Abstract
Fenofibrate is a peroxisome proliferator activated receptor alpha agonist that contains both pro and anti-inflammatory properties, and has been used in the treatment of dyslipidemia and diabetes for decades. Its receptors are expressed in the liver, skeletal muscle, cardiac, enteric, and renal cells, which allow it to provide systemic regulation of lipoprotein metabolism, fatty acid oxidation, and fatty acid transport. Hyperglycemia is a common complication found in the burn population because hepatic glucose production and catecholamine-mediated hepatic glycogenolysis are augmented. Insulin resistance occurs often in these patients and is associated with poor outcomes. In the pediatric burn population, fenofibrate has been found to ameliorate or decrease the number of hypoglycemic episodes when compared to management with insulin alone. Its mechanism of action is thought to involve an improvement in insulin signaling in skeletal muscle, as well as improvements in mitochondrial function, glucose oxidation, and insulin sensitivity. The long term use of fenofibrate in severely burned patients may improve hyperglycemia and insulin resistance, as well as improve wound healing, and reduce apoptosis, and oxidative stress.
Keywords: Fenofibrate, PPAR, burns, hypermetabolism, lipolysis, insulin
1. Physiology of peroxisome proliferator activated receptors
Peroxisome proliferator activated receptors (PPARs) are part of the nuclear steroid hormone receptor superfamily and consists of three different isoforms: PPAR-alpha, PPAR-gamma, and PPAR-delta. These hormone receptors contribute to the regulation of lipid and glucose homeostasis via activation by ligand-dependent and ligand-independent mechanisms involving fatty acids, anti-diabetic and anti-lipid drugs, and various metabolites [1–5]. PPARs also have both pro-inflammatory and anti-inflammatory properties, and play roles in the pathogenesis of atherosclerosis, diabetes, obesity and hypertension [3, 5]. They are highly expressed in the liver, where anti-lipid drugs such as fibrates act on these receptors to regulate lipoprotein metabolism, fatty acid oxidation, and fatty acid transport within hepatic cells [6].
PPAR-alpha acts as a transcription factor and is expressed in cells with fatty acid oxidative capacity. Animal studies have shown that this isoform regulates the oxidation of fatty acid and amino acid metabolism in the liver, as well as in cardiac, enteric, and renal cells [2, 4]. PPAR-alpha exerts activity in skeletal muscle, where more than eighty percent of whole body glucose disposal occurs [7]. PPAR-alpha regulates the effects of anti-lipid drugs, such as fibrates, on gene expression. In diabetic mice, PPAR-alpha influences glucose homeostasis by sensing feeding status and inducing metabolic adjustments to reduce endogenous glucose production [2]. Patsouris et al. suggest that PPAR-alpha regulates genes involved in gluconeogenesis and that free fatty acids (FFA) and glycerol bind to these receptors to activate glycerol’s entrance into hepatocytes [2].
