Abstract
Gamma-aminobutyric acid (GABA) is produced and secreted by adult pancreatic β-cells, which also express GABA receptors mediating autocrine signaling and regulating β-cell proliferation. However, whether the autocrine GABA signaling involves in β-cell progenitor development or maturation remains uncertain. By means of immunohistochemistry we analyzed the expression profiles of the GABA synthesizing enzyme glutamic acid decarboxylase (GAD) and the α1-subunit of type-A GABA receptor (GABAARα1) in the pancreas of mice at embryonic day 15.5 (E15.5), E18.5, postnatal day 1 (P1) and P7. Our data showed that at E15.5 the pancreatic and duodenum homeobox-1 (Pdx1) was expressed in the majority of cells in the developing pancreata. Notably, insulin immunoreactivity was identified in a subpopulation of pancreatic cells with a high level of Pdx1 expression. About 80% of the high-level Pdx-1 expressing cells in the pancreas expressed GAD and GABAARα1 at all pancreatic developmental stages. In contrast, only about 30% of the high-level Pdx-1 expressing cells in the E15.5 pancreas expressed insulin; i.e., a large number of GAD/GABAARα1-expressing cells did not express insulin at this early developmental stage. The expression level of GAD and GABAARα1 increased steadily, and progressively more GAD/GABAARα1-expressing cells expressed insulin in the course of pancreatic development. These results suggest that 1) GABA signaling proteins appear in β-cell progenitors prior to insulin expression; and 2) the increased expression of GABA signaling proteins may be involved in β-cell progenitor maturation.
Keywords: γ-aminobutyric acid, GABA receptor, embryonic pancreas, β-cell progenitor
Introduction
The development and lineage differentiation of pancreatic β-cells represents the culmination of a complex cell biological program [1]. Numerous studies show that γ-aminobutyric acid (GABA), a primary neurotransmitter of the central nervous system, is widely used as a signaling molecule by various non-neuronal cells [2-4], including β-cells in islets of Langerhans [5,6]. Specifically, adult pancreatic β-cells produce GABA [7] through the enzymatic activity of two isoforms of glutamic acid decarboxylase (GAD65 and GAD67) [8]; and they also express type-A GABA receptors (GABAARs) [5], mediating an autocrine signaling. This GABA signaling regulates insulin secretion by β-cells in vitro [5,6], and it is also involved in β-cell regeneration in the pancreas of adult rats [9]. It is known that GABA, through GABAARs, up-regulates neurogenesis in the brain [10] and modulates embryonic stem cell proliferation and differentiation in vitro [11]. However, whether this GABA signaling is involved in embryonic development of β-cell progenitors remains uncertain [12]. To investigate whether a GABA signaling system exists in β-cell progenitors during development, this study examined the expression profiles of GABA signaling molecules in the late-fetal and early-postnatal pancreas of mice.
Pancreatic-duodenal homeobox-1 (Pdx1) is a transcriptional factor that is important for pancreatic genesis [13], development and β-cell maturation [14]. In mice, Pdx1 protein is detected in early pancreatic progenitor cells and it is essential for specialization of β-cell phenotype [15]. During adulthood, Pdx1 expression becomes largely confined to the β-cells, where it functions to activate insulin gene transcription and to control β-cell regeneration [15,16]. All lines of evidence indicate that Pdx1 is a marker of pancreatic progenitors in both rodents and humans [16]. In this study, Pdx1 was used as a marker of pancreatic progenitors, while insulin was used as an indicator of pancreatic β-cells.
Materials and methods
Animals and tissue preparations
Our investigation conforms to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996). Use of animals was approved by the Animal Use Subcommittee at the University of Western Ontario, Canada. Pancreatic tissues were collected from C57BL/6 mice at embryonic day 15.5, 18.5 (E15.5, E18.5), and postnatal day 1, day 7 (P1, P7). Specifically, mouse dams at 15.5 and 18.5 days of gestation were anesthetized by ketamine and xylazine, and fetuses were harvested by Caesarean sections. P1 and P7 mouse pups were sacrificed by rapid cervical dislocation. Fetal abdominal trunks and postnatal pancreatic tissues were dissected and then fixed in 4% paraformaldehyde, embedded in paraffin and cut into 5 mm sections. The structure of the pancreas at each time point was identified by hematoxylin and eosin (H/E) staining.
Immunohistochemical staining and confocal microscopy
To study the expression profile of GAD and GABAAR in β-cell progenitors and/or β-cells, pancreatic sections from mice at four time points were immunostained using previously described protocols [17,18]. Specifically, the pancreatic slices were incubated overnight in phosphate buffered saline (PBS) containing rabbit anti-GAD 65/67 (1:1000 dilution, Catalog # G5163, Sigma, MO) or rabbit anti-GABAARα1 (GABAARα1) (1:100 dilution, Catalog # 06-868, Millipore, MA), followed by one hour incubation with CY3 conjugated anti-rabbit IgG secondary antibody (1:400 dilution, JacksonImmuno Research Laboratories, PA). The pancreatic sections were double-stained for mouse anti-insulin (1:1000 dilution, Catalog # I2018 Sigma, MO) or for goat anti-Pdx1 (1:1500 dilution, Catalog # ab47383, Abcam, MA) followed by FITC conjugated anti-mouse IgG or anti-goat IgG secondary antibodies (1:200 dilution, JacksonImmunoResearch Laboratories, PA). Omitting the primary antibody in the staining procedure was used as negative controls. Images were taken with the Zeiss LSM 510/ConfoCor 2 confocal microscope. The detecting threshold for the immunofluorescence of a specific protein was set above the fluorescent level of negative control. Multiple images were taken from each pancreatic slice with a focus on cells that displayed immunofluorescence of Pdx1 or insulin.
