Abstract
Objective: To study the possible effect of angiotensin II type 1 Receptor blocker (AT1 blocker) on renal function, arterial blood pressure and parathyroid hormone related protein over expression in cadmium induced nephrotoxicity in adult male rats. Forty five rats were divided randomly into a control (group I), group II, received cadmium chloride at a dose of 5 mg/kg/day, orally, for nine weeks, group III received telmisartan (TEL) treatment (1 mg/kg/day, orally) one week before cadmium administration and continued for ten weeks. Results: Telmisartan significantly reduced blood urea nitrogen (BUN) and serum creatinine levels which were increased significantly by cadmium. Telmisartan significantly suppressed lipid peroxidation, compensated deficits in the antioxidant defenses (super oxide dismutase (SOD) level and catalase activity), decreased the elevations of nitric oxide (NO) and cadmium ion concentrations in renal tissue observed in Cd-treated rats. Group III had a significant decrease of urinary levels of total protein, N-acetyl-β-d-glucosaminidase (NAG), alkaline phosphatase (ALP) and γ-glutamyl-transpeptidase (GGT) and urinary 8-isoprostanes than those of group II. Telmisartan decreased the systolic blood pressure significantly than those of group II. Histopathological examination revealed that cadmium-induced renal tissue damage was ameliorated by telmisartan treatment. Immunohistochemical analysis revealed that telmisartan significantly decreased the cadmium-induced overexpression of parathyroid hormone receptor 1 (PTHR1) in renal tissue. RT-PCR analysis showed that Cd increased renal expression of PTHrP; however telmisartan could decrease the expression of PTHrP in group III. Conclusion: Blocking AT1 receptors significantly decreases PTHrP over expression and ameliorates renal dysfunction in Cd induced nephrotoxicity. These data suggest that Ang II might contribute to pathophysiology and deleterious effects in cadmium nephrotoxicity.
Keywords: Cadmium, parathyroid hormone related protein, nephrotoxicity, telmisartan
Introduction
In the environment, heavy metals are present either in the form of industrial pollutants or naturally in soils, and they can contaminate food and drinking water [1]. Cd is an abundant transition metal of worldwide concern, because it accumulates in the environment as a result of its numerous industrial uses. In human non-occupational exposure to Cd results from smoking, air pollution [2]. Cigarette smoke is the main source of airborne Cd exposure in the environment as a single cigarette contains 1.5 μg of Cd [3].
Because of the long biological half-life of Cd (10–25 years) and its low rate of excretion, the body becomes a ‘sink’ as Cd accumulates and causes toxicity to many vital organs including the lungs, liver and kidneys [4]. Evidences indicate that oxidative stress and reactive oxygen species (ROS) formed in the presence of cadmium could be responsible for its toxic effects in many organs or cells [5]. Chronic exposure to Cd causes severe nephrotoxicity in humans and animals [6]. In those with occupational exposure to cadmium, the renal stones and glomerular damage have been found [7]. The most common effects of Cd on the kidney are impairment of renal tubular function, glomerular alterations and interstitial fibrosis. However, the substantial changes of renal injury in chronic Cd poisoning have not been fully established and the mechanism by which Cd administration causes such renal changes remains unclear [8].
Parathyroid hormone-related protein (PTHrP) is the peptide hormone responsible for most instances of humoral hypercalcemia of malignancy. Parathyroid hormone receptor-1 (PTHR1) is a second G protein-coupled PTH receptor, PTHR2, has been identified [9]. PTHrP binds to PTHR1 but not to PTHR2 [10].
In the adult kidney, both PTHrP and the parathyroid hormone receptor-1 (PTH1R) are abundant throughout the renal parenchyma, including the intrarenal vasculature [11]. PTHrP appears to modulate renal plasma flow, glomerular filtration rate, and induces proliferative effects on both glomerular mesangial and tubuloepithelial cells [12]. Renal PTHrP is over expressed in several experimental nephropathies, including a rat model of tubulointerstitial scarring after protein overload, associated with the development of proteinuria [12].
Clinical and experimental studies have suggested that angiotensin II plays an important role in the pathophysiology of various kidney diseases. Most studies have evaluated the effects of blockade of the renin-angiotensin system (RAS) in conferring renal protection using either an angiotensin-converting enzyme (ACE) inhibitor or an angiotensin type 1 (AT1) receptor antagonist [13]. The AT1 receptor is considered the major receptor mediating the actions of angiotensin II, whereas the AT2 receptor has no or a minimal role in renal pathology. Telmisartan is a highly selective AT1-receptor antagonist approved for treatment of hypertension. On the other hand, telmisartan acts as a partial agonist on the nuclear peroxisome proliferator-activated receptor-γ that has been reported to exert anti-oxidative and anti-inflammatory effects [14]. Clinical studies revealed the effectiveness and safety of telmisartan against diabetic nephropathy in patients with type 2 diabetes mellitus [15], as well as in non-diabetic patients with hypertensive nephropathy [16]. Fouad and Jresat found that telmisartan, through its antioxidant and anti-inflammatory actions, effectively prevented cadmium nephrotoxicity in mice [17].
This study aimed to assess the effect of angiotensin receptor 1 antagonist (telmisartan) on kidney function, blood pressure alternation and PTHr-P over expression in cadmium nephrotoxicity in adult male rats. Furthermore, to explore the possible role of Angiotensin II in the pathophysiology of the cadmium induced nephrotoxicity.
Material and methods
Animals
This study was carried out on forty five white male albino rats, 2-month old with a initial body weight of about 230 ± 30 gm. These animals were obtained and maintained in The Assiut University Animal Nutrition and Care House. The experimental protocol was approved by the Institutional Animal Research Committee of the Faculty of Medicine, Assiut University, Egypt, and followed the published guidelines and regulations. The animals were caged in metabolic cages and kept under standard conventional laboratory conditions at a temperature of 22°C ± 2°C, with a relative humidity of 50 ± 5% and a 12-h/12-h light/dark cycle. They had unlimited access to drinking water ad libitum and rat chow.
