Abstract
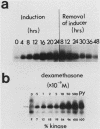
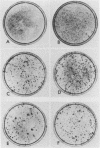
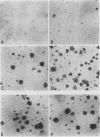
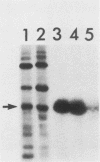
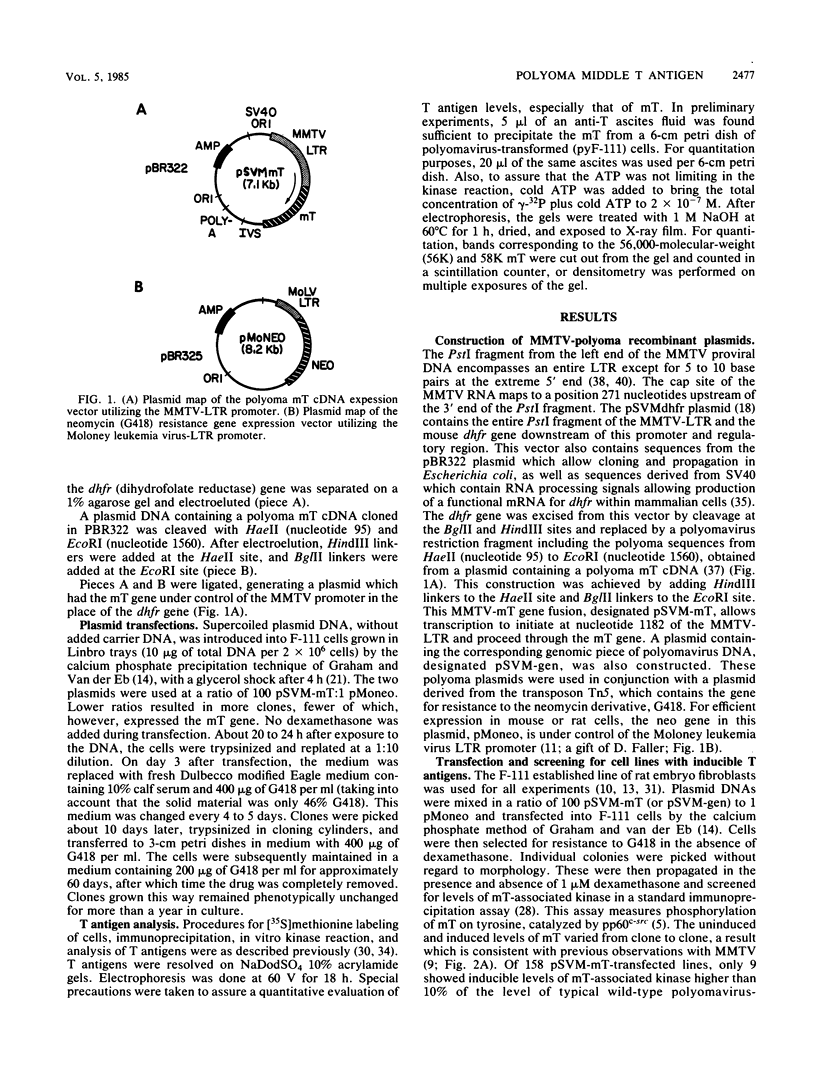
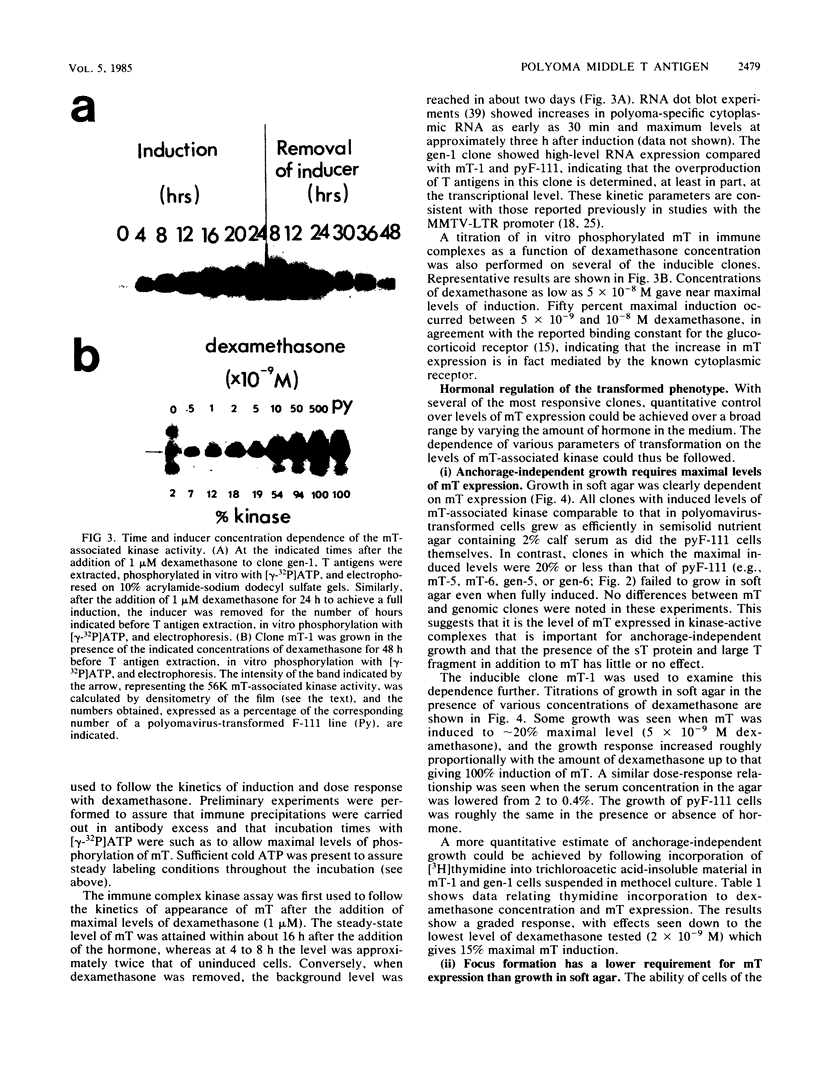
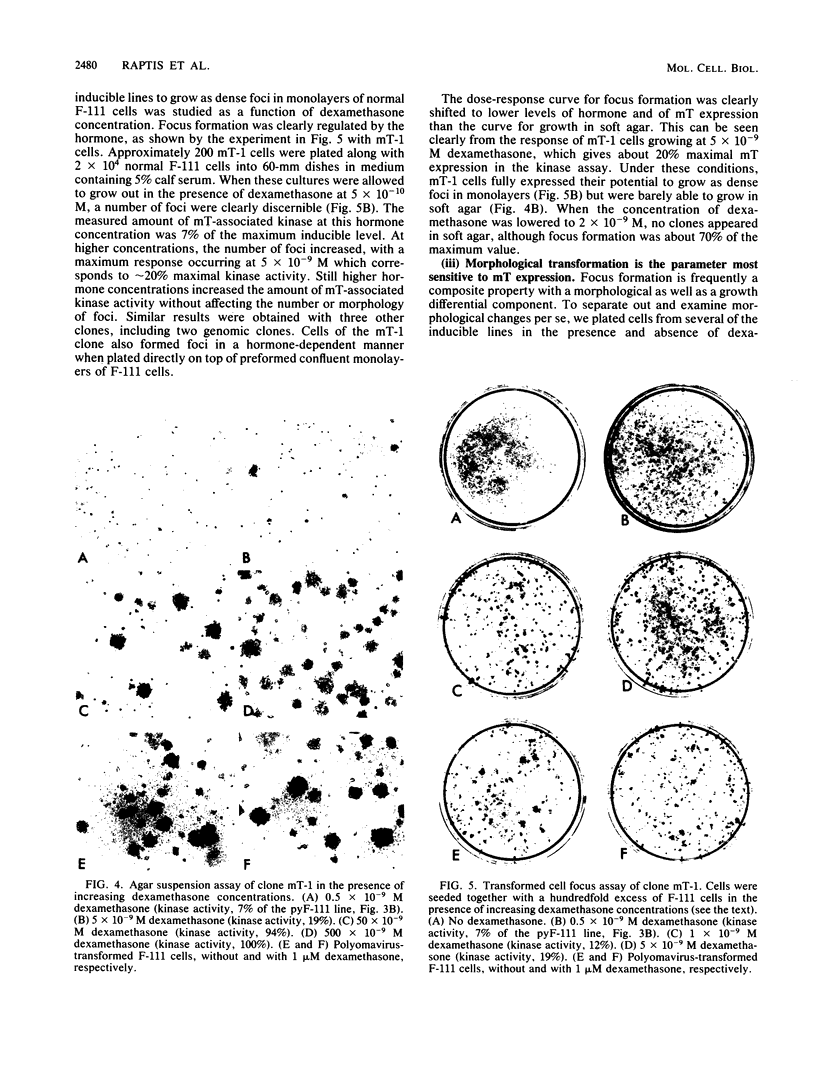
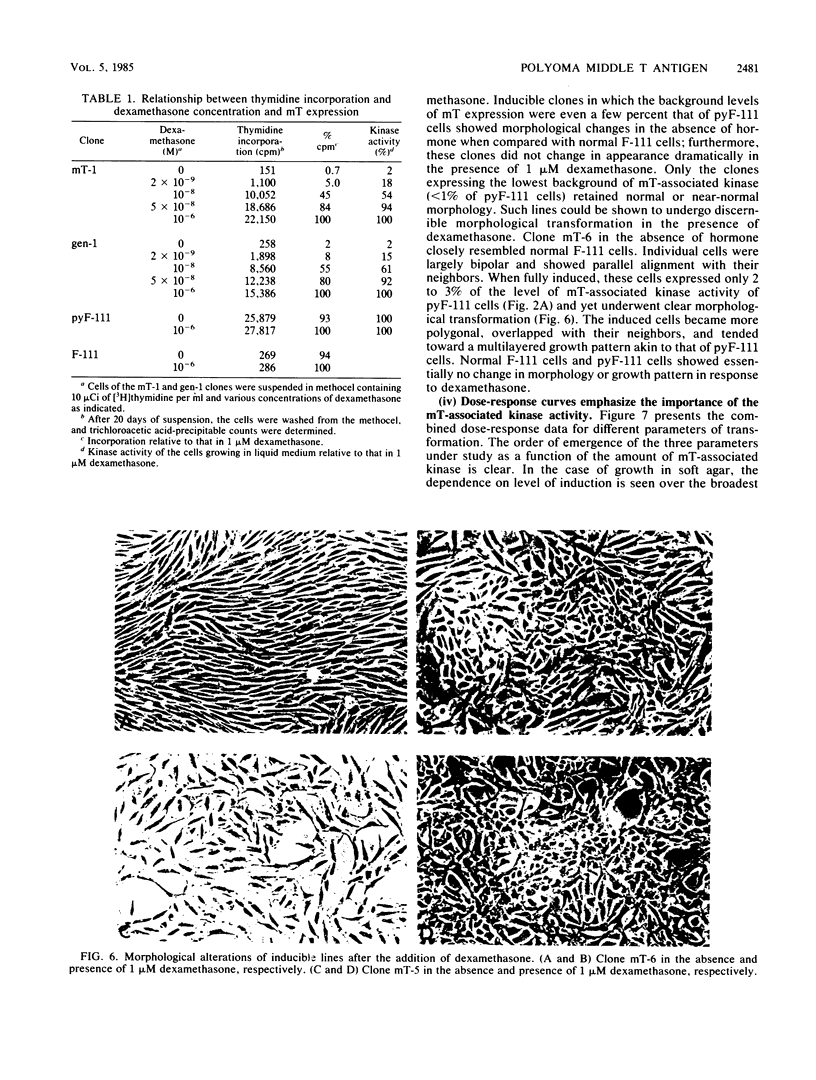
Polyoma middle T antigen (mT) was expressed in rat F-111 cells under control of the dexamethasone-regulatable mouse mammary tumor virus promoter. Graded phenotypic responses to levels of mT induction by the hormone were seen, with morphological transformation, focus formation, and anchorage-independent growth requiring increasing levels of mT expression. The ability of different clones to form tumors reflected their maximum level of induction of mT-associated kinase and their ability to grow in soft agar. Expression of transformation parameters and tumorigenicity correlates with the level of mT phosphorylated by pp60c-src in immune complexes and not with the total amount of mT determined by metabolic labeling. We suggest that cellular factors regulate mT activity by forming a kinase-active fraction of mT molecules that controls the transformed state.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Becker D., Kurth R., Critchley D., Friis R., Bauer H. Distinguishable transformation-defective phenotypes among temperature-sensitive mutants of Rous sarcoma virus. J Virol. 1977 Mar;21(3):1042–1055. doi: 10.1128/jvi.21.3.1042-1055.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin T. L. The hr-t gene of polyoma virus. Biochim Biophys Acta. 1982 Dec 21;695(2):69–95. doi: 10.1016/0304-419x(82)90018-x. [DOI] [PubMed] [Google Scholar]
- Bolen J. B., Thiele C. J., Israel M. A., Yonemoto W., Lipsich L. A., Brugge J. S. Enhancement of cellular src gene product associated tyrosyl kinase activity following polyoma virus infection and transformation. Cell. 1984 Oct;38(3):767–777. doi: 10.1016/0092-8674(84)90272-1. [DOI] [PubMed] [Google Scholar]