Image analyses and statistics
The subcellular colocalization or correlation of the double-stained proteins was analyzed by means of the image-analyzing program Image-J (http://rsb.info.nih.gov/ij) using a protocol described by Colin Rickman (http://www.calm.ed.ac.uk). Data were expressed as means ± SD.
Results
High-level Pdx1 is a marker of β-cell progenitors in developmental mouse pancreata
We first examined the general structure of mouse pancreata at each of four developmental time points (E15.5, E18.5, P1 and P7) by viewing the pancreatic sections with H/E staining. As demonstrated previously [19,20], no islet of Langerhans was identified in the E15.5 pancreas, whereas the structure of islets was spotted in most pancreatic sections of E18.5, P1 and P7 mice (Figure 1; upper row). We also collected the differential interference contrast (DIC) images of the pancreas during confocal microscopy. The general structure of pancreatic islets could be recognized by DIC microscopy, as the islet cells displayed a lighting scheme very different from the adjacent acinar cells (Figure 1; lower row). This optical feature of confocal DIC images allows for determining anatomic location of islet cells in the pancreas.
Figure 1.
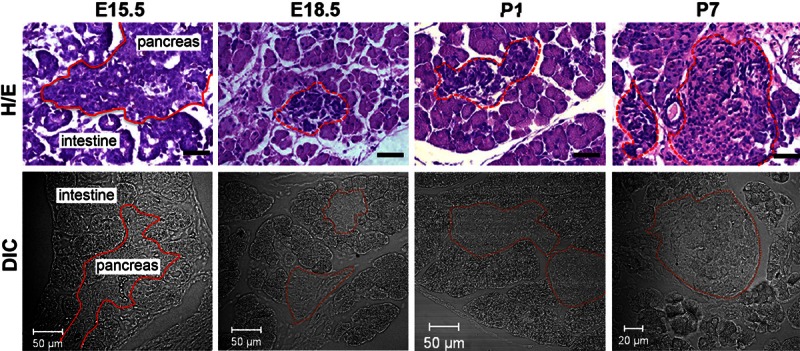
General structure of the mouse pancreas at late embryonic and early postnatal stages. Upper row: Typical pictures of hematoxylin and eosin (H/E) stained mouse pancreata at E15.5, E18.5, P1 and P7. Black bar represents 50 μm. Lower row: Shown are representative images of mouse pancreata at E15.5, E18.5, P1 and P7, collected by means of differential interference contrast (DIC) microscopy. Note: in both upper and lower rows, the solid lines in pictures of E15.5 tissue sections outline the border of the pancreas, and the dotted lines in pictures of E18.5, P1 and P7 pancreatic slices delineate islets of Langerhans.
We then examined the expression patterns of Pdx1 and insulin in the mouse pancreata of each developmental time point. Previous cell-tracing experiments show that both endocrine and exocrine cells derive from Pdx1-expressing progenitor cells [21]. Consistent with previous reports [20,22], immunofluorescence of Pdx1 was seen in the nuclei of E15.5 pancreatic cells (Figure 2A; the 2nd column). Interestingly, a subpopulation of pancreatic cells at E15.5 displayed a higher intensity of Pdx1 immunofluorescence in comparison to cells outside the pancreas, and they often clustered as groups (the 2nd column of Figure 2A). Conversely, from E18.5 to P7 the relatively high immunoreactivity of Pdx1 was only seen in cells compiled in the islets of Langerhans, which were identified in DIC images (the 1st column of Figure 2A). At E15.5 the immunofluorescence of insulin was observed in cells scattered in the pancreas, whereas from E18.5 to P7, the immunoreactivity of insulin was primarily restricted to the cells in the islets of Langerhans (Figure 2A; the 3rd column). Image analysis revealed that the immunofluorescence of insulin was highly correlated to cells that displayed a high level of Pdx1 (Figure 2B). However, at E15.5 only about 30% of pancreatic cells that displayed high-level Pdx1 displayed immunofluorescence of insulin, although the percentage of insulin-expressing cells (Figure 2C) and the expression level (Figure 2D) of insulin increased in this cell subpopulation along with pancreatic development. These results not only suggest that Pdx1, at high expression levels, is a relatively-reliable marker of β-cell progenitors in the developing pancreas, but also corroborate that the expression level of insulin increases in the course of β-cell maturation.
Figure 2.
