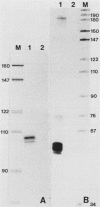
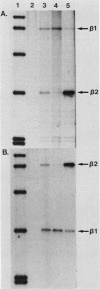
Abstract
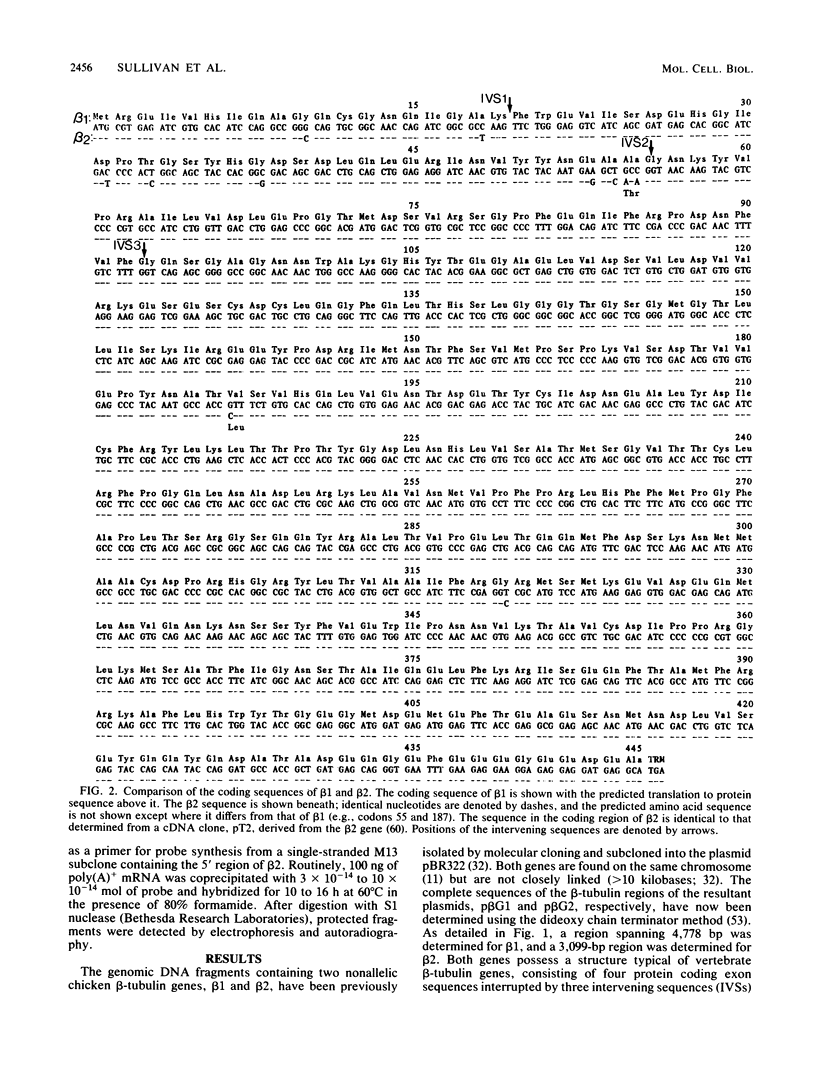
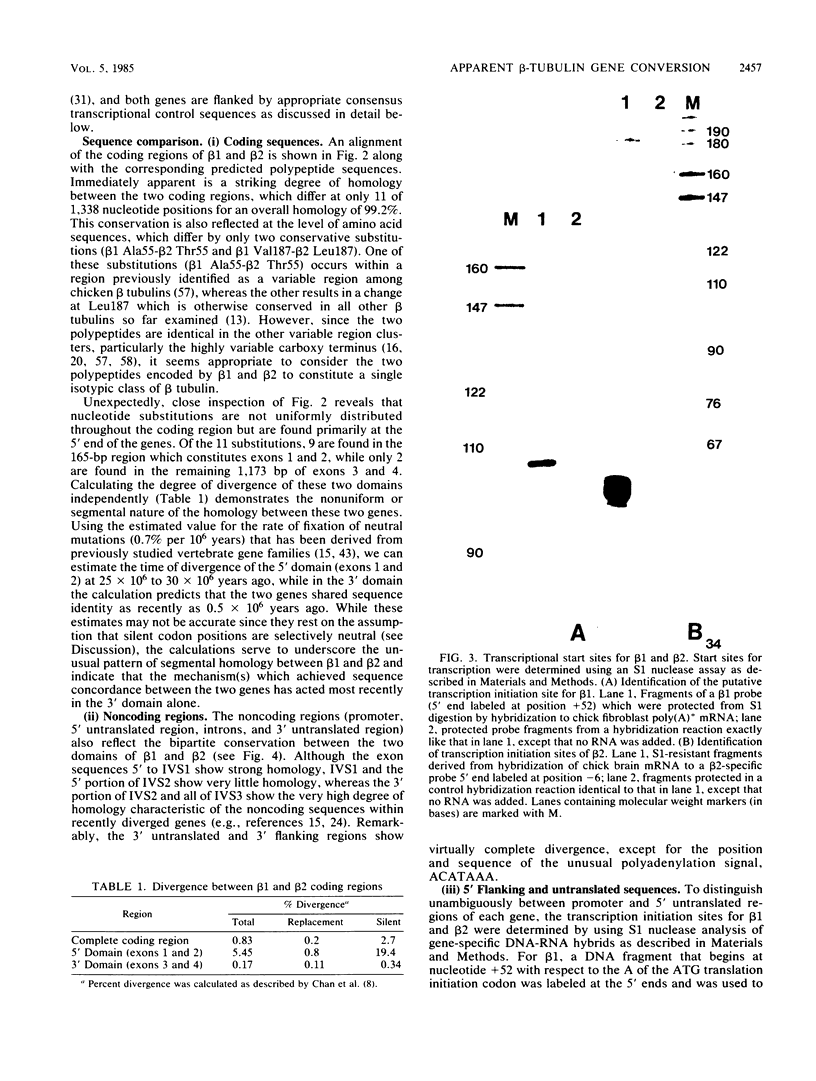
We have determined the complete nucleotide sequences of two chicken beta-tubulin genes, beta 1 and beta 2. These genes display an unusual pattern of segmental homology which indicates that they originally arose by gene duplication and have subsequently coevolved by a process that included localized gene conversion or intergenic recombination. Since the beta-tubulin polypeptides encoded by the two genes are virtually identical (99.5%), particularly in the major beta-tubulin isotype defining regions, they almost certainly constitute a single isotypic class of beta tubulin. However, the regulatory properties of the two genes are highly divergent as indicated by analysis of their patterns of expression in different chicken cell types. beta 1 is the major transcript detected in skeletal muscle myoblasts, whereas beta 2 is the major beta-tubulin transcript in cultured sympathetic neurons. The existence of these two genes appears to derive from a regulatory requirement whereby the expression of a single tubulin isotype is mediated through different regulatory programs in development and differentiation. These results thus provide direct experimental support for the hypothesis that gene conversion and intergenic recombination play an important role in evolution by uncoupling the evolution of structural genes from the regulatory sequences which control them.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandraki D., Ruderman J. V. Evolution of alpha q- and beta-tubulin genes as inferred by the nucleotide sequences of sea urchin cDNA clones. J Mol Evol. 1983;19(6):397–410. doi: 10.1007/BF02102315. [DOI] [PubMed] [Google Scholar]
- Baltimore D. Gene conversion: some implications for immunoglobulin genes. Cell. 1981 Jun;24(3):592–594. doi: 10.1016/0092-8674(81)90082-9. [DOI] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Spliced early mRNAs of simian virus 40. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1274–1278. doi: 10.1073/pnas.75.3.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Calos M. P., Miller J. H. Transposable elements. Cell. 1980 Jul;20(3):579–595. doi: 10.1016/0092-8674(80)90305-0. [DOI] [PubMed] [Google Scholar]