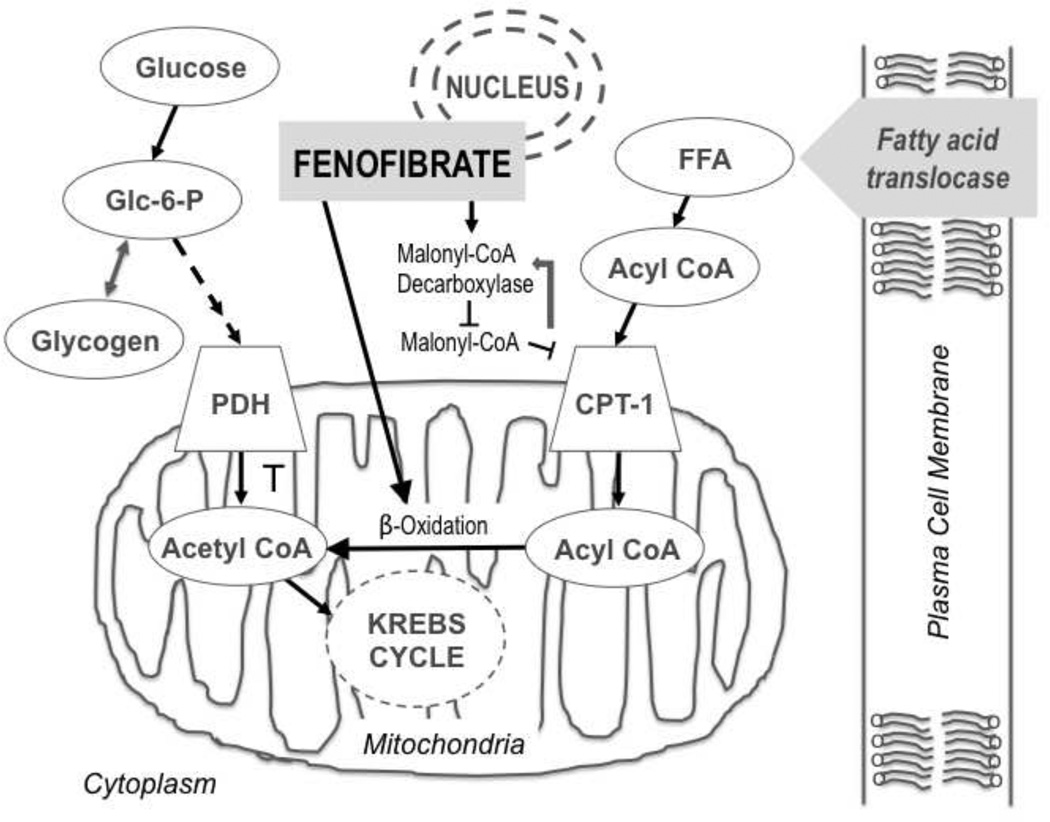
The PPAR-alpha subtype plays a central role in promoting the proliferation of intracellular organelles involved in fatty acid metabolism [8]. Upon binding to fatty acid ligands within the cytoplasm, these receptors then translocate to the nucleus and form dimers with 9-cis retinoic acid receptors (RXR), where the receptor complex binds to target gene promoter regions [9]. The activation of PPAR-alpha leads to an increase in malonyl-CoA decarboxylase activity, suppressing the release of carnitine palmitoyl transferase I (CPT-1) and malonyl-CoA release and ultimately causes fatty acyl groups to transfer into mitochondria. Subsequent increases in fatty acid oxidation and acetyl-CoA concentrations inhibit pyruvate dehydrogenase (PDH) activity, resulting in increased glycogenesis. PPAR-alpha can work synergistically with co-activators to boost fat catabolism in cardiac cells via the up-regulation of enzymes involved in fatty acid oxidation, leading to mitochondrial biogenesis and increasing FFA oxidation [10] (Figure 1).
Figure 1.
Intracellular PPAR-alpha activation cascade. The increased synthesis of free fatty acid (FFA) leads to increased plasma Acyl CoA, causing a flux in oxidation. PPAR-alpha (found within the nuclear membrane) activity increases malonyl-CoA decarboxylase activity, which inhibits malonyl-CoA, subsequently decreasing CPT-1 inhibition via a feedback loop (represented by white arrow). CPT-1 activity allows the transfer of free fatty acyl groups into the mitochondria, generating acetyl CoA via indirect measures, ultimately inhibiting pyruvate dehydrogenase kinase and shunting glucose metabolism in the opposite direction (toward glycogenesis).
2. The PPAR agonist fenofibrate
Fenofibrate is a fibric acid derivative that has been used clinically in the treatment of dyslipidemia for decades [11–12]. It is a protein-bound, lipophilic compound (2-(4((4-chlorobenzoyl) phenoxy)-2-methyl-propanoic acid, 1-methylethyl ester) that is activated via the hydrolysis of the compound’s ester bond [13]. It is inactivated by UDP-glucuronyltransferase into fenofibric acid glucuronide, which is mainly excreted in urine, but may accumulate in severe kidney disease. Blood lipid levels are modulated by fenofibric acid by lowering fasting and postprandial triglyceride concentrations, the exact mechanism of which remains to be determined. Under normal conditions, steady state appears to be achieved in five days, with a peak obtained within six to eight hours of treatment. When taken with meals, it is well absorbed throughout the gastrointestinal tract and has great bioavailability in healthy volunteers, with a half-life estimated to be up to sixteen hours [13]. No pharmacokinetic studies have been conducted in patients with hepatic impairment. Large clinical studies performed on diabetic patients have demonstrated that fenofibrate reduces coronary vascular disease risk and slows coronary atherosclerosis progression [13].