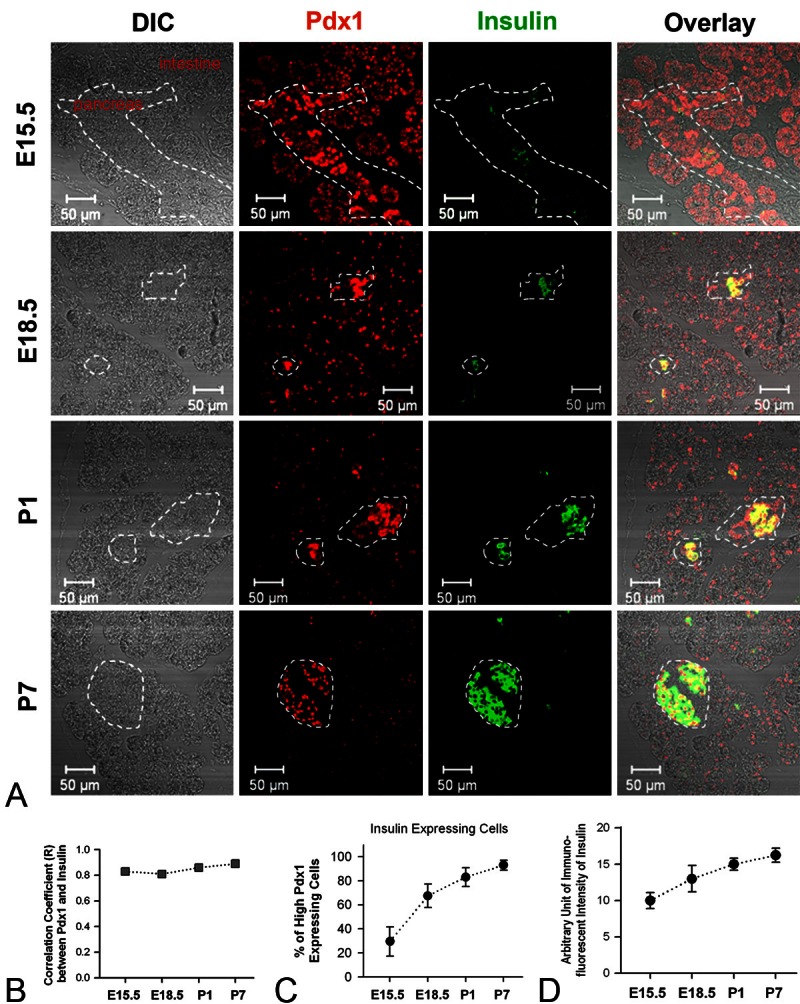
Expression patterns of Pdx1 and insulin in the late embryonic and early postnatal pancreas of mice. A. Representative pictures of double-staining of Pdx1 and insulin of mouse pancreata at E15.5, E18.5, P1 and P7. Note: the dotted lines in pictures of E15.5 tissue slice outline the border of pancreas, whereas the lines in E18.5, P1 and P7 pancreatic sections delineate islets of Langerhans. B. Shown are the correlation coefficient (R) between the immunofluorescence of Pdx1 and immunofluorescence of insulin in pancreatic tissue slices of mice at different developmental stages (at E15.5, E18.5, P1 and P7, R = 0.83, 0.81, 0.86 and 0.89, respectively). C. Graph summarizes the percentage of high-Pdx1 expressing cells that express insulin at different developmental stages (at E15.5, E18.5, P1 and P7, the percentage = 29.5 ± 12.2, 67.5 ± 9.7, 83.0 ± 7.7 and 93.0 ± 4.2, respectively). D. Graph shows the arbitrary unit of immunofluorescent intensity of insulin in pancreatic slices at E15.5, E18.5, P1 and P7 (the intensity unit = 10.0 ± 1.1, 13.0 ± 1.8, 15.0 ± 0.82 and 16.3 ± 0.96, respectively).
Expression of GAD and GABAARα1 is associated with Pdx1-positive cells
Next we studied the expression profiles of GAD and GABAARα1 in the developing β-cell progenitors and/or β-cells from E15.5 to P7, by double-staining Pdx1 and GAD or Pdx1 and GABAARα1, respectively. The immunofluorescence of GAD (Figure 3A; mid row) and GABAARα1 (Figure 4A; mid row) was observed in cells that expressed a high level of Pdx1 (Figures 3A and 4A; upper row) in pancreatic slices of mice at all developmental stages. Specifically, the GAD-positive cells and/or GABAARα1-positive cells were broadly distributed throughout the pancreas at E15.5; however, at E18.5, P1 and P7, these cells became associated only with cells within the islets. Notably, the immunofluorescence of GAD (Figure 3A and 3B) and GABAARα1 (Figure 4A and 4B) was highly correlated to cells that expressed high-level Pdx1 at each developmental stage. The GAD-expressing cells (Figure 3A and 3C) and/or GABAARα1-expressing cells (Figure 4A and 4C) were constantly above 80% and 75%, respectively, of the cells that expressed high-level Pdx1. Moreover, the expression levels of GAD (Figure 3A and 3D) and GABAARα1 (Figure 4A and 4D) increased steadily from E15.5 to P7. These results show that an autocrine GABAAR signaling system exists in β-cell progenitors. Next we investigated whether this autocrine GABA signaling is involved in β-cell development.
