Abstract
L-arginine is a semi-essential amino acid that found naturally in food. It has been shown that administration of large doses of L-arginine can induce acute pancreatitis. In the present study, we evaluated if simvastatin, a 3-hydroxy-methylglutaryl coenzyme A reductase (HMG-CoA reductase) inhibitor, might prevent acute pancreatitis induced by L-arginine. Thirty male Wistar rats were randomly allocated to five groups. Groups were: DMSO, saline, simvastatin, L-arginine, and simvastatin with L-arginine. Twenty four hours after the last dose, rats were sacrificed and their blood was collected from heart for biochemical analysis. Pancreatic tissues were obtained for analysis of glutathione peroxidase (GPx), glutathione s-transferase (GST), lipid peroxide levels (MDA) and histology analysis was examined for pancreas. Results indicated that treatment with simvastatin significantly enhanced levels of GPx and GST and decreased lipid peroxide levels induced by L-arginine compared to the vehicle. Moreover, histopathological analysis further confirmed that administration of simvastatin relatively prevented pancreatic acinar cell damage compared to those animals received L-arginine alone. These findings pointed out the protective role of simvastatin against acute pancreatitis induced by high doses of L-arginine.
Keywords: L-arginine, acute pancreatitis, simvastatin, lipid peroxidation, antioxidant enzymes
Introduction
Acute pancreatitis (AP) is an acute inflammatory disorder of the pancreas with variable involvement of other regional tissues [1]. The most common symptoms of AP are acute abdominal pain and increased concentration of serum lipase and amylase [2]. AP is a reversible inflammatory disorder that varies in severity, ranging from focal edema and fat necrosis to widespread hemorrhagic parenchymal necrosis [3,4]. It is relatively common, with an annual incidence of 10 to 20 per 100,000 people in the Western world. Approximately 80% of cases are attributed to either biliary tract disease or alcoholism.
The basic alterations in AP include microvascular disturbances causing edema, fat necrosis, acute inflammatory reaction, destruction of pancreatic parenchyma, and destruction of blood vessels leading to interstitial hemorrhage [4,5]. These alterations are largely due to activation of digestive proteases, proinflammatory cell infiltration, release of inflammatory cytokines, and generation of free radicals. In milder forms, histological alterations include interstitial edema and focal areas of fat necrosis in the pancreatic substance and peripancreatic fat. Fat necrosis results from enzymatic destruction of fat cells where the released fatty acids combine with calcium to form insoluble salts that precipitate in situ [4,5].
As a model, it has been shown that large doses of L-arginine induce acute pancreatitis [6]. A single dose of 500 mg/kg L-arginine is known to induce necrotizing pancreatitis in rats and it is found that such dose can selectively induce pancreatic acinar cell damage without any morphological changes in the islets of Langerhans [6,7]. L-arginine-induced AP model is highly reproducible and produces selective, dose-dependent acinar cell necrosis [8].
L-arginine is the precursor for the endogenous synthesis of nitric oxide (NO). NO is a highly reactive radical gas and an important messenger molecule that is involved in functions such as neurotransmission, inflammation, and regulation of gene expression. Additionally NO is a powerful vasodilator and can increase blood flow. The mechanism by which L-arginine causes AP is still unknown but it has been proposed that oxygen/nitric oxide, and inflammatory cytokines may be involved in the development of the disease [9].
HMG-CoA reductase is the enzyme that catalyses the conversion of HMG-CoA in mevalonate and this is the limiting step in the cholesterol synthesis. Statins are HMG-CoA reductase inhibitors used clinically in treatment of hyperlipidemia [10]. There are five statins in clinical use including lovastatin, simvastatin, pravastatin, atorvastatin, and fluvastatin [11].
In addition to their antihyperlipidemic effect, statins have antioxidant activity against lipid peroxidation, anti-inflammatory effects, induce nitric oxide levels, impeding thrombogenesis by inhibiting activation of extrinsic coagulation, produce beneficial effects in hypertension, improving endothelial dysfunction, and provide additional cardioprotective effects [12].
The purpose of the present study was to evaluate the protective effect of simvastatin against large dose L-arginine-induced acute toxicity of pancreas.