Drugs and chemicals
Cadmium chloride powder and telmisartan powder were obtained from Sigma Chemical Company, USA. Cadmium chloride was dissolved in normal saline, while telmisartan was prepared in 1% aqueous solution of Tween 80.
Experimental design
The rats were divided into three groups consisting of 45 rats each. Group I: included 15 rats received saline orally for 9 weeks. Group II: included 15 rats received cadmium orally (as CdCl2) (were purchased from Sigma-Aldrich (St. Louis, USA). in 0.5-ml sterile physiological saline at a dose of 5 mg/kg day for 9 weeks [18]. Group III: included 15 rats received cadmium orally in the same dose as group II and treated with the AT1 antagonist, telmisartan at a dose of 1 mg/kg/day orally [19], respectively, for ten weeks starting one week before cadmium administration.
During this experiment, daily food and water consumption of every cage were recorded during the study period. The 24 hr urine sample was collected; the volume was determined and recorded for each rat. Urine volume was measured (ml/min). Urine was centrifuged and the supernatant fluid was taken and stored by at -20°C. At the end of the study, the animals of all groups were weighed. Systolic arterial BP was measured in all rats (conscious and restrained) by the tail-cuff sphygmomanometer (NARCO Biosystems, Houston, TX). The BP value for each rat was calculated as the mean of three separate measurements at each session.
Preparation of kidney tissue homogenates
5 ml blood from each rat’s retro-orbital vein were collected in micro tubes and centrifuged immediately for 10 min at 5000 rpm to obtain clear sera which were stored at -20°C until analysis. Then, the rats were killed by decapitation. The kidneys were rapidly removed, weighted and kept frozen in liquid nitrogen. Tissues were stored in -80°C until processed.
The kidneys were minced, washed, and homogenized in a dounce glass homogenizer in 10 mM HEPES-KOH/1 mM EGTA buffer (pH 7.5) containing 250 mM sucrose and supplemented with protease and phosphatase inhibitors. The homogenates were spun down for 10 min at 2000 × g at 4°C. The supernatant was collected and stored at -20°C. Blood urea nitrogen (BUN) was measured using colorimetric assay kit according to the recommendations of the manufacturer (Stanbio Laboratory, USA). Serum creatinine level was measured by an enzymatic assay method (Roche Diagnostics GmbH, Germany) using a Hitachi 902 automatic analyzer (Hitachi Co. Ltd, Tokyo, Japan).
Measurement of urinary protein excretion
Urinary protein concentrations were measured with an automatic analyzer (Hitachi 911; Boehringer Mannheim) by indirect potentiometry with ion-selective electrodes and colorimetric reactions.
Determination of nitric oxide, lipid peroxidation, protein carbonyl contents and 8-isoprostane
Determination of renal NO was done by evaluation of its oxidant products, nitrates and nitrites by using Griess reaction [20]. The levels of Lipid peroxidation in the renal tissue was estimated colorimetrically by measuring thiobarbituric acid reactive substances (TBARSs) as described by Niehiaus and Samuelsson [21]. As a hallmark of protein oxidation, total protein carbonyl content was determined in the kidney by a spectrophotometric method described by Levine [22] and expressed as nmol/mg protein.
8-Isoprostane, a marker for oxidative stress, is produced via the random oxidation of tissue phospholipids by oxygen radicals. Urinary 8-isoprostane was analyzed by an enzyme-linked immunosorbent assay kit [23] according to the manufacturer’s instruction (Cayman).
Assay of antioxidant enzymes
Superoxide dismutase (SOD) activity was determined by the method of Marklund and Marklund [24] in which the inhibition of formation of NADH-phenazine methosulfate nitroblue tetrazolium formazan was measured spectrophotometrically at 560 nm. Catalase (CAT) activity was assayed calorimetrically as described by Sinha [25] using dichromate acetic acid reagent.
Urinary markers of renal toxicity
The activities of N-acetyl-β-d-glucosaminidase (NAG), alkaline phosphatase (ALP) and γ-glutamyl-transpeptidase (GGT) in urine were determined colorimetrically [26] using commercial Kits (Roche Diagnostics, Mannheim, Germany).
Cadmium measurement in kidney tissues
Cadmium concentration in the kidney was determined as described in the previous study [27] using an atomic absorption spectrophotometer using a Zeeman furnace system (Solaar 969, Thermo Optek).
Measuring angiotensin II (Ag II) in serum and kidney
Ang II concentrations in serum and renal tissue were determined using Ang II radioimmunoassay kit (Beijing North Institute of Biological Technology, China) [28].
Morphological examination
Immediately after euthanasia, one kidney of each animal was removed, and fixed immediately in 10% buffered formalin, embedded in paraffin, prepared as 5-μm-thick sections and stained with hematoxylin and eosin (HE). Stained sections were examined under light microscope (Olympus CX31, Japan) and photographed using digital camera (Olympus, Camedia C-5060, Japan). Other kidneys specimens were kept frozen in liquid nitrogen and stored in -80°C until processed.
Immunohistochemical analysis
The slides from kidneys of all experimental groups were deparaffinized with three changes of xylene and rehydrated through a graded ethanol series to distilled water. Antigen retrieval was performed by placing the slides in 10 mM citrate buffer (pH 3.0), heating them in a microwave oven for 20 min, and allowing them to cool to room temperature for 20 min. Slides were rinsed once with phosphate buffered saline (PBS), and the endogenous peroxidase activity was blocked by incubating the samples for 30 min in the blocking solution (3% H2O2 in PBS), followed by rinsing three times with PBS. Non specific binding was blocked by incubating the slides for 30 min in PBS containing 2% normal goat serum (Vector Laboratories, Burlingame, CA) and 1% Triton X-100, followed by incubation with specific polyclonal anti parathyroid hormone receptor-1.