Figure 3.
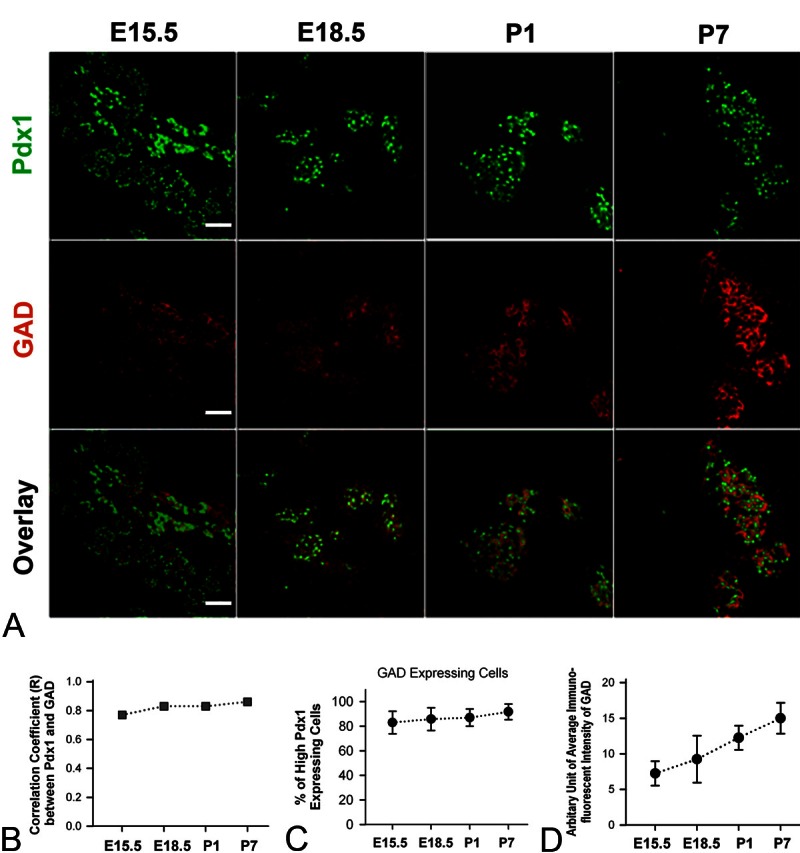
Expression profiles of Pdx1 and GAD in developing mouse pancreas. A. Shown are illustrative pictures of double-staining of Pdx1 and GAD of mouse pancreata at E15.5, E18.5, P1 and P7. White bar = 50 μm. B. Correlation coefficient (R) between the Pdx1 immunoluorescence and GAD immunofluorescence in pancreatic tissue slices of mice at E15.5, E18.5, P1 and P7 (R = 0.77, 0.83, 0.83 and 0.86, respectively). C. The percentage of high-Pdx1 expressing cells that express GAD at E15.5, E18.5, P1 and P7 (The percentage = 83.0 ± 9.2, 85.8 ± 9.3, 87.0 ± 7.0 and 91.8 ± 6.3, respectively). D. Graph shows the arbitrary unit of immunofluorescent intensity of GAD in pancreatic slices at E15.5, E18.5, P1 and P7 (the intensity unit = 7.3 ± 1.7, 9.3 ± 3.3, 12.2 ± 1.7 and 15.0 ± 2.2, respectively).
Figure 4.
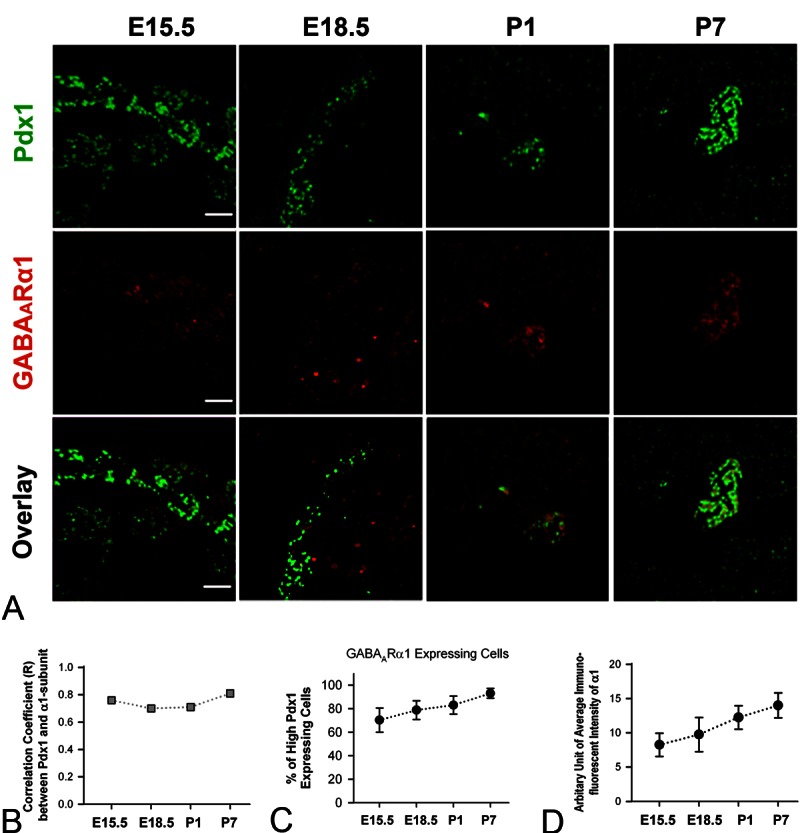
Expression profiles of Pdx1 and GABAARα1 in developing mouse pancreas. A. Representative pictures of double-staining of Pdx1 and GABAARα1 of mouse pancreata at E15.5, E18.5, P1 and P7. White bar = 50 μm. B. Correlation coefficient (R) between the Pdx1 immunoluorescence and GABAARα1 immunofluorescence in mouse pancreata at E15.5, E18.5, P1 and P7 (R = 0.76, 0.70, 0.71 and 0.81, respectively). C. The percentage of high-Pdx1 expressing cells that express GABAARα1 at E15.5, E18.5, P1 and P7 (The percentage = 70.3 ± 10.2, 78.8 ± 7.9, 83.0 ± 7.7 and 93.0 ± 4.2, respectively). D. The arbitrary unit of immunofluorescent intensity of GABAARα1 in pancreatic tissues at E15.5, E18.5, P1 and P7 (the intensity unit = 8.3 ± 1.7, 9.8 ± 2.5, 12.3 ± 1.7 and 14.0 ± 1.8, respectively).