Materials and methods
Animals and animal’s procedures
Male Sprague Dawley rats, weighing 160-210 g, aged 8-12 weeks obtained from the central animal house of Jordan University of Science & Technology were used in this study. Animals were maintained at a constant temperature (23 ± 2°C) with light-dark cycles of 12/12 hour and free access to water and standard laboratory chow. Animals were randomly divided into five groups of six in each and experiments performed after 12 hour fasting. Approval of the study was conducted by Institutional Animal Care Committee at Jordan University of Science and Technology (Irbid, Jordan).
Drugs administration
Drugs were administered orally using a ball tipped stainless steel gavage attached to a syringe. Simvastatin was dissolved in DMSO and L-arginine was dissolved in normal saline. Five experimental groups were established: Group I: 0.1% DMSO as vehicle, Group II: saline as normal control, Group III: simvastatin (1 mg/kg), Group IV L-arginine (500 mg/kg), Group V: L-arginine (500 mg/kg) and simvastatin (1 mg/kg). Animals in all groups received their medication on a daily basis except those in Group IV received L-arginine on the third day of the experiment and in Group V received daily simvastatin and given L-arginine on the third day of the experiment.
Serum analysis
Animals in all groups were scarified in an ether chamber after 24 hr from the last application of the treatments. Blood samples were taken by intracardiac puncture and collected into heparinized tubes. Samples were centrifuged at 3000 rpm for 10 min. The serum amylase and lipase were determined by routine colorimetric methods using commercial kits (Al-Far Medical Company, Irbid, Jordan) and expressed as U/L.
Determination of glutathione peroxidase (GPx) and glutathione s-transferase (GST)
Kidneys were removed from each rat. One kidney was homogenized with phosphate buffered solution (pH 7.2) to obtain a 1:5 (w/v) homogenate. The homogenate was stored at -80°C, later thawed, and GST, catalase activities, and lipid peroxidation levels (MDA) were determined. GST activity was measured by monitoring the rate of 1-chloro-2,4-dinitrobenzene conjugation with reduced glutathione at 340 nm [13]. Catalase activity was measured by monitoring the rate of hydrogen peroxide decomposition at 240 nm [14]. Level of lipid peroxidation was determined at 532 nm by a method described earlier [15]. GST, catalase activities and lipid peroxidation levels were expressed in terms of protein content. Total protein concentration (mg/ml) was estimated according to Bradford method (Figure 1) using bovine serum albumin standard curve [16].
Figure 1.
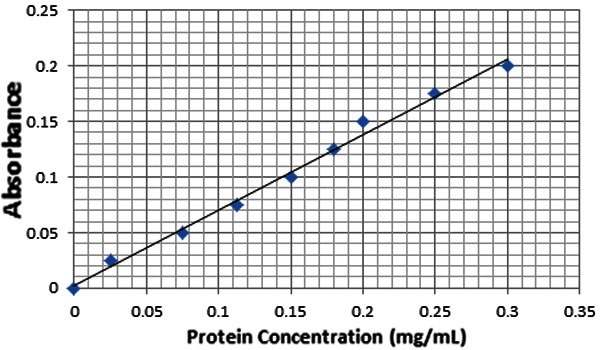
Bradford standard curve for total protein concentration, using eight different concentrations of bovine serum albumin along with blank containing Bradford solution and same volume of buffer. The standard curve is linear with equation (Y=0.6543X+0.0069) and goodness of fit (R2= 0.9878).
Determination of lipid peroxidation
Following homogenization of pancreatic tissues in (0.1 M) phosphate-buffered saline (PBS), levels of lipid peroxide in the homogenate samples were determined by a previously described method [15]. Briefly, 4 ml of a mixture containing 1.5 ml of 20% acetic acid, 0.2 ml of 8.1% SDS, 1.5 ml of 0.8% TBA, 0.7 ml distilled water and 10 μl of the crude was prepared and incubated at 95°C for one hour. After cooling, samples were centrifuged at 2500 rpm for 8 minutes. The optical density of the chromogen malondialdehyde (MDA) was then read at 532 nm against blank containing distilled water instead of sample.