Reverse transcription-polymerase chain reaction RT-PCR)
Total RNA was isolated from rat kidney homogenates obtained using an RNeasy Tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The RNA aliquots were stored at -80°C until use. Total RNA from either rat kidney, was reverse transcribed, and resulting cDNA was amplified, using a Qiagen one step RT-PCR kits using specific primers for the rat PTHrP (sense 5΄-TGGTGTTCCTGCTCAGCTA-3΄, antisense 5΄-CCTCGTCGTCTGACCCAAA-3΄). These primers yield PCR amplification products of 266 bp. The housekeeping gene; glyceraldhyde-3-phosphate dehydrogenase (GAPDH) was amplified using specific primers (5΄-CCTTCATTGACCTCAACTACATG-3΄; reverse, 5΄-CTTCTCCATGGTGGTGAAGAC-3΄) as a constitutive control. The reaction mixture (10 μL), with 0.5 μCi (α 32P) dCTP (3000 Ci/mmol; NEN Life Science Products, Zaventem, Belgium), was incubated for 45 minutes at 48°C and two minutes at 95°C, followed by 30 cycles of one minute at 95°C, one minute at 60°C, and two minutes at 68°C, with a final extension of seven minutes at 68°C. Preliminary experiments established that these conditions provided a linear cDNA amplification in each case. Each PCR product was loaded onto agarose gel, visualized by ethidium bromide staining under ultraviolet light, and analyzed by scanning densitometry (ImageQuant; Molecular Dynamics, Sunnyvale, CA, USA) of the different PCR products were. The results are normalized against those of the corresponding GAPDH. Band intensities of RT–PCR products were quantified using Biometre Image software.
Statistical analysis
Data are expressed as mean ± SD for all parameters. The data were analyzed using Graph Pad Prism data analysis program (Graph Pad Software, Inc., San Diego, CA, USA). For comparison of statistical significance between different groups Student Newman-Keuls t-test for paired data were used. For multiple comparisons, one-way analysis of variance (ONE-WAY-ANOVA) test followed by the least Significant Difference (LST). Correlations were assessed using Spearman’s non-parametric correlation coefficient ƍ as described by Knapp and Miller [29]. A value of P ≤ 0.05 was considered statistically significant.
Results
Effect of AT1 antagonist on body weight gain
Cd intake had no significant effect on food intake of group I, II and III (25.46 ± 1.02, 25.92 ± 1.26 and 26.39 ± 1.23 g, respectively). No significant differences of water consumption between group I, II and III were detected (25.74 ± 0.52, 25.51 ± 0.72 and 25.14 ± 0.83 ml respectively). As a high Cd intake had no significant effect on food or water consumption, no significant differences between the studied groups have been observed for the body weight gain. In addition, Kidney weights of rats of all groups were not significantly different (Table 1).
Table 1.
Body weight gain and kidney weight of all studied groups
| Group I | Group II | Group III | |
|---|---|---|---|
| Body Weight gain (gm) | 31.55 ± 1.13 | 31.68 ± 0.66 | 31.36 ± 0.69 |
| Kidney Weight (gm) | 0.487 ± 0.007 | 0.486 ± 0.008 | 0.489 ± 0.012 |
Values are means ± SD. No significant differences have been observed between the body weight gain and kidney weights of all studied animals through the experiment.
Effect of AT1 antagonist on BUN and serum creatinine
Rats which received cadmium chloride alone, showed significant increase in BUN and serum creatinine as compared to the control group (p<0.001). However, significant reduction of these levels were observed in group III treated with telmisartan as compared to group II (p<0.01), but these levels were still significantly higher than those of control group (p<0.05) (Table 2).
Table 2.
Effect of telmisartan on Cd-induced changes in 24 hr urine volume, BUN and serum creatinine
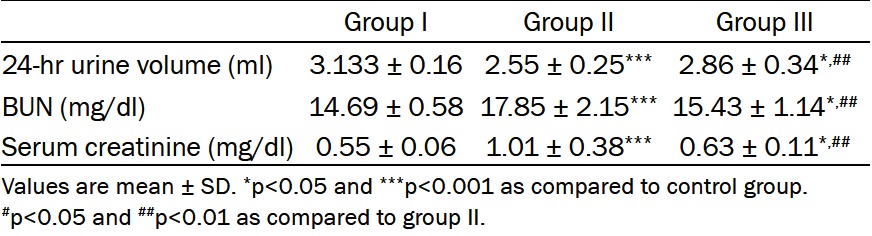
|
Effect of AT1 antagonist renal biochemical analysis
Telmisartan treatment significantly suppressed renal tissues levels of NO, lipid peroxidation (TBARS) and protein carbonyl compared to those of the group II (p<0.05, p<0.01 and p<0.01 respectively). In addition telmisartan attenuated the depletion of the antioxidant defense mechanisms (SOD and catalase activity) compared to Cd treated rats (p<0.01 and p<0.05 respectively) (Table 3).
Table 3.