- Carmichael G. G., Schaffhausen B. S., Dorsky D. I., Oliver D. B., Benjamin T. L. Carboxy terminus of polyoma middle-sized tumor antigen is required for attachment to membranes, associated protein kinase activities, and cell transformation. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3579–3583. doi: 10.1073/pnas.79.11.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. Polyoma virus transforming protein associates with the product of the c-src cellular gene. Nature. 1983 Jun 2;303(5916):435–439. doi: 10.1038/303435a0. [DOI] [PubMed] [Google Scholar]
- Cuzin F. The polyoma virus oncogenes. Coordinated functions of three distinct proteins in the transformation of rodent cells in culture. Biochim Biophys Acta. 1984 Apr 5;781(3):193–204. doi: 10.1016/0167-4781(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Eckhart W., Hutchinson M. A., Hunter T. An activity phosphorylating tyrosine in polyoma T antigen immunoprecipitates. Cell. 1979 Dec;18(4):925–933. doi: 10.1016/0092-8674(79)90205-8. [DOI] [PubMed] [Google Scholar]
- Feinstein S. C., Ross S. R., Yamamoto K. R. Chromosomal position effects determine transcriptional potential of integrated mammary tumor virus DNA. J Mol Biol. 1982 Apr 15;156(3):549–565. doi: 10.1016/0022-2836(82)90266-2. [DOI] [PubMed] [Google Scholar]
- Fluck M. M., Benjamin T. L. Comparisons of two early gene functions essential for transformation in polyoma virus and SV-40. Virology. 1979 Jul 15;96(1):205–228. doi: 10.1016/0042-6822(79)90185-5. [DOI] [PubMed] [Google Scholar]
- Flyer D. C., Burakoff S. J., Faller D. V. Cytotoxic T lymphocyte recognition of transfected cells expressing a cloned retroviral gene. 1983 Oct 27-Nov 2Nature. 305(5937):815–818. doi: 10.1038/305815a0. [DOI] [PubMed] [Google Scholar]
- Freedman V. H., Shin S. I. Cellular tumorigenicity in nude mice: correlation with cell growth in semi-solid medium. Cell. 1974 Dec;3(4):355–359. doi: 10.1016/0092-8674(74)90050-6. [DOI] [PubMed] [Google Scholar]