Pancreatic histopathology
One kidney was placed in 10% formaldehyde solution for histopathology examination by light microscopy. Fixed materials were washed in 70% ethanol repeatedly. Dehydration was performed by passing the materials in upgraded ethanol concentrations as follows: 80%, 90%, 95% and absolute (two hours in each change), and then cleared in xylene for 20 min. Infiltration was carried out using paraffin wax (melting point 49°C), embedding of the samples with pure melted paraffin wax was achieved by pouring it in cassettes in order to make blocks. Blocks were then trimmed and they were serially sectioned using a wild microtome at 5 μm thickness. Glass slides were used to collect the serial sections floating in distilled water and placed on a hot plate for stretching. Serial sections were stained using Ehrlich hematoxylin and Eosin stains (H & E). The stained sections were mounted with Canada balsam and covered with cover slip, examined on CX31 microscope. Images were captured using DP20 camera set from Olympus™. DP20 is a 2 Megapixel color CCD digital microscope camera, with a resolution of 1600 x 1200 pixels, and pixel size of 4.2 μm x 4.2 μm.
Statistical analysis
Results were analyzed using the SPSS software, and expressed as a mean ± Standard error (SE). The data was analyzed using one-way analysis of variance (ANOVA). A P-value of less than 0.05 was considered statistically significant.
Results
Results in the present study showed no mortality in animals during the study period. Figures 2 and 3 demonstrated that administration of L-arginine has significantly (P<0.05) increased plasma lipase activity with a slight increase in plasma amylase activity in comparison with saline group. While pre-treatment with simvastatin at 1 mg/kg has significantly (P<0.05) inhibited these increases in lipase and amylase levels, simvastatin at 0.3 mg/kg did not affect their levels in animals treated with L-arginine (data not shown).
Figure 2.
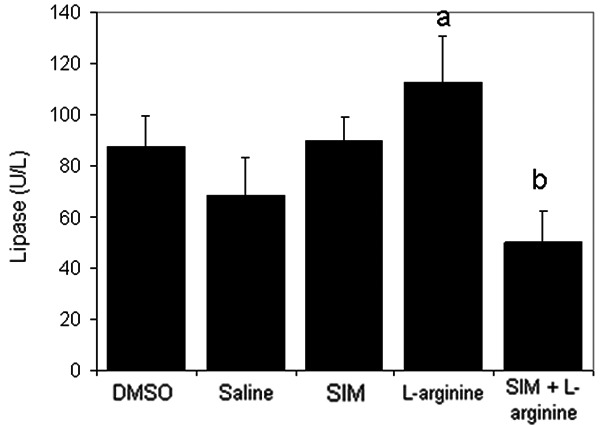
The effect of L-arginine (500 mg/Kg) and/or simvastatin (1 mg/Kg) on plasma lipase activity for 5 days. (n=6). Values are means ± SEM. Analysis of variance (ANOVA), aP<0.05 compared with saline group, bP<0.05 compared with the L-arginine group.
Figure 3.
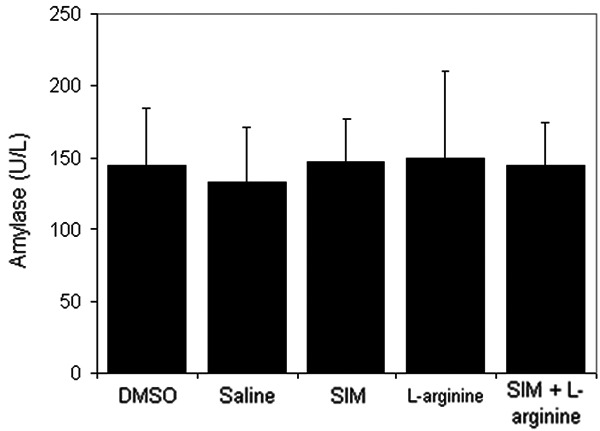
The effect of L-arginine (500 mg/Kg) and/or simvastatin (1 mg/Kg) on plasma amylase activity for 5 days. (n=6). Values are means ± SEM. Analysis of variance (ANOVA).
Figures 4 and 5 depict the effect of L-arginine on the antioxidant enzymes GPx and GST. Results indicated that L-arginine significantly (P<0.05) decreased their levels compared to the control group. Pre-treatment with simvastatin at 1 mg/kg significantly (P<0.05) enhanced levels of both GST and GPx in rats receiving L-arginine.
Figure 4.