Expression of GAD and GABAARa1 is associated with insulin-positive cells
We reasoned that if this autocrine GABA signaling was involved in β-cell progenitor maturation, the related GABA signaling proteins should be expressed in β-cell progenitors prior to insulin expression. Thus we made double-staining of insulin and GAD, or double-staining of insulin and GABAARα1. Our assays showed that insulin expressing cells were scattered in the mouse pancreata at E15.5, and they became primarily assembled in pancreatic islets after E18.5 (Figures 5A and 6A, upper row). Notably, at E15.5 a large number of pancreatic cells that expressed GAD (Figure 5A) or GABAARα1 (Figure 6A) did not express insulin; although increasingly more GAD-expressing cells (Figure 5A and 5B) or GABAARα1-expressing cells (Figure 6A and 6B) exhibited insulin immunofluorescence along with pancreatic development. After E18.5, the immunofluorescence of insulin was always highly correlated to cells that expressed GAD (Figure 5A and 5C) or to the cells that expressed GABAARα1 (Figure 6A and 6C).
Figure 5.
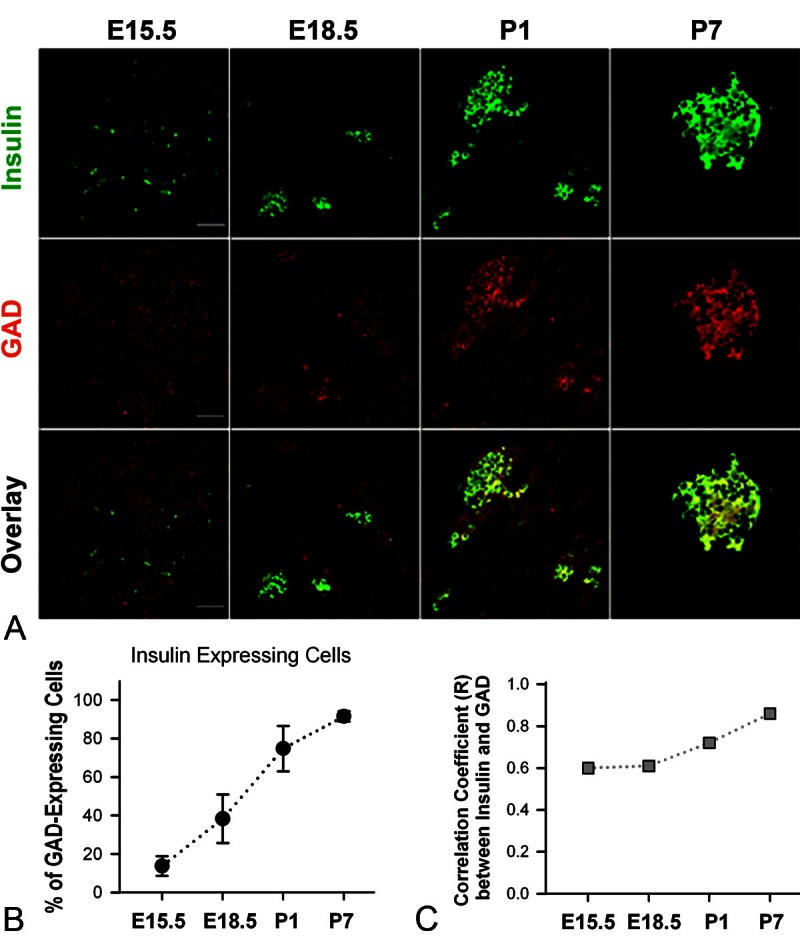
Expression patterns of GAD and insulin in developing mouse pancreas. A. Typical pictures of double-staining of GAD and insulin of mouse pancreata at E15.5, E18.5, P1 and P7. White bar = 50 μm. B. The percentage of GAD-expressing cells that express insulin at E15.5, E18.5, P1 and P7 (The percentage = 13.8 ± 5.1, 38.3 ± 12.6, 74.8 ± 11.8 and 91.5 ± 2.6, respectively). C. Correlation coefficient (R) between the GAD immunoluorescence and insulin immunofluorescence in mouse pancreata at E15.5, E18.5, P1 and P7 (R = 0.60, 0.61, 0.72 and 0.86, respectively).