The lack of hypoglycemic events has lead to the examination of fenofibrate use in the management of hyperglycemia, hyperlipidemia and insulin resistance over conventional medications such as insulin [14]. Both human and animal studies have shown that muscle triglyceride content is negatively related to insulin action. Thus, one possible mechanism by which fenofibrate may increase insulin sensitivity is to lower muscle triglyceride content by decreasing intra-muscular fatty acid influx, while decreasing circulating lipid levels [6]. Two recent and large cohort studies provide safety data regarding long-term fenofibrate use. The Fenofibrate Intervention for Event Lowering in Diabetes (FIELD) study evaluated type two diabetic patients on fenofibrate over a five-year period and found that fenofibrate use was associated with a decrease in non-fatal myocardial infarctions and revascularization procedures compared to controls [15]. The Action to Control Cardiovascular Risk in Diabetes (ACCORD) study evaluated the combination of fenofibrate and simvastatin on cardiovascular disease in type 2 diabetic patients with high risk and found that over a 5 year period, combination therapy did not decrease the occurrence of cardiac events as compared to simvastatin alone [16]. While these studies did not focus on the use of fenofibrate in surgical patients or in the management of critical illness, they suggest that long-term use of fenofibrate is not associated with an increase in adverse events.
3. Effects of fenofibrate in non-burn injury
Lower plasma triglycerides, increased insulin sensitivity, and heightened fat oxidation are associated with fibrate use [17]. Fibrates are primarily used alone and sometimes in combination with lipid lowering agents in the management of diabetes mellitus [14, 18]. Fenofibrate is the most commonly used fibrate and has been found to work well in combination with statins.
Preliminary evidence in non-burn populations such as cardiovascular, trauma, traumatic brain injury, diabetes, pancreatitis, and peripheral vascular disease patients demonstrates that fenofibrate use is safe and efficacious [19]. Fenofibrate acts as an anti-inflammatory agent in cardiac patients by inhibiting endothelial monocyte adhesion and cell adhesion molecule expression [19]. In addition, fenofibrate suppresses pro-inflammatory, pro-hypertrophic and pro-fibrotic gene expression, leading to a reduction in cardiac hypertrophy, fibrosis, and dysfunction [19]. Animal studies by Besson et al. have shown that fenofibrate use in the traumatic brain injury population leads to a decrease in neuroinflammatory responses and thus decreases the expression of neurologic deficits, cerebral edema, and brain lesions [20]. Finally, in hypertriglyceride induced pancreatitis, fenofibrate’s anti-lipid properties have enabled management of elevated triglyceride levels via the modulation of hepatic secretion of very low density lipoprotein [21].
The use of fenofibrate has been associated with various side effects such as gastrointestinal complaints, gallstones, skin reactions, and hematological disturbances. It has also been associated with the rare risk of acute renal failure secondary to monotherapy-induced rhabdomyolysis, of which only sixteen cases have been documented in association with fenofibrate monotherapy or fenofibrate-statin combination therapy [22]. Incidences of other side effects such as dehydration in the elderly, myopathy, venous thrombosis, hepatic injury, renal injury requiring replacement, and pancreatitis are also rare, but have been documented with both monotherapy and combination therapy [23]. Clinical trials have not found fenofibrate to be associated with an increased risk for renal failure in individuals without pre-existing medical conditions.
The use of fenofibrate in the non-cardiac/non-diabetic population has been fairly limited, particularly in burn patients. This relatively new drug also has anti-inflammatory properties [20] in addition to its anti-lipid profile. Severely burned patients are in a hyper-inflammatory state and are severely insulin resistant [24], making the use of this drug in burn patients interesting on several levels. Here we will discuss post-burn hyperglycemia and insulin resistance and the use of fenofibrate to counteract the extreme, prolonged reduction in insulin sensitivity.