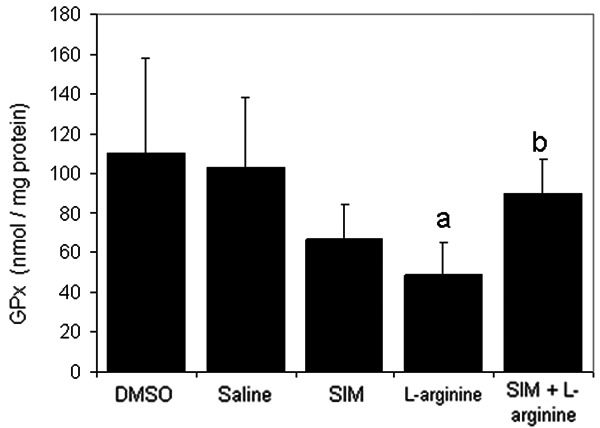
The effect of L-arginine (500 mg/Kg) and/or simvastatin (1 mg/Kg) on GPx activity for 5 days. (n=6). Values are means ± SEM. Analysis of variance (ANOVA), aP<0.05 compared with saline group, bP<0.05 compared with the L-arginine group.
Figure 5.
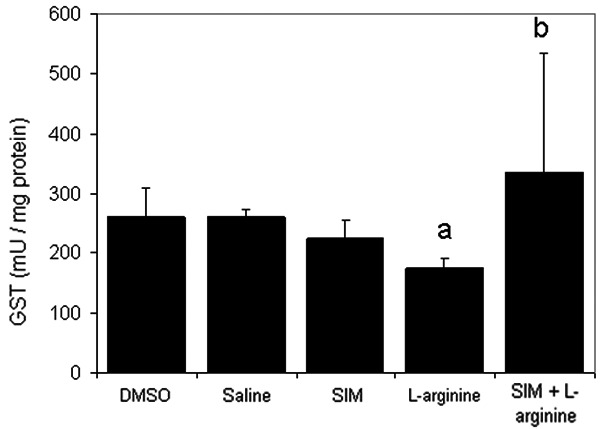
The effect of L-arginine (500 mg/Kg) and/or simvastatin (1 mg/Kg) on GST activity for 5 days. (n=6). Values are means ± SEM. Analysis of variance (ANOVA), aP<0.05 compared with saline group, bP<0.05 compared with the L-arginine group.
In order to assess pancreatic damage, levels of malondialdehyde (MDA), an end-product of lipid peroxidation, were measured in animals received different drug combinations. Results shown in (Figure 6) revealed that MDA levels in the L-arginine-induced acute pancreatitis group were significantly (P<0.05) higher when compared to the saline-treated group. Pre-treatment with simvastatin at 1 mg/kg reduced the increase in MDA levels induced by L-arginine.
Figure 6.
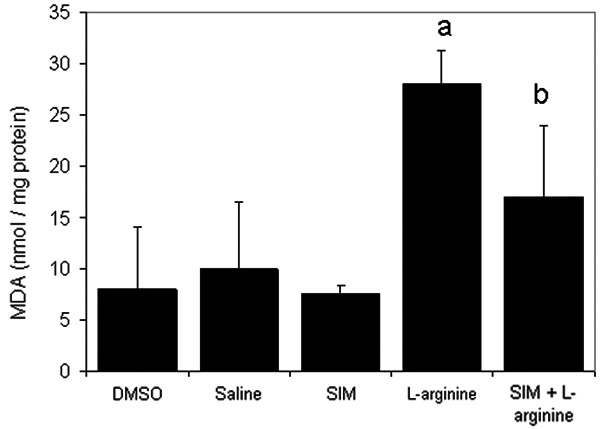
The effect of L-arginine (500 mg/Kg) and/or simvastatin (1 mg/Kg) on MDA levels for 5 days. (n=6). Values are means ± SEM. Analysis of variance (ANOVA), aP<0.05 compared with saline group, bP<0.05 compared with the L-arginine group.
Histological examination of pancreatic specimens from animals treated with DMSO, saline, or simvastatin at 1 mg/kg presented no histological alterations within acinar cells (Figure 7). Administration of L-arginine resulted in a significant disruption of normal architecture with cell vacuolization (Figure 7D) and fat necrosis in adipose tissues (Figure 7E). Simvastatin at 1 mg/kg protected pancreas from histological damage induced by L-arginine and pathological scores were significantly decreased (Figure 7F).