Effects of telmisartan (TEL) treatment on renal tissue levels of cadmium, NO, TBARS, protien carbonyl and some enzymatic antioxidants in rats with nephrotoxicity induced by cadmium (Cd) chloride
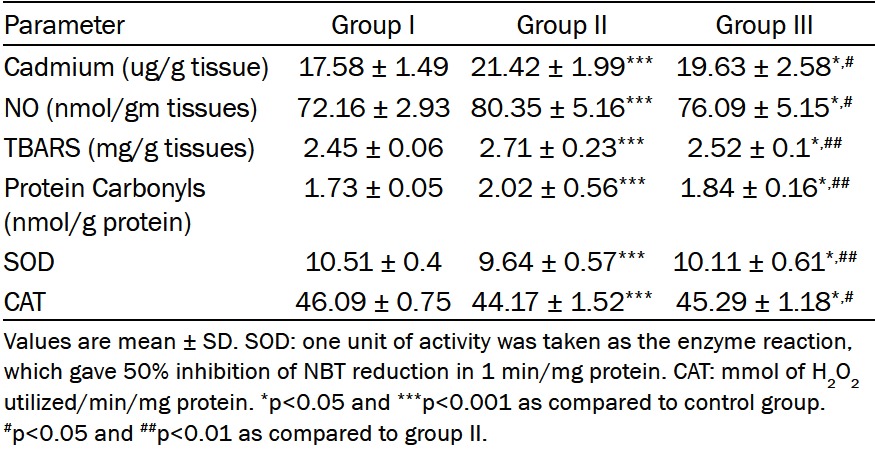
|
Effect of AT1 antagonist on renal toxicity markers
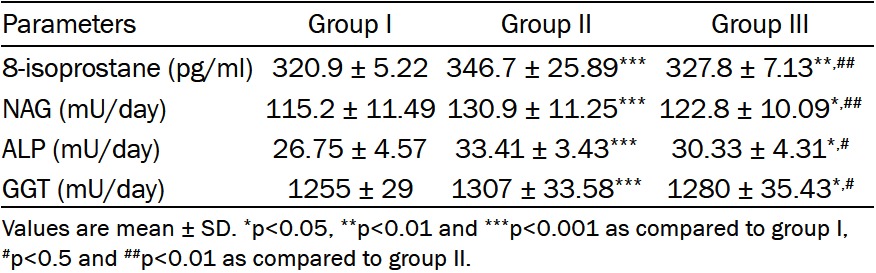
Group II had significant elevations in urinary levels of total protein (Figure 1B), 8-isoprostane, NAG, ALP and GGT compared with those of control rats (p<0.001). Telmisartan treatment significantly suppressed these elevations compared to those of group II (p<0.01, p<0.01, p<0.01, p<0.05 and p<0.05 respectively) (Table 4).
Figure 1.
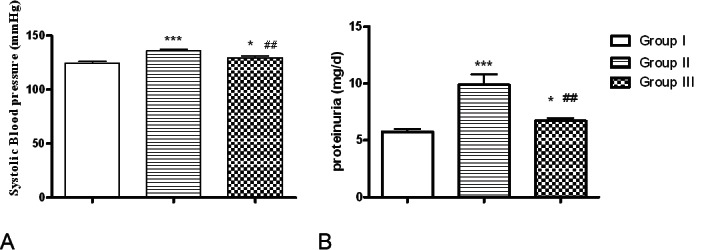
Systolic BP (A) and proteinuria (B) in control, group II and group III. Data are shown as mean ± SD. *P<0.05, ***P<0.001 versus control; ##P<0.01 versus group II.
Table 4.
Effect of telmisartan treatment on urinary levels of 8-sopostane, NAG, ALP and GGT in all studied groups
|
Effects of AT1 antagonist on renal cadmium concentrations
Rats that received cadmium chloride showed significant increase in cadmium ion concentrations as compared to the control animals (p<0.001). However, telmisartan treated rats had a significantly lower renal cadmium level in comparison with the group II (Table 3).
Effects of AT1 antagonist on serum and renal Ang II levels
Figure 2 showed that serum and renal Ang II levels in group II were significantly higher than those of group I (p<0.001). These levels were significantly lowered in group III after telmisartan treatment, but they were still significantly higher than those of control group (Figure 2).
Figure 2.
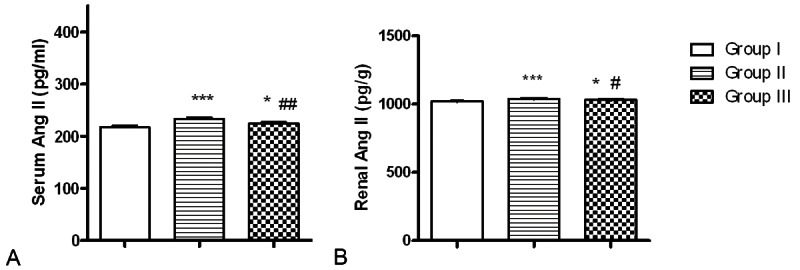
Effect of telmisartan treatment on serum and renal tissue levels of Angiotensin II of rats treated with cadmium for nine weeks. Values are mean ± SD. *p<0.05, **p<0.01 and ***p<0.001 as compared to control group. ##p<0.01 and #p<0.05 as compared to group II.
Effects of AT1 antagonist on systolic blood pressure
Systolic blood pressure of rats received Cd for nine weeks was significantly increased than those of Group I (p<0.001), whereas this increase was markedly lower in rats of group III compared to group II (p<0.01) (Figure 1A).
Effects of AT1 antagonist on renal histology
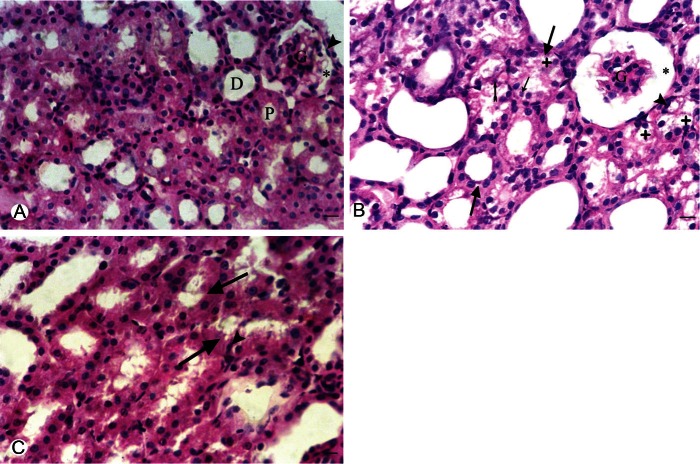
In general, the morphological examination of HE-stained sections of the kidneys of Cd treated rats showed that Cd effects were found largely confined to the proximal tubules; Cadmium intake for nine weeks caused widespread dilatation, vacuolar degeneration and necrosis (pyknotic and karyoylsed nuclei and others show exfoliation) in the cytoplasm of the proximal convoluted tubules. There was also glomerular atrophy with widening of Bowman’s space. The histopathological renal damage induced by cadmium was markedly ameliorated in animals received telmisartan treatment (Figure 3).