- Chan S. J., Episkopou V., Zeitlin S., Karathanasis S. K., MacKrell A., Steiner D. F., Efstratiadis A. Guinea pig preproinsulin gene: an evolutionary compromise? Proc Natl Acad Sci U S A. 1984 Aug;81(16):5046–5050. doi: 10.1073/pnas.81.16.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Hughes S. H., Stubblefield E., Kirschner M. W., Varmus H. E. Multiple alpha and beta tubulin genes represent unlinked and dispersed gene families. J Biol Chem. 1981 Mar 25;256(6):3130–3134. [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Sullivan K. F. Molecular biology and genetics of tubulin. Annu Rev Biochem. 1985;54:331–365. doi: 10.1146/annurev.bi.54.070185.001555. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W. The tubulins: from DNA to RNA to protein and back again. Cell. 1983 Sep;34(2):330–332. doi: 10.1016/0092-8674(83)90366-5. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Goad W. B., Kanehisa M. I. Pattern recognition in nucleic acid sequences. I. A general method for finding local homologies and symmetries. Nucleic Acids Res. 1982 Jan 11;10(1):247–263. doi: 10.1093/nar/10.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Cowan N. J. Structural features and restricted expression of a human alpha-tubulin gene. Nucleic Acids Res. 1985 Jan 11;13(1):207–223. doi: 10.1093/nar/13.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havercroft J. C., Cleveland D. W. Programmed expression of beta-tubulin genes during development and differentiation of the chicken. J Cell Biol. 1984 Dec;99(6):1927–1935. doi: 10.1083/jcb.99.6.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden J. H., Allen R. D. Detection of single microtubules in living cells: particle transport can occur in both directions along the same microtubule. J Cell Biol. 1984 Nov;99(5):1785–1793. doi: 10.1083/jcb.99.5.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Jones C. W., Kafatos F. C. Accepted mutations in a gene family: evolutionary diversification of duplicated DNA. J Mol Evol. 1982;19(1):87–103. doi: 10.1007/BF02100227. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. alpha-Tubulin genes of Drosophila. Cell. 1981 Apr;24(1):97–106. doi: 10.1016/0092-8674(81)90505-5. [DOI] [PubMed] [Google Scholar]
- Karfunkel P. The mechanisms of neural tube formation. Int Rev Cytol. 1974;38(0):245–271. doi: 10.1016/s0074-7696(08)60927-4. [DOI] [PubMed] [Google Scholar]
- Kataoka T., Miyata T., Honjo T. Repetitive sequences in class-switch recombination regions of immunoglobulin heavy chain genes. Cell. 1981 Feb;23(2):357–368. doi: 10.1016/0092-8674(81)90131-8. [DOI] [PubMed] [Google Scholar]
- Kemphues K. J., Kaufman T. C., Raff R. A., Raff E. C. The testis-specific beta-tubulin subunit in Drosophila melanogaster has multiple functions in spermatogenesis. Cell. 1982 Dec;31(3 Pt 2):655–670. doi: 10.1016/0092-8674(82)90321-x. [DOI] [PubMed] [Google Scholar]