Figure 7.
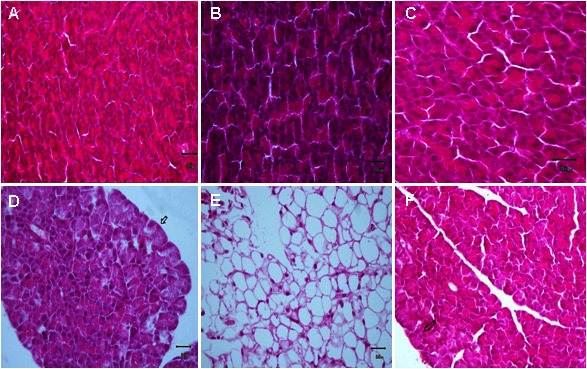
Effect of different drug combination on acinar cells and fat tissues. Specimen of pancreas showing effects of DMSO (A), saline (B), simvastatin (C), L-arginine on acinar cells and fat tissues (D & E), and simvastatin with L-arginine (F). (H & E, X 400).
Discussion
The present study aimed to investigate the potential of large dose of L-arginine (as a model) to induce acute pancreatitis in rats and the protective effect of simvastatin. In accordance with the previous reports [17,18], L-arginine induced acute pancreatitis as evidenced by a dramatic increase in plasma lipase levels, and to a lesser degree in amylase levels in L-arginine treated group. It has been shown early that lipase might be more specific biochemical marker than amylase for diagnosis of acute pancreatitis [19]. The increase in lipase levels was reversed by pre-administration of simvastatin indicating that simvastatin might inhibit some signaling pathway involved in the development of AP.
Following AP, lipids are one of the main targets for free radicals damage. The later will induce lipid peroxidation by removing one hydrogen atom from polyunsaturated fatty acids and form hydroperoxides. As a result, perturbations in cellular fluidity and membrane integrity lead to disintegration of cells and necrotic cell death. Consequently, subcellular structures released into the extracellular media will induce several inflammatory events and further worsen the ongoing damage [1-4]. The MDA level in the simvastatin-treated pancreatitis group was found to be significantly lower compared to the L-arginine-treated group (p<0.05). These results suggest a protective effect against free radical-induced damage through inhibition of lipid peroxidation.
GST and GPx are known to protect the cell from oxidative injuries by neutralizing free radicals and peroxides. Tissue GST levels are rapidly decreased during pancreatic damage [6]. In our study, GST and GPx levels were significantly lower in the L-arginine-treated group than in the saline-treated group (p<0.05). These results were similar to those presented previously where antioxidant activity of simvastatin explored [6,8,20]. These effects might due to the potential of GST to conjugate free radicals including hydroxyl radicals, single oxygen, nitric oxide and peroxynitrite by formation thioether bond, thus masking their reactivity. In addition, the antioxidant activity of GPx might due to neutralization of hydrogen peroxide into water, thus perturbing its oxidative activity.
Statins, which are 3-hydroxy-methylglutaryl coenzyme A reductase (HMG-CoA reductase) inhibitors, have been clinically used for treatment of hyperlipidemia. The HMG-CoA reductase is the enzyme that catalyses the conversion of HMG-CoA in mevalonate, the limiting step in the cholesterol synthesis. Moreover, it has been suggested that statins might alter the inflammatory response independently of their lipid lowing effect. They can modulate the production of acute-phase proteins, endothelial leukocyte adherence, NO and macrophage activation, besides their immunomodulatory role [21,22].
Several studies have reported that simvastatin is among the drugs that increase risk of AP [23-28]. The incidence of simvastatin potential to induce AP seems clearly to be very rare, dose and treatment time-dependent [29]. In contrast, other reports explored the anti-inflammatory effects of simvastatin. These effects were thought to be mediated by inhibition of mononuclear cell adhesiveness, suppression of T-cell response, reduced expression of class II major histocompatibility complexes on antigen-presenting cells, reduced chemokine synthesis in peripheral blood mononuclear cells and blocking the LFA-1/ICAM-1 interaction [30-33]. Thus, the differential effect of simvastatin might depend on simvastatin dose, biological system used, and duration of treatment.