Figure 3.
A. Representive photomicroscopy of a section in the kidney of a control rat showing the renal corpuscle with the parietal layer of Bowmman’s capsule (arrow head). glomerular and normal Bowmann’s space (*). Normal appearance of the proximal (P) and distal (D) in control convulated tubules. B. Group II showing apparent glomerular atrophy (G) with widened Bowman’s space (*). Swelling of the renal tubules is observed (thick arrows). The proximal tubules showed marked vaculation of the cytoplasm (+), some nuclei in the proximal tubules appear pyknotic (arrow head) or karyolsed (thin rrow) and others show exfoliation (tailed arrow). C. Group III showing swelling of the renal tubules (thick arrows) and some proximal tubules showed pyknotic nuclei (arrow head). (H&E) X 400. Bar = 50 μm.
Effects of telmisartan on renal immunohistochemistry
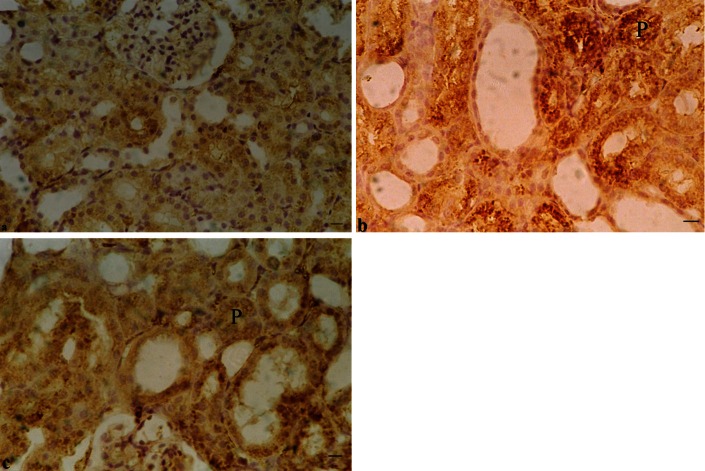
Immunohistochemical examinations of rats’ kidney revealed that cadmium administration caused marked increases in the immunoreactivity (highly positive reaction) of PTHR1 in the kidney of group II in the cytoplasm of proximal tubular cells as compared to the control group. On the other hand, the detection of PTHR1 in the renal tissues of group III demonstrates a weak positivity slightly higher than the control group (Figure 4).
Figure 4.
Immunohistochemical staining of sections from rat kidneys (X400). A. Normal expression of PTHR1 in the tubular epithelium of group I The glomerulus showed no positivity for PTHR1. B. Marked activation of PTHR1 (highly positive reaction) in the proximal tubules (P) by cadmium intake to adult male rats of group II for nine weeks. C. The detection of PTHR1 in the renal tissues of group III demonstrates a weak positivity slightly higher than the control group. Bar = 50 μm.
Effects of telmisartan on PTHrP expression
RT-PCR was used to investigate the effect of telmisartan administration to adult male rats on PTHrP transcripts in rat’s kidney. As shown in Figure 5A, Cd administration to adult male rats of group II augmented PTHR1 mRNA significantly than those of group I and III (p<0.001 and p<0.01 respectively). Significant differences between renal PTHrP mRNA of group I and III were detected (p<0.05. Specific bands of predicted length and increasing exponentially (at 266 bp) were obtained with PTHrP specific primers of predicted length in group II and this band decreased markedly in group III compared to group II (Figure 5B).
Figure 5.
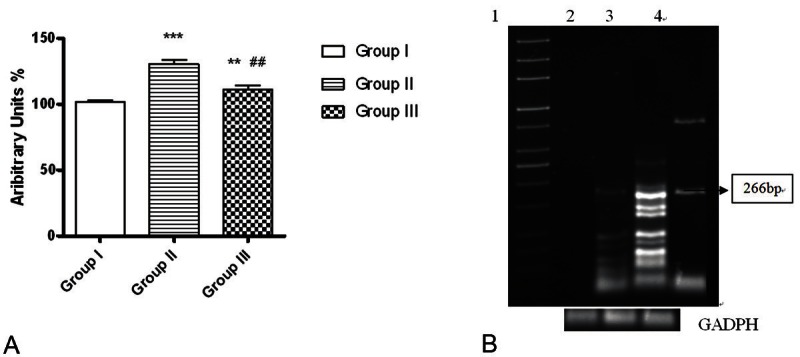
Representative graph corresponding to PTHrP mRNA changes (evaluated by RT-PCR) in the kidney of all studied groups. A. Telmisartan treatment decreased PTHrP mRNA in renal tissues of group III. B. PhosphorImager analysis of RT-PCR amplification using specific oligonucleotide primers for PTHrP mRNA in the kidney of control, group II and group III. Total RNA isolated from all Kidneys was used for RT-PCR reactions as described in Materials and Methods. Lane 1 from the left is the 1-kb ladder control; lane 2: group I, lanes 3: group II, and 4: group III correspond to the primers specific for PTHr-P at 266 bp. GAPDH mRNA was included as a constitutive control. *p<0.05,***p<0.001 as compared to control group. ##p<0.01 as compared to group II.
Discussion
The present study showed that telmisartan treatment significantly protected against renal tissue injury induced by cadmium in adult male rats. Also, the present work, in agreement with previous studies, clearly demonstrated that oxidative stress with increased lipid peroxidation (TARBS), depletion of antioxidant defenses play a crucial role in the pathogenesis of cadmium nephrotoxicity [30-32]. The results of the present study showed that renal levels of TARBS and protein carbonyl were significantly increased in group II than those of control rats suggesting increased renal oxidative stress of Cd treated rats. Telmisartan could decrease these levels significantly in group III. In addition telmisartan increased the levels of antioxidants in group III significantly than those of group II.