4. Hypermetabolism in burns
The hypermetabolic response is a phenomenon that occurs post injury and involves persistent elevations in total urine cortisol levels, serum cytokines, stress hormones, catecholamines, basal energy expenditure, and impaired glucose metabolism [25]. The severity, duration, and magnitude of the changes induced by a severe burn injury exposes the patient to a prolonged stress response [24]. Once the catecholamine induced sympathetic stress cascade is initiated, substrates bind to their respective G protein-coupled receptors (GPCRs) to initiate down-stream responses via second messengers such as calcium and cyclic adenosine monophosphate. Canonical signaling, down-stream of the catecholamine receptors, ultimately increase the expression and secretion of pro-inflammatory cytokines, with consequent increases in inflammation, immune dysfunction, susceptibility to infection, and organ dysfunction [26]. While post burn muscle catabolism, insulin resistance, and the hypermetabolic response continue for up to two years, the duration of tissue-specific mechanisms induced by systemic catecholamine elevation remains unclear [27–29].
Hyperglycemia is one of several important metabolic changes associated with the hypermetabolic response in burns, trauma, stroke, surgery, and critical illness [30]. Burn patients, particularly those with burn injuries greater than forty percent of total body surface area (TBSA), experience glycolysis, proteolysis, lipolysis, and elevated body temperatures. The induction of these processes occurs secondary to changes in energy metabolism during injury and mainly provides glucose to fuel vital organs. In burn patients, hepatic glucose production is augmented, as is catecholamine-mediated hepatic glycogenolysis [14]. The normal regulatory action of insulin-mediated glucose uptake is impaired following the down regulation of genes encoding glucose-6-phosphatase transport and glucose transporter- 4 (GLUT-4) [31]. Great interest lies in minimizing the occurrence of hyperglycemia because when it occurs in the critically ill, it is associated with poor clinical outcomes such as impaired wound healing, decreased tensile strength, skin graft loss, increased muscle protein catabolism, infections, and increased mortality [31–32].
Insulin resistance occurs when the insulin signaling cascade is disrupted. Activation of insulin receptor signaling begins with the ligand-bound tyrosine kinase receptor phosphorylating intracellular substrate proteins via two main pathways regulated by phosphatidylinositol 3-kinase (PI3K) or rat sarcoma (RAS) [33]. In one pathway, after insulin binds to its receptor, the insulin receptor substrate (IRS) proteins are phosphorylated and its residues are targeted by PI3K, which then go on to phosphorylate and activate Akt via the PI3K/Akt cascade. The second pathway involves activation of Ras/mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK/ERK) and is less affected by injury [34]. In skeletal muscle, activation of PI3K/Akt is necessary for GLUT4 insulin-induced translocation [35]. In the absence of PI3K/Akt cascade activation, the insulin-stimulated glucose transport/phosphorylation activity is defective, which has been linked to insulin resistance [35]. The process of insulin resistance also involves the activation of glycogen and lipid synthesis, lipolysis inhibition, and the apoptosis of lipocytes [36–37].
5. Lipolysis
The hypermetabolic response during critical illness induces elevated levels of glucocorticoids and lipolysis, which leads to increased levels of circulating free fatty acids and glycerol [38]. Fat induced insulin resistance is initiated by the entrance of excessive amounts of free fatty acids into skeletal muscle cells. Once inside, free fatty acids are converted to fatty acyl CoA and diacylglycerol (DAG) and subsequently activate protein kinase C, resulting in phosphorylation of IRS-1 serine residues. This action inhibits insulin induced PI 3-kinase activity, ultimately causing a reduction in AKT2 activity. When AKT2 activity is decreased, GLUT4 translocation is inhibited, resulting in a decrease in insulin –induced glucose uptake [35, 39]. Associated with this process of lipolysis is an elevation in glycolysis, with a resultant increase in lactate production. The gluconeogenic substrates of glycerol, alanine and lactate are ultimately transported to the liver to augment hepatic glucose production. In addition, catecholamine and cytokine secretion also mediate glucose production via increases in gluconeogenesis and glycogenolysis [35, 39].