- Freeman A. E., Gilden R. V., Vernon M. L., Wolford R. G., Hugunin P. E., Huebner R. J. 5-Bromo-2'-deoxyuridine potentiation of transformation of rat-embryo cells induced in vitro by 3-methylcholanthrene: induction of rat leukemia virus gs antigen in transformed cells. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2415–2419. doi: 10.1073/pnas.70.8.2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hager G. L. Expression of a viral oncogene under control of the mouse mammary tumor virus promoter: a new system for the study of glucocorticoid regulation. Prog Nucleic Acid Res Mol Biol. 1983;29:193–203. doi: 10.1016/s0079-6603(08)60447-x. [DOI] [PubMed] [Google Scholar]
- Huang A. L., Ostrowski M. C., Berard D., Hager G. L. Glucocorticoid regulation of the Ha-MuSV p21 gene conferred by sequences from mouse mammary tumor virus. Cell. 1981 Dec;27(2 Pt 1):245–255. doi: 10.1016/0092-8674(81)90408-6. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Majors J. E., Varmus H. E. Hormonal regulation of the Rous sarcoma virus src gene via a heterologous promoter defines a threshold dose for cellular transformation. Cell. 1984 Oct;38(3):757–765. doi: 10.1016/0092-8674(84)90271-x. [DOI] [PubMed] [Google Scholar]
- Lee F., Mulligan R., Berg P., Ringold G. Glucocorticoids regulate expression of dihydrofolate reductase cDNA in mouse mammary tumour virus chimaeric plasmids. Nature. 1981 Nov 19;294(5838):228–232. doi: 10.1038/294228a0. [DOI] [PubMed] [Google Scholar]
- Liang T. J., Carmichael G. G., Benjamin T. L. A polyoma mutant that encodes small T antigen but not middle T antigen demonstrates uncoupling of cell surface and cytoskeletal changes associated with cell transformation. Mol Cell Biol. 1984 Dec;4(12):2774–2783. doi: 10.1128/mcb.4.12.2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J., Ringold G. M. Use of the mouse mammary tumor virus long terminal repeat to promote steroid-inducible expression of v-mos. J Virol. 1984 Nov;52(2):420–430. doi: 10.1128/jvi.52.2.420-430.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rassoulzadegan M., Cowie A., Carr A., Glaichenhaus N., Kamen R., Cuzin F. The roles of individual polyoma virus early proteins in oncogenic transformation. Nature. 1982 Dec 23;300(5894):713–718. doi: 10.1038/300713a0. [DOI] [PubMed] [Google Scholar]