It has been demonstrated that increased NO production is implicated in cadmium-mediated cytotoxicity and oxidative damage [17,33]. This was supported by results of this study as levels of renal tissues NO of group II were significantly higher than those of control group. Telmisartan could decrease these levels significantly than those of Cd-induced nephrotoxicity. In the present study, telmisartan significantly attenuated the increase in cadmium concentration in renal tissue resulted from cadmium administration. This was explained the fact that cadmium can be bound with high affinity to the thiol in glutathione and metallothionein molecules which are essential for intracellular heavy metal detoxification [34,35]. It could be also stated that telmisartan, through its antioxidant activity, decreased the cadmium burden in renal tissue which results in an additional protective effect against cadmium nephrotoxicity.
This study also showed that there was a significant reduction in the 24 h urine volume of group II. This is in consonance with previous studies and confirm that cadmium administration causes declined renal function, in the form of increased serum creatinine and BUN [36,37]. Administration of telmisartan for group III improved cadmium- induced renal dysfunction. This is consistent with Fouad and Jersat [17] and could suggest that AT1 blocker improves renal function by preventing renal damage.
Urine NAG, ALP and ALP enzymes activities mainly come from the renal tubules and are regarded as sensitive indexes of renal tubular function [38]. The results of this experiment showed that the levels of these enzymes activities in the Cd only group were significantly higher than those in the control group at the end of ninths week. These results indicated that Cd treatment alone had caused appreciable kidney damage. However, the study revealed that telmisartan has a marked protective effect on renal tubular toxicity, as rats receiving TEL in addition to Cd showed a markedly decreased excretion of NAG, GGT and ALP, thus suggesting a decreased tubular damage. This protective effect of TEL was confirmed when renal tissues were examined histologically, as minor tubular cellular alterations were observed in these animals, compared with those that has received Cd alone.
Varoni et al [39] found that the activation of the renin–angiotensin system has been indicated to be involved in the pathophysiology of tissue injury induced by cadmium. These facts were supported by the present results as telmisartan decreased the elevated renal and serum levels of angiotensin II in group III. Ang II, the most active factor in this system [40] is considered to be the main source of cadmium-induced production of reactive oxygen species [41,42]. The elevation of Ang II levels in Cd administrated rats was explained by the interplay between the kallikrein-kinin system and the RAS which caused various hemodynamic alterations, resulting in elevated RAS activity in these animals. Moreover, the low levels of kallikrein displayed elevated concentrations of plasma AII and initial alterations in the renal glomerulotubular system [39].
Treatment with telmisartan could significantly decrease the elevated blood pressure recorded in Cd treated rats. This could be explained by that telmisartan lowers the elevated levels of Ang II in Cd treated rats, leading to decrease significantly the blood pressure of group III.
The present study observed increased both the renal PTHrP and PTHR1 expression of Cd group. Telmisartan treatment significantly inhibited the cadmium-induced expression of PTHrP and PTHR1. This was explained by Ortego et al [43] who reported that Ang II interacts with its type 1 receptor (AT1) which activates mitogen-activated protein kinase and the transcription factor cAMP-responsive element binding protein, leading to increased PTHrP expression. In group III the Ang II antagonists (TEL) improved the renal functions and this improvement was associated with inhibition of PTHrP and PTHR1 expression. Thus, PTHrP and its receptors appears to exert a reciprocal control on, some Ang II effects in the damaged kidney [44]. In addition, Lorenzo et al [45] reported a significant correlation between PTHrP and tubular damage in the rat kidney after systemic Ang II infusion. These data suggest that Ang II considered a responsible factor for PTHrP up-regulation in Cd treated rats, and this might contribute to the deleterious effects of Ang II in the Cd induced nephrotoxicity.
Conclusions
Collectively, the present study indicates that AT1 blocker significantly protected against cadmium-induced renal dysfunction. The antioxidant effect of telmisartan, and its role in inhibition of PTHrP overexpression can be considered the main factors responsible for the renoprotective effect of telmisartan. Therefore, telmisartan represents a potential therapeutic option to prevent renal tissue damage and dysfunction resulting from cadmium intoxication in adult male rats. These data suggest that Ang II might contribute to the deleterious effects of cadmium induced nephrotoxicity.
Acknowledgments
The author wishes to thanks Dr. Mostafa Gaber Mohamed, Professor of Medical Physiology, Faculty of Medicine, Assiut University, Egypt for his careful revision of the manuscript. Also the author thanks Dr. Heba Kamal Mohamed, Lecturer of Anatomy, Anatomy Department, Faculty of Medicine, Assiut University, Assiut, Egypt for her kind help in morphological and histological assessments.