6. Effects of fenofibrate on insulin resistance and burn injury
While the relationship between fat metabolism and insulin sensitivity remains to be completely understood in the burn population, the presence of insulin resistance is well documented [27]. In burn patients, insulin resistance persists for at least nine months and even up to three years after hospital discharge [40–41]. Peripheral insulin resistance in the burn population is associated with increased hepatic gluconeogenesis and triglyceride deposition, as well as a three-fold increase in free fatty acid cycling [34, 42]. A similar mechanism is also observed in obesity related insulin resistance, which suggests that it is secondary to impaired insulin signaling as a result of the accumulation of toxic lipid intermediates within tissue, as well as from an oversupply of lipids [43].
Post-burn fat and glucose metabolism may be related to changes in insulin resistance. While overall levels of free fatty acids are variable, there is an existing, continuous cycle of breakdown and formation of triglycerides that does not appear to be used for energy production, but seems related to hyperglycemia. In addition, lipolysis is increased during the flow phase and appears to be related to the presence of elevated levels of catecholamines. While the mechanistic basis of this relationship remains unclear, the amount of subcutaneous fat removed during fascial excision for skin grafting may play a role. Mitochondria in burned tissue sustain significant damage, leading to dysfunction in the form of decreased oxidative capacity when compared to healthy tissue. The presence of hyperglycemia is also known to inhibit the oxidation of fatty acids. All of these interactions seem to be minimized by controlling the development of insulin resistance, thus leading to an improvement in overall outcomes following injury [27].
We have successfully used glucose-lowering drugs such as insulin, metformin, and fenofibrate to treat hyperglycemia in severely burned children. In a prospective, double blind, randomized, controlled clinical trial, fenofibrate or placebo were given to 18 children with TBSA burns >40% for two weeks. Fenofibrate treatment led to a significant increase in the phosphorylation of insulin receptors and IRS-1 in muscle tissue during hyperinsulinemic clamps [44]. This finding was clinically significant in its demonstration that insulin resistance could be ameliorated in pediatric burn patients without the complication of hypoglycemic events associated with the use of regular insulin. Magnetic resonance spectroscopy (MRS) was also used to compare muscle and liver triglycerides and muscle biopsies were performed to determine mitochondrial palmitate oxidation, diacylglycerol, fatty acyl Co-A and fatty acyl carnitine concentrations. In addition to improving insulin signaling in the muscle, the mitochondrial palmitate oxidative capacity of pyruvate was also improved. By decreasing plasma glucose concentrations, significant improvements in mitochondrial function, glucose oxidation and insulin sensitivity resulted. No adverse effects were observed, indicating that fenofibrate may be safe to use short term in the burn population [44–45]. Similar findings have been seen in studies on non-burned, obese rats [46], but in burn patients, this was the first study to demonstrate such a finding.
During burn injury, PPAR-alpha gene expression is down regulated in skeletal muscle, leading to mitochondrial uncoupling and dysfunction [5, 47]. Burn injury also causes the down regulation of genes encoding proteins in cell and organelle organization and biogenesis, wound response, external stimulus response, apoptosis regulation, intracellular signaling and stress response, as well as in genes dealing with oxidative stress [48]. This information suggests that as a PPAR-alpha agonist, fenofibrate use in burn patients may be beneficial in improving not only hyperglycemia and insulin resistance, but also in improving outcomes such as wound healing, apoptosis reduction, and oxidative stress reduction. Other studies evaluating long-term fenofibrate use demonstrated that there is no associated increase in adverse outcomes. Short term use of fenofibrate has proven to be promising at our institution. The long-term use of fenofibrate (up to one year post injury) is now being explored.
Acknowledgements
This work was financially supported by the Shriners of North America grant # 71002.