- Kirschner M. W. Microtubule assembly and nucleation. Int Rev Cytol. 1978;54:1–71. doi: 10.1016/s0074-7696(08)60164-3. [DOI] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Max E. E., Seidman J. G., Leder P. Sequences of five potential recombination sites encoded close to an immunoglobulin kappa constant region gene. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3450–3454. doi: 10.1073/pnas.76.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain D. A., D'Eustachio P., Edelman G. M. Role of surface modulating assemblies in growth control of normal and transformed fibroblasts. Proc Natl Acad Sci U S A. 1977 Feb;74(2):666–670. doi: 10.1073/pnas.74.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Michelson A. M., Orkin S. H. Boundaries of gene conversion within the duplicated human alpha-globin genes. Concerted evolution by segmental recombination. J Biol Chem. 1983 Dec 25;258(24):15245–15254. [PubMed] [Google Scholar]
- Modiano G., Battistuzzi G., Motulsky A. G. Nonrandom patterns of codon usage and of nucleotide substitutions in human alpha- and beta-globin genes: an evolutionary strategy reducing the rate of mutations with drastic effects? Proc Natl Acad Sci U S A. 1981 Feb;78(2):1110–1114. doi: 10.1073/pnas.78.2.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy D. B., Tilney L. G. The role of microtubules in the movement of pigment granules in teleost melanophores. J Cell Biol. 1974 Jun;61(3):757–779. doi: 10.1083/jcb.61.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A. Molecular genetics of yeast mating type. Annu Rev Genet. 1982;16:439–500. doi: 10.1146/annurev.ge.16.120182.002255. [DOI] [PubMed] [Google Scholar]
- Nikaido T., Nakai S., Honjo T. Switch region of immunoglobulin Cmu gene is composed of simple tandem repetitive sequences. Nature. 1981 Aug 27;292(5826):845–848. doi: 10.1038/292845a0. [DOI] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Ollo R., Rougeon F. Gene conversion and polymorphism: generation of mouse immunoglobulin gamma 2a chain alleles by differential gene conversion by gamma 2b chain gene. Cell. 1983 Feb;32(2):515–523. doi: 10.1016/0092-8674(83)90471-3. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perler F., Efstratiadis A., Lomedico P., Gilbert W., Kolodner R., Dodgson J. The evolution of genes: the chicken preproinsulin gene. Cell. 1980 Jun;20(2):555–566. doi: 10.1016/0092-8674(80)90641-8. [DOI] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponstingl H., Krauhs E., Little M., Kempf T. Complete amino acid sequence of alpha-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 May;78(5):2757–2761. doi: 10.1073/pnas.78.5.2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radding C. M. Homologous pairing and strand exchange in genetic recombination. Annu Rev Genet. 1982;16:405–437. doi: 10.1146/annurev.ge.16.120182.002201. [DOI] [PubMed] [Google Scholar]