Figure 6.
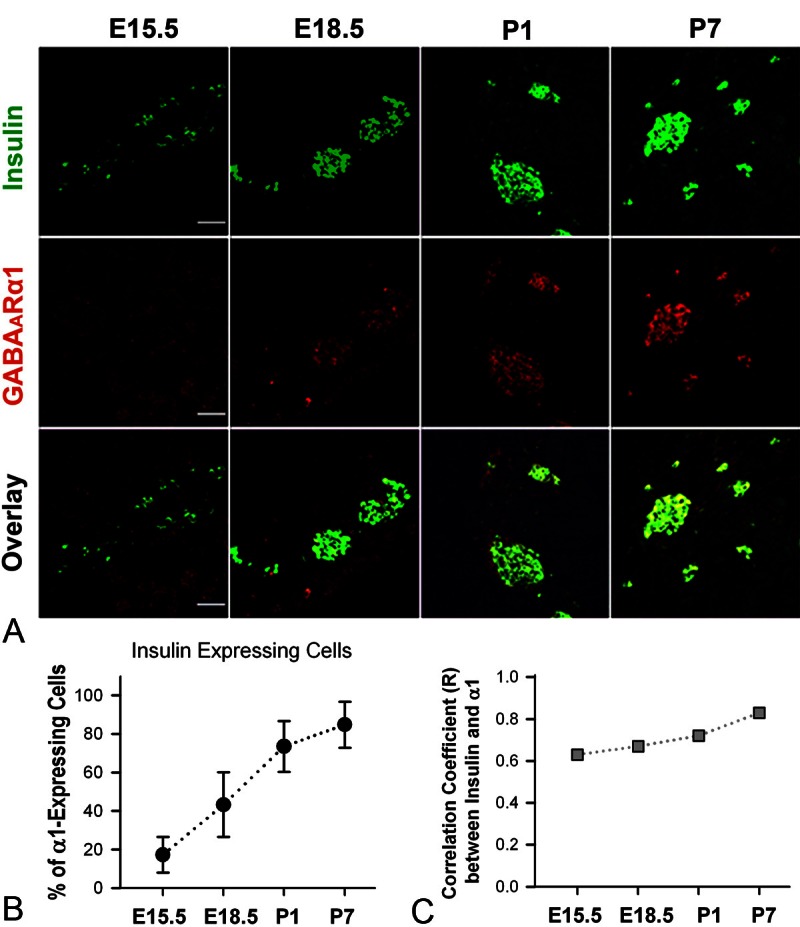
Expression of GABAARα1 and insulin in developing mouse pancreas. A. Illustrative images of double-staining of GABAARα1 and insulin of mouse pancreata at E15.5, E18.5, P1 and P7. White bar = 50 μm. B. The percentage of GABAARα1-expressing cells that express insulin at E15.5, E18.5, P1 and P7 (The percentage = 17.3 ± 9.3, 43.3 ± 16.8, 73.5 ± 13.2 and 84.8 ± 12.0, respectively). C. Correlation coefficient (R) between the GABAARα1 immunoluorescence and insulin immunofluorescence in mouse pancreata at E15.5, E18.5, P1 and P7 (R = 0.63, 0.67, 0.72 and 0.83, respectively).
Discussion
The β-cell differentiation and/or maturation is a complex process involving multiple signaling pathways, and identifying these molecular regulators of β-cell differentiation may provide new therapeutic targets for diabetes [23]. An autocrine GABA signaling exists in adult rodent and human pancreatic β-cells [5,7,8]. Our previous studies showed that GABA regulates β-cell function [5,6] and enhances β-cell proliferation/regeneration in adult rodents [9]. However, the role of GABA signaling in β-cell progenitors remains unclear. It is well known that GABA, via GABAARs, modulates the development of neuronal progenitors, including their proliferation, differentiation and migration [10]; and that GABA regulates the activity of neuroprogenitors by increasing their intracellular Ca2+ concentrations [24]. Interestingly, GABA also increases intracellular Ca2+ concentrations in β-cells [5]. Although it has been proposed that GABA regulates islet cell development, evidence in support of this hypothesis remains elusive. For example, knocking-out either GAD65 or GAD67 at the initial embryonic stage does not affect the development of islet cells or the general morphology of islets [12]. Arguably, lacking the function of one isoform of GAD could be compensated by the other isoform. On the other hand, a recent study demonstrates that GABA enhances pancreatic β-cell proliferation in vivo [9]. This new finding navigates us to determine a potential role of GABA signaling in the process of β-cell progenitor differentiation and maturation.