Abbreviations
- PPAR
peroxisome proliferator activated receptor
- FFA
free fatty acid
- RXR
retinoic acid receptors
- CPT-1
carnitine palmitoyl transferase I
- PDH
pyruvate dehydrogenase
- GPCRs
G protein-coupled receptors
- TBSA
total body surface area
- GLUT-4
glucose transporter-4
- PI3K
phosphatidylinositol 3-kinase
- RAS
rat sarcoma
- IRS
insulin receptor substrate
- MEK/ERK
mitogen-activated protein kinase/extracellular signal-regulated kinase
- DAG
diacylglycerol
- MRS
Magnetic resonance spectroscopy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors declare that they have no competing interests.
References
- 1.Weindl G, Schafer-Korting M, Schaller M, Korting HC. Peroxisome proliferator-activated receptors and their ligands: entry into the post-glucocorticoid era of skin treatment? Drugs. 2005;65(14):1919–1934. doi: 10.2165/00003495-200565140-00002. [DOI] [PubMed] [Google Scholar]
- 2.Patsouris D, Mandard S, Voshol PJ, Escher P, Tan NS, Havekes LM, et al. PPARalpha governs glycerol metabolism. The Journal of clinical investigation. 2004;114(1):94–103. doi: 10.1172/JCI20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paukkeri EL, Leppanen T, Sareila O, Vuolteenaho K, Kankaanranta H, Moilanen E. PPARalpha agonists inhibit nitric oxide production by enhancing iNOS degradation in LPS-treated macrophages. Br J Pharmacol. 2007;152(7):1081–1091. doi: 10.1038/sj.bjp.0707477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burns KA, Vanden Heuvel JP. Modulation of PPAR activity via phosphorylation. Biochim Biophys Acta. 2007;1771(8):952–960. doi: 10.1016/j.bbalip.2007.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzika AA, Mintzopoulos D, Mindrinos M, Zhang J, Rahme LG, Tompkins RG. Microarray analysis suggests that burn injury results in mitochondrial dysfunction in human skeletal muscle. Int J Mol Med. 2009;24(3):387–392. doi: 10.3892/ijmm_00000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furuhashi M, Ura N, Murakami H, Hyakukoku M, Yamaguchi K, Higashiura K, et al. Fenofibrate improves insulin sensitivity in connection with intramuscular lipid content, muscle fatty acid-binding protein, and beta-oxidation in skeletal muscle. J Endocrinol. 2002;174(2):321–329. doi: 10.1677/joe.0.1740321. [DOI] [PubMed] [Google Scholar]
- 7.Yoon M. PPARalpha in Obesity: Sex Difference and Estrogen Involvement. PPAR Res. 2010;2010 doi: 10.1155/2010/584296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson E, Grieve DJ. Significance of peroxisome proliferator-activated receptors in the cardiovascular system in health and disease. Pharmacol Ther. 2009;122(3):246–263. doi: 10.1016/j.pharmthera.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Kliewer SA, Umesono K, Noonan DJ, Heyman RA, Evans RM. Convergence of 9-cis retinoic acid and peroxisome proliferator signalling pathways through heterodimer formation of their receptors. Nature. 1992;358(6389):771–774. doi: 10.1038/358771a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarma S, Ardehali H, Gheorghiade M. Enhancing the metabolic substrate: PPAR-alpha agonists in heart failure. Heart Fail Rev. 2010 doi: 10.1007/s10741-010-9208-0. [DOI] [PubMed] [Google Scholar]
- 11.Kraja AT, Province MA, Straka RJ, Ordovas JM, Borecki IB, Arnett DK. Fenofibrate and metabolic syndrome. Endocr Metab Immune Disord Drug Targets. 2010;10(2):138–148. doi: 10.2174/187153010791213047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staels B, Maes M, Zambon A. Fibrates and future PPARalpha agonists in the treatment of cardiovascular disease. Nat Clin Pract Cardiovasc Med. 2008;5(9):542–553. doi: 10.1038/ncpcardio1278. [DOI] [PubMed] [Google Scholar]