- Raff E. C. Genetics of microtubule systems. J Cell Biol. 1984 Jul;99(1 Pt 1):1–10. doi: 10.1083/jcb.99.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roninson I. B., Ingram V. M. Gene evolution in the chicken beta-globin cluster. Cell. 1982 Mar;28(3):515–521. doi: 10.1016/0092-8674(82)90206-9. [DOI] [PubMed] [Google Scholar]
- Rossignol J. L., Paquette N., Nicolas A. Aberrant 4:4 asci, disparity in the direction of conversion, and frequencies of conversion in Ascobolus immersus. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 2):1343–1352. doi: 10.1101/sqb.1979.043.01.153. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schon E. A., Wernke S. M., Lingrel J. B. Gene conversion of two functional goat alpha-globin genes preserves only minimal flanking sequences. J Biol Chem. 1982 Jun 25;257(12):6825–6835. [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Sobell H. M. A mechanism to activate branch migration between homologous DNA molecules in genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):279–283. doi: 10.1073/pnas.72.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Sequence of a highly divergent beta tubulin gene reveals regional heterogeneity in the beta tubulin polypeptide. J Cell Biol. 1984 Nov;99(5):1754–1760. doi: 10.1083/jcb.99.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Wu R. Unequal crossing over in the ribosomal DNA of Saccharomyces cerevisiae. Nature. 1980 Apr 3;284(5755):426–430. doi: 10.1038/284426a0. [DOI] [PubMed] [Google Scholar]
- Sánchez F., Natzle J. E., Cleveland D. W., Kirschner M. W., McCarthy B. J. A dispersed multigene family encoding tubulin in Drosophila melanogaster. Cell. 1980 Dec;22(3):845–854. doi: 10.1016/0092-8674(80)90561-9. [DOI] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]
- Weiss E. H., Mellor A., Golden L., Fahrner K., Simpson E., Hurst J., Flavell R. A. The structure of a mutant H-2 gene suggests that the generation of polymorphism in H-2 genes may occur by gene conversion-like events. Nature. 1983 Feb 24;301(5902):671–674. doi: 10.1038/301671a0. [DOI] [PubMed] [Google Scholar]
- Wilbur W. J., Lipman D. J. Rapid similarity searches of nucleic acid and protein data banks. Proc Natl Acad Sci U S A. 1983 Feb;80(3):726–730. doi: 10.1073/pnas.80.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousaf S. I., Carroll A. R., Clarke B. E. A new and improved method for 3'-end labelling DNA using [alpha-32P]ddATP. Gene. 1984 Mar;27(3):309–313. doi: 10.1016/0378-1119(84)90075-1. [DOI] [PubMed] [Google Scholar]