As an initial effort, the present study examined the expression profile of GABA signaling proteins such as GAD and a GABAAR subunit in the pancreas of fetal and early postnatal mice. Since no specific marker for β-cell progenitors has been identified, we attempted to label pancreatic β-cell progenitors by immunostaining Pdx1 in the present study. This is because 1) early pancreatic progenitors express Pdx-1, and 2) Pdx1 expression is down-regulated in acinar and ductal cells beginning at approximately E13.0, but is maintained in differentiated endocrine cells and up-regulated specifically in β-cells throughout life [25]. Consistent with previous studies, our analyses revealed that at E15.5, Pdx1 was widely expressed in intestinal duodenal and pancreatic cells (Figure 2). However, a higher expression level of Pdx1 was always seen in a subpopulation of cells in the pancreas at all developmental stages. In particular, pancreatic cells that expressed a high-level of Pdx1 mainly compiled in the islets of Langerhans; and increasingly more of them expressed insulin along pancreatic development (Figure 2). Our results support the notion that high-level Pdx1 is likely a reliable marker of β-cell progenitors in the developing pancreata of mice.
As summarized in Figure 7, our results demonstrated that as early as E15.5 the majority of pancreatic cells displaying a high-level of Pdx1 express GABA signaling proteins GAD and/or GABAARα1. In contrast, at the same developmental stage only about 30% of these pancreatic progenitors express insulin. These findings suggest that an autocrine GABA signaling system occurs in pancreatic β-cell progenitors prior to insulin expression. Our data also showed that the expression level of GAD and GABAARα1 increases progressively from E15.5 to P7. Remarkably, during the same time course of pancreatic development more and more β-cell progenitors express insulin, and the expression level of insulin rises steadily.
Figure 7.
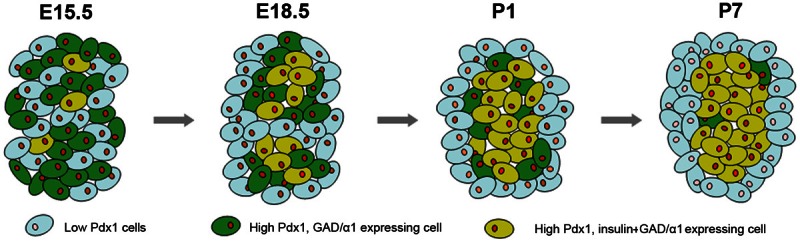
Expression profiles of Pdx1, GAD/GABAARα1 and insulin in mouse β-cell progenitors at E15.5, E18.5, P1 and P7. During development, cells in the pancreas express different levels of Pdx1. At E15.5, pancreatic cells that display a high level of Pdx1 express GAD and GABAARα1 (illustrated as green cells), and some of them also express insulin (shown as yellow cells). From E18.5 to P7, the high Pdx1-expressing cells tend to cluster as groups in the islet; and increasingly more of them express insulin.
Taken together, our data show that at the developmental stages from E15.5 to P7, an autocrine GABA signaling system exists in pancreatic β-cell progenitors and/or β-cells. Since the GABA signaling proteins appear prior to insulin expression, this autocrine GABA signaling system is possibly involved in the process of β-cell progenitor maturation. However, a definitive role of GABA signaling in β-cell development is not examined in the present study and requires further investigations.
Acknowledgments
The authors thank Dr. Xiangru Lu for her assistance in mouse tissue collection and mouse pancreatic slice preparation. This study is supported by the Canadian Institutes of Health Research grant (MOP-84517) to WYL.
Declaration of conflict of interest
None.
References
- 1.Murtaugh LC. Pancreas and beta-cell development: from the actual to the possible. Development. 2007;134:427–38. doi: 10.1242/dev.02770. [DOI] [PubMed] [Google Scholar]
- 2.Xiang YY, Wang S, Liu M, Hirota JA, Li J, Ju W, Fan Y, Kelly MM, Ye B, Orser B, O’Byrne PM, Inman MD, Yang X, Lu WY. A GABAergic system in airway epithelium is essential for mucus overproduction in asthma. Nat Med. 2007;13:862–7. doi: 10.1038/nm1604. [DOI] [PubMed] [Google Scholar]
- 3.Wang S, Xiang YY, Ellis R, Wattie J, Feng M, Inman MD, Lu WY. Effects of furosemide on allergic asthmatic responses in mice. Clin Exp Allergy. 2011;41:1456–67. doi: 10.1111/j.1365-2222.2011.03811.x. [DOI] [PubMed] [Google Scholar]
- 4.Chen X, Li M, Xiong ZG, Orser BA, MacDonald JF, Lu WY. An anti-coagulation agent Futhan preferentially targets GABA(A) receptors in lungepithelia: implication in treating asthma. Int J Physiol Pathophysiol Pharmacol. 2011;3:249–56. [PMC free article] [PubMed] [Google Scholar]