- 13.Moutzouri E, Kei A, Elisaf MS, Milionis HJ. Management of dyslipidemias with fibrates, alone and in combination with statins: role of delayed-release fenofibric acid. Vasc Health Risk Manag. 2010;6:525–539. doi: 10.2147/vhrm.s5593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gauglitz GG, Herndon DN, Jeschke MG. Insulin resistance postburn: underlying mechanisms and current therapeutic strategies. J Burn Care Res. 2008;29(5):683–694. doi: 10.1097/BCR.0b013e31818481ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366(9500):1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- 16.Ginsberg HN, Elam MB, Lovato LC, Crouse JR, 3rd, Leiter LA, Linz P, et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N Engl J Med. 2010;362(17):1563–1574. doi: 10.1056/NEJMoa1001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cree MG, Newcomer BR, Read LK, Sheffield-Moore M, Paddon-Jones D, Chinkes D, et al. Plasma triglycerides are not related to tissue lipids and insulin sensitivity in elderly following PPAR-alpha agonist treatment. Mech Ageing Dev. 2007;128(10):558–565. doi: 10.1016/j.mad.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, et al. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363(3):233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang WP, Yin WH, Chen JW, Jen HL, Young MS, Lin SJ. Fenofibrate attenuates endothelial monocyte adhesion in chronic heart failure: an in vitro study. Eur J Clin Invest. 2009;39(9):775–783. doi: 10.1111/j.1365-2362.2009.02176.x. [DOI] [PubMed] [Google Scholar]
- 20.Besson VC, Chen XR, Plotkine M, Marchand-Verrecchia C. Fenofibrate, a peroxisome proliferator-activated receptor alpha agonist, exerts neuroprotective effects in traumatic brain injury. Neurosci Lett. 2005;388(1):7–12. doi: 10.1016/j.neulet.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Whitten AE, Lorenz RP, Smith JM. Hyperlipidemia-associated pancreatitis in pregnancy managed with fenofibrate. Obstet Gynecol. 2011;117(2 Pt 2):517–519. doi: 10.1097/AOG.0b013e31820755b5. [DOI] [PubMed] [Google Scholar]
- 22.Danis R, Akbulut S, Ozmen S, Arikan S. Rhabdomyolysis-induced acute renal failure following fenofibrate therapy: a case report and literature review. Case Report Med. 2010;2010 doi: 10.1155/2010/537818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT. Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. American Journal of Cardiology. 2010;106(11):1594–1601. doi: 10.1016/j.amjcard.2010.07.041. [DOI] [PubMed] [Google Scholar]
- 24.Finnerty CC, Herndon DN, Przkora R, Pereira CT, Oliveira HM, Queiroz DM, et al. Cytokine expression profile over time in severely burned pediatric patients. Shock. 2006;26(1):13–19. doi: 10.1097/01.shk.0000223120.26394.7d. [DOI] [PubMed] [Google Scholar]
- 25.Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401. doi: 10.1097/SLA.0b013e3181856241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kulp GA, Herndon DN, Lee JO, Suman OE, Jeschke MG. Extent and magnitude of catecholamine surge in pediatric burned patients. Shock. 2010;33(4):369–374. doi: 10.1097/SHK.0b013e3181b92340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cree MG, Wolfe RR. Postburn trauma insulin resistance and fat metabolism. Am J Physiol Endocrinol Metab. 2008;294(1):E1–E9. doi: 10.1152/ajpendo.00562.2007. [DOI] [PubMed] [Google Scholar]
- 28.Hart DW, Wolf SE, Chinkes DL, Gore DC, Mlcak RP, Beauford RB, et al. Determinants of skeletal muscle catabolism after severe burn. Ann Surg. 2000;232(4):455–465. doi: 10.1097/00000658-200010000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, et al. Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11(4):R90. doi: 10.1186/cc6102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363(9424):1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 31.Mecott GA, Al-Mousawi AM, Gauglitz GG, Herndon DN, Jeschke MG. The role of hyperglycemia in burned patients: evidence-based studies. Shock. 2010;33(1):5–13. doi: 10.1097/SHK.0b013e3181af0494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Worthley MI, Holmes AS, Willoughby SR, Kucia AM, Heresztyn T, Stewart S, et al. The deleterious effects of hyperglycemia on platelet function in diabetic patients with acute coronary syndromes mediation by superoxide production, resolution with intensive insulin administration. J Am Coll Cardiol. 2007;49(3):304–310. doi: 10.1016/j.jacc.2006.08.053. [DOI] [PubMed] [Google Scholar]