Consistent with previous histological studies of pancreas, results in here showed that administration of L-arginine caused cytoplasmic vacuolation within acinar cells, also the adipose tissue around the pancreas showed fat necrosis [6]. Pretreatment with simvastatin inhibited L-arginine-induced inflammatory events in pancreas and pancreatic damage. These results emphasized that administration of simvastatin reduced the severity of inflammation and promotes the spontaneous regeneration process of the pancreatic tissue as supported by histological examination.
Conclusion
The present study demonstrated that simvastatin treatment provided a significant protective effect against L-arginine induced acute pancreatitis. Therefore, simvastatin can be considered a potential candidate to minimize acute pancreatitis induced by oxidative stress which is a major clinical problem with L-arginine intoxication.
Acknowledgments
We would like to acknowledge the Jordan University of Science & Technology, Irbid, Jordan, for the financial support (Grant number 144-2011).
Declaration of conflict of interest
None.
References
- 1.Al-Bahrani AZ, Ammori BJ. Clinical laboratory assessment of acute pancreatitis. Clin Chim Acta. 2005;362:26–48. doi: 10.1016/j.cccn.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 2.Frossard JL, Steer LM, Paster CM. Acute pancreatitis. Lancet. 2008;173:143–152. doi: 10.1016/S0140-6736(08)60107-5. [DOI] [PubMed] [Google Scholar]
- 3.Bhatia M, Wong FL, Cao Y, Lau H, Huang J, Puneet P, Chevali L. Pathophysiology of acute pancreatitis. Pancreatology. 2005;5:132–144. doi: 10.1159/000085265. [DOI] [PubMed] [Google Scholar]
- 4.Gandotra DK, Ananda A, Verma S, Singh J, Gupta R, Gupta V. Acute pancreatitis. JK Science. 2004;6:182–186. [Google Scholar]
- 5.Cappell SM. Acute Pancreatitis: Etiology, Clinical Presentation, Diagnosis, and Therapy. Med Clin N Am. 2008;92:889–923. doi: 10.1016/j.mcna.2008.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Czako L, Takacs T, Varga I, Tiszlavicz L, Hai D, Hegyi P, Matkovics B, Lonovics J. Involvement of oxygen-derived free radicals in L-arginine induced acute pancreatitis. Dig Dis Sci. 1998;43:1770–1777. doi: 10.1023/a:1018839821176. [DOI] [PubMed] [Google Scholar]
- 7.Biczo G, Hegyi P, Sinevita R, Berczi S, Dosa S, Siska A, Ivanyi B, Venglovecz V, Takacs T, Alhonen L, Rakonczay Z. Characterization of polyamine homeostasis in L-ornithine-induced acute pancreatitis in rats. Pancreas. 2010;39:868–874. doi: 10.1097/MPA.0b013e3181d3cdf0. [DOI] [PubMed] [Google Scholar]
- 8.Hegyi P, Rakonczay J, Sári R, Góg C, Lonovics J, Takacs Z, Czako L. L-arginine-induced experimental pancreatitis. World J Gastroenterol. 2004;10:2003–2009. doi: 10.3748/wjg.v10.i14.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Su KH, Cuthbertson C, Christophi C. Review of experimental animal models of acute pancreatitis. HPB (Oxford) 2006;8:264–286. doi: 10.1080/13651820500467358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Almeida JL, Sampietre SN, Mendonça Coelho AM, Trindade Molan NA, Machado MC, Monteiro da Cunha JE, Jukemura J. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9:431–439. [PubMed] [Google Scholar]
- 11.Stancu C, Sima A. Statins: mechanism of action and effects. J Cell Mol Med. 2001;5:378–387. doi: 10.1111/j.1582-4934.2001.tb00172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tandon V, Gupta B, Tandon R. Non-lipids actions of statins. JK Science. 2004;6:124–126. [Google Scholar]