- 5.Dong H, Kumar M, Zhang Y, Gyulkhandanyan A, Xiang YY, Ye B, Perrella J, Hyder A, Zhang N, Wheeler M, Lu WY, Wang Q. Gamma-aminobutyric acid up- and downregulates insulin secretion from beta cells in concert with changes in glucose concentration. Diabetologia. 2006;49:697–705. doi: 10.1007/s00125-005-0123-1. [DOI] [PubMed] [Google Scholar]
- 6.Bansal P, Wang S, Liu S, Xiang YY, Lu WY, Wang Q. GABA coordinates with insulin in regulating secretory function in pancreatic INS-1 beta-cells. PLoS One. 2011;6:e26225. doi: 10.1371/journal.pone.0026225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gilon P, Campistron G, Geffard M, Remacle C. Immunocytochemical localisation of GABA in endocrine cells of the rat entero-pancreatic system. Biol Cell. 1988;62:265–73. [PubMed] [Google Scholar]
- 8.Garry DJ, Appel NM, Garry MG, Sorenson RL. Cellular and subcellular immunolocalization of L-glutamate decarboxylase in rat pancreatic islets. J Histochem Cytochem. 1988;36:573–80. doi: 10.1177/36.6.2896676. [DOI] [PubMed] [Google Scholar]
- 9.Soltani N, Qiu H, Aleksic M, Glinka Y, Zhao F, Liu R, Li Y, Zhang N, Chakrabarti R, Ng T, Jin T, Zhang H, Lu WY, Feng ZP, Prud’homme GJ, Wang Q. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc Natl Acad Sci U S A. 2011;108:11692–7. doi: 10.1073/pnas.1102715108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ge S, Pradhan DA, Ming GL, Song H. GABA sets the tempo for activity-dependent adult neurogenesis. Trends Neurosci. 2007;30:1–8. doi: 10.1016/j.tins.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Andang M, Hjerling-Leffler J, Moliner A, Lundgren TK, Castelo-Branco G, Nanou E, Pozas E, Bryja V, Halliez S, Nishimaru H, Wilbertz J, Arenas E, Koltzenburg M, Charnay P, El MA, Ibanez CF, Ernfors P. Histone H2AX-dependent GABA(A) receptor regulation of stem cell proliferation. Nature. 2008;451:460–4. doi: 10.1038/nature06488. [DOI] [PubMed] [Google Scholar]
- 12.Kash SF, Condie BG, Baekkeskov S. Glutamate decarboxylase and GABA in pancreatic islets: lessons from knock-out mice. Horm Metab Res. 1999;31:340–4. doi: 10.1055/s-2007-978750. [DOI] [PubMed] [Google Scholar]
- 13.Ahlgren U, Jonsson J, Edlund H. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–16. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–32. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Madsen OD, Jensen J, Petersen HV, Pedersen EE, Oster A, Andersen FG, Jorgensen MC, Jensen PB, Larsson LI, Serup P. Transcription factors contributing to the pancreatic beta-cell phenotype. Horm Metab Res. 1997;29:265–70. doi: 10.1055/s-2007-979035. [DOI] [PubMed] [Google Scholar]
- 16.Fujimoto K, Polonsky KS. Pdx1 and other factors that regulate pancreatic beta-cell survival. Diabetes Obes Metab. 2009;11(Suppl 4):30–7. doi: 10.1111/j.1463-1326.2009.01121.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bae JJ, Xiang YY, Martinez-Canabal A, Frankland PW, Yang BB, Lu WY. Increased transforming growth factor-beta1 modulates glutamate receptor expression in the hippocampus. Int J Physiol Pathophysiol Pharmacol. 2011;3:9–20. [PMC free article] [PubMed] [Google Scholar]
- 18.Dong H, Xiang YY, Farchi N, Ju W, Wu Y, Chen L, Wang Y, Hochner B, Yang B, Soreq H, Lu WY. Excessive expression of acetylcholinesterase impairs glutamatergic synaptogenesis in hippocampal neurons. J Neurosci. 2004;24:8950–60. doi: 10.1523/JNEUROSCI.2106-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003;228:185–93. doi: 10.1002/dvdy.10368. [DOI] [PubMed] [Google Scholar]
- 20.Artner I, Blanchi B, Raum JC, Guo M, Kaneko T, Cordes S, Sieweke M, Stein R. MafB is required for islet beta cell maturation. Proc Natl Acad Sci U S A. 2007;104:3853–8. doi: 10.1073/pnas.0700013104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–22. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 22.Nammo T, Yamagata K, Hamaoka R, Zhu Q, Akiyama TE, Gonzalez FJ, Miyagawa J, Matsuzawa Y. Expression profile of MODY3/HNF-1alpha protein in the developing mouse pancreas. Diabetologia. 2002;45:1142–53. doi: 10.1007/s00125-002-0892-8. [DOI] [PubMed] [Google Scholar]
- 23.Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, Gaiarsa JL, Tyzio R, Khazipov R. GABA: a pioneer transmitter that excites immature neurons and generates primitive oscillations. Physiol Rev. 2007;87:1215–84. doi: 10.1152/physrev.00017.2006. [DOI] [PubMed] [Google Scholar]
- 25.Guz Y, Montminy MR, Stein R, Leonard J, Gamer LW, Wright CV, Teitelman G. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121:11–8. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]