- 33.Barthel A, Schmoll D. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am J Physiol Endocrinol Metab. 2003;285(4):E685–E692. doi: 10.1152/ajpendo.00253.2003. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Messina JL. Acute insulin resistance following injury. Trends Endocrinol Metab. 2009;20(9):429–435. doi: 10.1016/j.tem.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004;10(7–12):65–71. doi: 10.2119/2005-00029.Saltiel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Saltiel AR, Pessin JE. Insulin signaling in microdomains of the plasma membrane. Traffic. 2003;4(11):711–716. doi: 10.1034/j.1600-0854.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 38.Slavin BG, Ong JM, Kern PA. Hormonal regulation of hormone-sensitive lipase activity and mRNA levels in isolated rat adipocytes. J Lipid Res. 1994;35(9):1535–1541. [PubMed] [Google Scholar]
- 39.Shulman GI. Cellular mechanisms of insulin resistance. The Journal of clinical investigation. 2000;106(2):171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hart DW, Wolf SE, Mlcak R, Chinkes DL, Ramzy PI, Obeng MK, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128(2):312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 41.Cree MG, Fram RY, Barr D, Chinkes D, Wolfe RR, Herndon DN. Insulin resistance, secretion and breakdown are increased 9 months following severe burn injury. Burns. 2009;35(1):63–69. doi: 10.1016/j.burns.2008.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wolfe RR, Herndon DN, Jahoor F, Miyoshi H, Wolfe M. Effect of severe burn injury on substrate cycling by glucose and fatty acids. N Engl J Med. 1987;317(7):403–408. doi: 10.1056/NEJM198708133170702. [DOI] [PubMed] [Google Scholar]
- 43.Thyfault JP, Cree MG, Zheng D, Zwetsloot JJ, Tapscott EB, Koves TR, et al. Contraction of insulin-resistant muscle normalizes insulin action in association with increased mitochondrial activity and fatty acid catabolism. Am J Physiol Cell Physiol. 2007;292(2):C729–C739. doi: 10.1152/ajpcell.00311.2006. [DOI] [PubMed] [Google Scholar]
- 44.Cree MG, Zwetsloot JJ, Herndon DN, Qian T, Morio B, Fram R, et al. Insulin sensitivity and mitochondrial function are improved in children with burn injury during a randomized controlled trial of fenofibrate. Ann Surg. 2007;245(2):214–221. doi: 10.1097/01.sla.0000250409.51289.ca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cree MG, Newcomer BR, Herndon DN, Qian T, Sun D, Morio B, et al. PPAR-alpha agonism improves whole body and muscle mitochondrial fat oxidation, but does not alter intracellular fat concentrations in burn trauma children in a randomized controlled trial. Nutr Metab (Lond) 2007;4:9. doi: 10.1186/1743-7075-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Murakami K, Tobe K, Ide T, Mochizuki T, Ohashi M, Akanuma Y, et al. A novel insulin sensitizer acts as a coligand for peroxisome proliferator-activated receptor-alpha (PPAR-alpha) and PPAR-gamma: effect of PPAR-alpha activation on abnormal lipid metabolism in liver of Zucker fatty rats. Diabetes. 1998;47(12):1841–1847. doi: 10.2337/diabetes.47.12.1841. [DOI] [PubMed] [Google Scholar]
- 47.Padfield KE, Astrakas LG, Zhang Q, Gopalan S, Dai G, Mindrinos MN, et al. Burn injury causes mitochondrial dysfunction in skeletal muscle. Proc Natl Acad Sci U S A. 2005;102(15):5368–5373. doi: 10.1073/pnas.0501211102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Parihar A, Parihar MS, Milner S, Bhat S. Oxidative stress and anti-oxidative mobilization in burn injury. Burns. 2008;34(1):6–17. doi: 10.1016/j.burns.2007.04.009. [DOI] [PubMed] [Google Scholar]