301. [DOI] [PubMed] [Google Scholar]
- 9.Kao CH, Hsieh JF, Tsai SC, Ho YJ, Yen RF. Comparison and discrepancy of 18F-2-deoxyglucose positron emission tomography and Tc-99m MDP bone scan to detect bone metastases. Anticancer Res. 2000;20:2189–2192. [PubMed] [Google Scholar]
- 10.Beyer T, Townsend DW, Brun T, et al. A combined PET/CT scanner for clinical oncology. J Nucl Med. 2000;41:1369–1379. [PubMed] [Google Scholar]
- 11.Kluetz PG, Meltzer CC, Villemagne VL, et al. Combined PET/CT imaging in oncology. Impact on patient management. Clin Positron Imaging. 2000;3:223–230. doi: 10.1016/s1095-0397(01)00055-3. [DOI] [PubMed] [Google Scholar]
- 12.Kaim AH, Burger C, Ganter CC, et al. PET-CT-guided percutaneous puncture of an infected cyst in autosomal dominant polycystic kidney disease: case report. Radiology. 2001;221:818–821. doi: 10.1148/radiol.2213010445. [DOI] [PubMed] [Google Scholar]
- 13.Metser U, Lerman H, Blank A, Lievshitz G, Bokstein F, Even-Sapir E. Malignant involvement of the spine: assessment by 18F-FDG PET/CT. J Nucl Med. 2004;45:279–284. [PubMed] [Google Scholar]
- 14.Nakamoto Y, Cohade C, Tatsumi M, Hammoud D, Wahl RL. CT appearance of bone metastases detected with FDG PET as part of the same PET/CT examination. Radiology. 2005;237:627–634. doi: 10.1148/radiol.2372031994. [DOI] [PubMed] [Google Scholar]
- 15.Kim JK, Learch TJ, Colletti PM, Lee JW, Tran SD, Terk MR. Diagnosis of vertebral metastasis, epidural metastasis, and malignant spinal cord compression: are T(1)-weighted sagittal images sufficient? Magn Reson Imaging. 2000;18:819–824. doi: 10.1016/s0730-725x(00)00181-8. [DOI] [PubMed] [Google Scholar]
- 16.Kim HJ, Ryu KN, Choi WS, Choi BK, Choi JM, Yoon Y. Spinal involvement of hematopoietic malignancies and metastasis: differentiation using MR imaging. Clin Imaging. 1999;23:125–133. doi: 10.1016/s0899-7071(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 17.Mehta RC, Marks MP, Hinks RS, Glover GH, Enzmann DR. MR evaluation of vertebral metastases: T1-weighted, short-inversion-time inversion recovery, fast spin-echo, and inversion-recovery fast spin-echo sequences. AJNR Am J Neuroradiol. 1995;16:281–288. [PMC free article] [PubMed] [Google Scholar]
- 18.Yuh WT, Zachar CK, Barloon TJ, Sato Y, Sickels WJ, Hawes DR. Vertebral compression fractures: distinction between benign and malignant causes with MR imaging. Radiology. 1989;172:215–218. doi: 10.1148/radiology.172.1.2740506. [DOI] [PubMed] [Google Scholar]
- 19.Resnick D. Skeletal metastasis. In: Resnick D, editor. Bone and joint imaging. Philadelphia, PA: W.B. Saunders Company; 1996. pp. 1076–1091. [Google Scholar]
- 20.Hawkins RA, Hoh CK. PET bone imaging. In: Collier D, Fogelman I, Rosenthall L, editors. Skeletal nuclear medicine. St. Louis, MO: Mosby; 1996. pp. 435–442. [Google Scholar]
- 21.Cheran SK, Herndon JE, 2nd, Patz EF., Jr Comparison of whole-body FDG-PET to bone scan for detection of bone metastases in patients with a new diagnosis of lung cancer. Lung Cancer. 2004;44:317–325. doi: 10.1016/j.lungcan.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 22.Abe K, Sasaki M, Kuwabara Y, et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19:573–579. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 23.Cook GJ, Houston S, Rubens R, Maisey MN, Fogelman I. Detection of bone metastases in breast cancer by 18FDG PET: differing metabolic activity in osteoblastic and osteolytic lesions. J Clin Oncol. 1998;16:3375–3379. doi: 10.1200/JCO.1998.16.10.3375. [DOI] [PubMed] [Google Scholar]
- 24.Eubank WB, Mankoff DA. Evolving role of positron emission tomography in breast cancer imaging. Semin Nucl Med. 2005;35:84–99. doi: 10.1053/j.semnuclmed.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 25.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35:135–142. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Shreve PD, Grossman HB, Gross MD, Wahl RL. Metastatic prostate cancer: initial findings of PET with 2-deoxy-2-[F-18]fluoro-D-glucose. Radiology. 1996;199:751–756. doi: 10.1148/radiology.199.3.8638000. [DOI] [PubMed] [Google Scholar]
- 27.Morris MJ, Akhurst T, Osman I, et al. Fluorinated deoxyglucose positron emission tomography imaging in progressive metastatic prostate cancer. Urology. 2002;59:913–918. doi: 10.1016/s0090-4295(02)01509-1. [DOI] [PubMed] [Google Scholar]
- 28.Galasko CS. Skeletal metastases. London: Butterwoths; 1986. [Google Scholar]
- 29.Clavo AC, Brown RS, Wahl RL. Fluorodeoxyglucose uptake in human cancer cell lines is increased by hypoxia. J Nucl Med. 1995;36:1625–1632. [PubMed] [Google Scholar]