References
- 1.Jacquillet G, Barbier O, Rubera I, Tauc M, Borderie A, Namorado MC, Martin D, Sierra G, Reyes JL, Poujeo P, Cougnon M. Cadmium causes delayed effects on renal function in the offspring of cadmium-contaminated pregnant female rats. Am J Physiol Renal Physiol. 2007;293:F1450–F1460. doi: 10.1152/ajprenal.00223.2007. [DOI] [PubMed] [Google Scholar]
- 2.Jarup L, Hellstrom L, Alfven T, Carlsson MD, Grubb A, Persson B. Low level exposure to cadmium and early kidney damage. The OSCAR Study. Occup Environ Med. 2000;57:668–672. doi: 10.1136/oem.57.10.668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chia SE, Xu B, Ong CN, Tsakok FM, Lee ST. Effect of cadmium and cigarette smoking on human semen quality. Int J Fertil Menopausal Stud. 1994;39:292–298. [PubMed] [Google Scholar]
- 4.Lee Wk, Thѐvenod F. Novel roles for ceramides, calpains and caseases in kidney proximal tubule cell apoptosis: Lessons from in vitro cadmium toxicity studies. Biochem Pharmacol. 2008;76:1323–1332. doi: 10.1016/j.bcp.2008.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Fang J, Leonard SS, Rao KMK. Cadmium inhibits electron transfer chain and induced reactive oxygen species. Free Radical Biol Med. 2004;36:1434–1443. doi: 10.1016/j.freeradbiomed.2004.03.010. [DOI] [PubMed] [Google Scholar]
- 6.Brzoska MM, Kaminski M, Supernak-Bobko D. Changes in the structure and function of the kidney of rats chronically exposed to Cd. I Biochemical and histopathological studies. Arch Toxicol. 2003;77:344–352. doi: 10.1007/s00204-003-0451-1. [DOI] [PubMed] [Google Scholar]
- 7.Hu H. Exposure to metals. Prim Care. 2000;27:983–996. doi: 10.1016/s0095-4543(05)70185-8. [DOI] [PubMed] [Google Scholar]
- 8.Asar M, kayisli UA, Izgüt-uysal VN, Akkoyunlu G. Immunohistochemical and ultrastructural changes in the renal cortex of cadmium-treated rats. Biol Trace Elem Res. 2004;79:249–263. doi: 10.1385/BTER:97:3:249. [DOI] [PubMed] [Google Scholar]
- 9.Usdin TB, Gruber C, Bonner TI. Identification and functional expression of a receptor selectively recognizing parathyroid hormone, the PTH2 receptor. J Biol Chem. 1995;270:15455–15458. doi: 10.1074/jbc.270.26.15455. [DOI] [PubMed] [Google Scholar]
- 10.Jüppner H, Abou-Samra A, Freeman MW, Kong XF, Schipani E, Richards J, Kolakowski LF, Hock J, Potts JT, Kronenberg HM, Segre GV. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991;254:1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- 11.Clemens TL, Cormier S, Eichinger A, Endlich K, Fiaschi-Taesch N, Fischer E, Friedman PA, Karaplis AC, Massfelder T, Rossert J, Schlüter KD, Silve C, Stewart AF, Takane K, Helwig JJ. Parathyroid hormone-related protein and its receptors: nuclear functions and roles in the renal and cardiovascular systems, the placental trophoblasts and the pancreatic islets. Br J Pharmacol. 2001;134:1113–1136. doi: 10.1038/sj.bjp.0704378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Endlich N, Nobiling R, Kriz W, Endlich K. Expression and signaling of parathyroid hormone-related protein in cultured podocytes. Exp Nephrol. 2001;9:436–443. doi: 10.1159/000052643. [DOI] [PubMed] [Google Scholar]
- 13.Cao Z, Bonnet F, Candido R, Nesteroff SP, Wendy C, Burns WC, Kawachi H, Shimizu F, Carey RM, Gasparo MD, Cooper ME. Angiotensin Type 2 Receptor Antagonism Confers Renal Protection in a Rat Model of Progressive Renal Injury. J Am Soc Nephrol. 2002;13:1773–1787. doi: 10.1097/01.asn.0000019409.17099.33. [DOI] [PubMed] [Google Scholar]
- 14.Benson SC, Pershadsingh HA, Ho CI, Chittiboyina A, Desai P, Pravenec M, Qi N, Wang J, Avery MA, Kurtz TW. Identification of telmisartan as a unique angiotensin II receptor antagonist with selective PPARγ-modulating activity. Hypertension. 2004;43:993–1002. doi: 10.1161/01.HYP.0000123072.34629.57. [DOI] [PubMed] [Google Scholar]
- 15.Barnett AH. Preventing renal complications in diabetic patients: the diabetics exposed to telmisartan and enalapril (DETAIL) study. Acta Diabetol. 2005;42:S42–49. doi: 10.1007/s00592-005-0180-4. [DOI] [PubMed] [Google Scholar]
- 16.Aranda P, Segura J, Ruilope LM, Aranda FJ, Frutos MA, López V, López de Novales E. Long-term renoprotective effects of standard versus high doses of telmisartan in hypertensive non-diabetic nephropathies. Am J Kidney Dis. 2005;46:1074–1079. doi: 10.1053/j.ajkd.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 17.Fouad AA, Jresat I. Protective effect of telmisartan against cadmium-induced nephrotoxicity in mice. Life Sci. 2011;89:29–35. doi: 10.1016/j.lfs.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 18.Renugadevi J, Prabu SM. Naringenin protects against cadmium-induced oxidative renal dysfunction in rats. Toxicology. 2009;256:128–134. doi: 10.1016/j.tox.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 19.Tsunenari I, Ohmura T, Seidler R, Chachin M, Hayashi T, Konomi A, Matsumaru T, Sumida T, Hayashi N, Horie Y. Renoprotective effects of telmisartan in the 5/6 nephrectomised rats. J Renin Angiotensin Aldosterone Syst. 2007;8:93–100. doi: 10.3317/jraas.2007.017. [DOI] [PubMed] [Google Scholar]
- 20.Ding AH, Nathan CF, Stuehr DJ. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988;14:2407–2412. [PubMed] [Google Scholar]
- 21.Niehiaus WG, Samuelsson D. Formation of malondialdehyde from phospholipid arachidonate during microsomal lipid peroxidation. Eur J Biochem. 1968;6:126–30. doi: 10.1111/j.1432-1033.1968.tb00428.x. [DOI] [PubMed] [Google Scholar]
- 22.Levine RL, Garland D, Oliver CN, Amic A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER. Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol. 1990;186:464–478. doi: 10.1016/0076-6879(90)86141-h. [DOI] [PubMed] [Google Scholar]