- Rassoulzadegan M., Naghashfar Z., Cowie A., Carr A., Grisoni M., Kamen R., Cuzin F. Expression of the large T protein of polyoma virus promotes the establishment in culture of "normal" rodent fibroblast cell lines. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4354–4358. doi: 10.1073/pnas.80.14.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risser R., Pollack R. A nonselective analysis of SV40 transformation of mouse 3T3 cells. Virology. 1974 Jun;59(2):477–489. doi: 10.1016/0042-6822(74)90457-7. [DOI] [PubMed] [Google Scholar]
- Robertson D. L., Varmus H. E. Dexamethasone induction of the intracellular RNAs of mouse mammary tumor virus. J Virol. 1981 Dec;40(3):673–682. doi: 10.1128/jvi.40.3.673-682.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Benjamin T. L. Phosphorylation of polyoma T antigens. Cell. 1979 Dec;18(4):935–946. doi: 10.1016/0092-8674(79)90206-x. [DOI] [PubMed] [Google Scholar]
- Schaffhausen B. S., Dorai H., Arakere G., Benjamin T. L. Polyoma virus middle T antigen: relationship to cell membranes and apparent lack of ATP-binding activity. Mol Cell Biol. 1982 Oct;2(10):1187–1198. doi: 10.1128/mcb.2.10.1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B. S., Silver J. E., Benjamin T. L. Tumor antigen(s) in cell productively infected by wild-type polyoma virus and mutant NG-18. Proc Natl Acad Sci U S A. 1978 Jan;75(1):79–83. doi: 10.1073/pnas.75.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L. Comparison of phosphorylation of two polyoma virus middle T antigens in vivo and in vitro. J Virol. 1981 Oct;40(1):184–196. doi: 10.1128/jvi.40.1.184-196.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaffhausen B., Benjamin T. L., Lodge J., Kaplan D., Roberts T. M. Expression of polyoma early gene products in E. coli. Nucleic Acids Res. 1985 Jan 25;13(2):501–519. doi: 10.1093/nar/13.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel R., Benjamin T. L. Cellular alterations dependent upon the polyoma virus Hr-t function: separation of mitogenic from transforming capacities. Cell. 1978 Jul;14(3):587–599. doi: 10.1016/0092-8674(78)90244-1. [DOI] [PubMed] [Google Scholar]