- 13.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 14.Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126. doi: 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- 15.Ohkawa H, Ohishi Ν, Yagi Κ. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 16.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 17.GOLDBERG RC, CHAIKOFF IL. Selective pancreatic acinar destruction by diethionine. AMA Arch Pathol. 1951;52:230–238. [PubMed] [Google Scholar]
- 18.Mizunuma T, Kawamura S, Kishino Y. Effects of injecting excess Arginine on rat pancreas. J Nutr. 1984;114:467–471. doi: 10.1093/jn/114.3.467. [DOI] [PubMed] [Google Scholar]
- 19.Baddeley R, Skipworth J, Pereira S. Acute pacreatitis. Medicine. 2010;39:108–115. [Google Scholar]
- 20.Prokopeva N, Gluyaeva L. Glutathione S-transferase activity in the liver in acute pancreatitis terms of disease and during the treatment with inducers. Bull Exp Biol Med. 2000;129:458–459. doi: 10.1007/BF02439801. [DOI] [PubMed] [Google Scholar]
- 21.Tandon V, Bano G, Khajuria V, Parihar A, Gupta S. Pleiotropic effects of statins. Indian J Pharmacol. 2005;37:77–85. [Google Scholar]
- 22.Rosenson R, Tangney C. Antiatherothrombotic properties of statins. JAMA. 1998;279:1643–1650. doi: 10.1001/jama.279.20.1643. [DOI] [PubMed] [Google Scholar]
- 23.Nitsche CJ, Jamieson N, Lerch MM, Mayerle JV. Drug induced pancreatitis. Best Pract Res Clin Gastroenterol. 2010;24:143–55. doi: 10.1016/j.bpg.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 24.Chen J, Cai QP, Shen PJ, Yan RL, Wang CM, Yang DJ, Fu HB, Chen XY. Netrin-1 protects against L-Arginine-induced acute pancreatitis in mice. PLoS One. 2012;7:e46201. doi: 10.1371/journal.pone.0046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sakai A, Nishiumi S, Shiomi Y, Kobayashi T, Izumi Y, Kutsumi H, Hayakumo T, Azuma T, Yoshida M. Metabolomic analysis to discover candidate therapeutic agents against acute pancreatitis. Arch Biochem Biophys. 2012;522:107–20. doi: 10.1016/j.abb.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Onur E, Paksoy M, Baca B, Akoglu H. Hyperbaric oxygen and N-acetylcysteine treatment in L-arginine-induced acute pancreatitis in rats. J Invest Surg. 2012;25:20–8. doi: 10.3109/08941939.2011.593694. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Wang Y, Dong M, Cui J, Rong D, Dong Q. Oxymatrine ameliorates L-arginine-induced acute pancreatitis in rats. Inflammation. 2012;35:605–13. doi: 10.1007/s10753-011-9352-2. [DOI] [PubMed] [Google Scholar]
- 28.Melo CM, Carvalho KM, Neves JC, Morais TC, Rao VS, Santos FA, Brito GA, Chaves MH. alpha, beta-amyrin, a natural triterpenoid ameliorates L-arginine-induced acute pancreatitis in rats. World J Gastroenterol. 2010;16:4272–80. doi: 10.3748/wjg.v16.i34.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Almeida JL, Sampietre SN, Mendonça Coelho AM, Trindade Molan NA, Machado MC, Monteiro da Cunha JE, Jukemura J. Statin pretreatment in experimental acute pancreatitis. JOP. 2008;9:431–9. [PubMed] [Google Scholar]
- 30.Almog Y. Statins, inflammation, and sepsis: hypothesis. Chest. 2003;124:740–3. doi: 10.1378/chest.124.2.740. [DOI] [PubMed] [Google Scholar]
- 31.Blanco-Colio LM, Tuñón J, Martín-Ventura JL, Egido J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003;63:12–23. doi: 10.1046/j.1523-1755.2003.00744.x. [DOI] [PubMed] [Google Scholar]
- 32.Liappis AP, Kan VL, Rochester CG, Simon GL. The effect of statins on mortality in patients with bacteremia. Clin Infect Dis. 2001;33:1352–57. doi: 10.1086/323334. [DOI] [PubMed] [Google Scholar]
- 33.Merx MW, Liehn EA, Janssens U, Lütticken R, Schrader J, Hanrath P, Weber C. HMG-CoA reductase inhibitor simvastatin profoundly improves survival in a murine model of sepsis. Circulation. 2004;109:2560–5. doi: 10.1161/01.CIR.0000129774.09737.5B. [DOI] [PubMed] [Google Scholar]