- 23.Badr KF, Abi-Antoun TE. Isoprostanes and the kidney. Antioxid Redox Signal. 2005;7:236–243. doi: 10.1089/ars.2005.7.236. [DOI] [PubMed] [Google Scholar]
- 24.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur J Biochem. 1975;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 25.Sinha AK. Calorimetric assay of catalase. Anal Biochem. 1972;47:389–394. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 26.Morales AI, Vicente-Sa´nchez C, Santiago Sandoval JM, Egido J, Mayoral P, Are´valo MA, Ferna´ndez-Tagarro M, Lo´pez-Novoa JM, Pe´rez-Barriocanal F. Protective effect of quercetin on experimental chronic cadmium nephrotoxicity in rats is based on its antioxidant properties. Food Chem Toxicol. 2006;44:2092–2100. doi: 10.1016/j.fct.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 27.Haouem S, Sakly R. Lactational transfer of cadmium from Meriones shawi shawi mothers to their pups and its effects on calcium homoeostasis and bone calcium in pups. Ann Nutr Metab. 2005;49:296–299. doi: 10.1159/000087296. [DOI] [PubMed] [Google Scholar]
- 28.Danser A, Van Kats JP, Admiraln P, Derkx F, Lamers J, Verdouw PD, Saxena PR, Schalekamp MA. Cardiac renin and angiotensins: uptake from plasma versus in situ synthesis. Hypertension. 1994;24:37–48. doi: 10.1161/01.hyp.24.1.37. [DOI] [PubMed] [Google Scholar]
- 29.Knapp GR, Miller MC. Clinical Epidemiology and Biostatistics. 1st Edition. Baltimoe, Maryland: Williams and Wilkins; 1992. Tests of statistical significance: Regression and Correlation; pp. 255–274. [Google Scholar]
- 30.Thévenod F. Nephrotoxicity and the proximal tubule. Insights from cadmium. Nephron Physiol. 2003;93:p87–93. doi: 10.1159/000070241. [DOI] [PubMed] [Google Scholar]
- 31.Manna P, Sinha M, Sil PC. Taurine plays a beneficial role against cadmium-induced oxidative renal dysfunction. Amino Acids. 2009;36:417–428. doi: 10.1007/s00726-008-0094-x. [DOI] [PubMed] [Google Scholar]
- 32.Fouad AA, Qureshi HA, Al-Sultan AI, Yacoubi MT, Ali AA. Protective effect of hemin against cadmium-induced testicular damage in rats. Toxicology. 2009;257:153–160. doi: 10.1016/j.tox.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 33.Chen L, Zhou J, Gao W, Jiang YZ. Action of NO and TNF-alpha release of rats with cadmium loading in malfunction of multiple system organ. Sheng Li Xue Bao. 2003;55:535–540. [PubMed] [Google Scholar]
- 34.Satoh M, Shimada A, Zhang B, Tohyama C. Renal toxicity caused by cisplatinum in glutathione-depleted metallothionein-null mice. Biochem Pharmacol. 2000;60:1729–1734. doi: 10.1016/s0006-2952(00)00478-0. [DOI] [PubMed] [Google Scholar]
- 35.Rooney JP. The role of thiols, dithiols, nutritional factors and interacting ligands in the toxicology of mercury. Toxicology. 2007;234:145–156. doi: 10.1016/j.tox.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Akesson AT, Lundh M. Tubular and glomerular kidney effects in Swedish women with low environmental cadmium exposure. Environ Health Perspect. 2005;113:1627–1631. doi: 10.1289/ehp.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ige SF, Salawu EO, Olaleye SB, Adeeyo OA, Badmus J, Adeteke AA. Onion (Alluim cepa) extract prevents cadmium-induced renal dysfunction. Indian J Nephrol. 2009;19:140–144. doi: 10.4103/0971-4065.59334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu B, Xu ZF, Deng Y, Yang JH. Protective effects of Chlorpromazine and Verapamil against cadmium-induced kidney damage in vivo. Exp Toxicol Pathol. 2010;62:27–34. doi: 10.1016/j.etp.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 39.Varoni MV, Palomba D, Macciotta NP, Antuofermo E, Deiana G, Baralla E. Brain renin–angiotensin system modifies the blood pressure response to intracerebroventricular cadmium in rats. Drug Chem Toxicol. 2010;33:302–309. doi: 10.3109/01480540903418496. [DOI] [PubMed] [Google Scholar]
- 40.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 41.Souza V, Escobar Mdel C, Bucio L, Hernández E, Gómez-Quiroz LE, Gutiérrez Ruiz MC. NADPH oxidase and ERK1/2 are involved in cadmium induced-STAT3 activation in HepG2 cells. Toxicol Lett. 2009;187:180–186. doi: 10.1016/j.toxlet.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 42.Banakou E, Dailianis S. Involvement of Na+/H+ exchanger and respiratory burst enzymes NADPH oxidase and NO synthase, in Cd-induced lipid peroxidation and DNA damage in hemocytes of mussels. Comp Biochem Physiol C Toxicol Pharmacol. 2010;152:346–352. doi: 10.1016/j.cbpc.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 43.Ortega A, Rámila D, Izquierdo A, González L, Barat A, Gazapo R, Bosch RJ, Esbrit P. Role of the renin–angiotensin system on the parathyroid hormone-related protein overexpression induced by nephrotoxic acute renal failure in the rat. J Am Soc Nephrol. 2005;16:939–949. doi: 10.1681/ASN.2004040328. [DOI] [PubMed] [Google Scholar]
- 44.Izquierdo A, López-Luna P, Ortega A, Romero M, Guitiérrez-Tarrés MA, Arribas I, Alvarez MJ, Esbrit P, Bosch RJ. The parathyroid hormone-related protein system and diabetic nephropathy outcome in streptozotocin-induced diabetes. Kidney Int. 2006;69:2171–7. doi: 10.1038/sj.ki.5000195. [DOI] [PubMed] [Google Scholar]
- 45.Lorenzo O, Ruiz-Ortega M, Esbrit P, Rupérez M, Ortega A, Santos S, Blanco J, Ortega L, Egido J. Angiotensin II increases parathyroid hormone-related protein (PTHrP) and the type 1 PTH/PTHrP receptor in the kidney. J Am Soc Nephrol. 2002;13:1595–1607. doi: 10.1097/01.asn.0000015622.33198.bf. [DOI] [PubMed] [Google Scholar]