- Segawa K., Ito Y. Differential subcellular localization of in vivo-phosphorylated and nonphosphorylated middle-sized tumor antigen of polyoma virus and its relationship to middle-sized tumor antigen phosphorylating activity in vitro. Proc Natl Acad Sci U S A. 1982 Nov;79(22):6812–6816. doi: 10.1073/pnas.79.22.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seif R., Cuzin F. Temperature-sensitive growth regulation in one type of transformed rat cells induced by the tsa mutant of polyoma virus. J Virol. 1977 Dec;24(3):721–728. doi: 10.1128/jvi.24.3.721-728.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Schaffhausen B., Benjamin T. Tumor antigens induced by nontransforming mutants of polyoma virus. Cell. 1978 Oct;15(2):485–496. doi: 10.1016/0092-8674(78)90018-1. [DOI] [PubMed] [Google Scholar]
- Subramani S., Mulligan R., Berg P. Expression of the mouse dihydrofolate reductase complementary deoxyribonucleic acid in simian virus 40 vectors. Mol Cell Biol. 1981 Sep;1(9):854–864. doi: 10.1128/mcb.1.9.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Templeton D., Eckhart W. Characterization of viable mutants of polyomavirus cold sensitive for maintenance of cell transformation. J Virol. 1984 Mar;49(3):799–805. doi: 10.1128/jvi.49.3.799-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. A., Bancroft F. C. Cytoplasmic dot hybridization. Simple analysis of relative mRNA levels in multiple small cell or tissue samples. J Biol Chem. 1982 Aug 10;257(15):8569–8572. [PubMed] [Google Scholar]
- Yamamoto K. R., Payvar F., Firestone G. L., Maler B. A., Wrange O., Carlstedt-Duke J., Gustafsson J. A., Chandler V. L. Biological activity of cloned mammary tumor virus DNA fragments that bind purified glucocorticoid receptor protein in vitro. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):977–984. doi: 10.1101/sqb.1983.047.01.111. [DOI] [PubMed] [Google Scholar]