Abstract
We report the case of a symptomatic intracerebral haemorrhage (ICH) in an elderly woman, secondary to cerebral amyloid angiopathy (CAA), and present the relevant imaging. A few months before, our patient experienced multiple, stereotyped, brief episodes of spreading paraesthesias, which were considered to be transient ischaemic attacks (TIAs) and treated with antithrombotic agents. In this case report, we explore CAA, a highly prevalent but under-recognised form of small vessel cerebrovascular disease and common cause of ICH. We then briefly discuss the clinical significance of transient focal neurological episodes in the context of CAA, as potential warning signs of future ICH. An important clinical message is that misdiagnosis of CAA-related focal neurological symptoms as TIAs (and prescribing antithrombotic drugs) could lead to potentially avoidable ICH. We also provide the current evidence base for the acute and secondary prevention treatment of patients with lobar ICH attributed to CAA, and discuss the prognosis.
Background
We report the case of a symptomatic intracerebral haemorrhage (ICH) in an elderly woman, secondary to clinically probable cerebral amyloid angiopathy (CAA), and present the relevant imaging. A few months before, our patient experienced multiple, stereotyped, brief episodes of spreading paraesthesias, which were considered to be transient ischaemic attacks (TIAs) and treated with antithrombotic agents. In this case report, first we explore CAA, a highly prevalent but under-recognised form of small vessel cerebrovascular disease and common cause of ICH. We then briefly discuss transient focal neurological episodes as a clinical feature of CAA and potential warning sign of ICH; the clinical importance here is that ICH might in some cases be preventable if antithrombotic drugs are avoided in the context of these episodes where MRI shows changes consistent with CAA.1 Our case emphasises the value of brain imaging using MRI with specific blood-sensitive sequences in the investigation of transient focal neurological episodes. Finally, we outline the current evidence base for the acute and secondary prevention treatment of patients with lobar ICH attributed to CAA, and discuss the prognosis.
Case presentation
A 78-year-old woman presented to her general practitioner (GP) complaining about episodes of paraesthesias. She described five stereotyped episodes of ‘pins and needles’ over 1 week. These episodes evolved over several minutes, starting on the scalp and spreading to the left side of her face and mouth, and lasted up to 10 min. During the same period she reported one episode of limb-shaking, affecting her left leg, lasting 1–2 h.
Significant medical history included an uncomplicated surgical removal of a right occipital lobe meningioma 13 years prior, a symptomatic lobar ICH 6 years prior, a myocardial infarction and congestive heart failure. At this time the patient was mobile with a stick and lived at home with limited support from family and carers (modified Rankin score: 1). She was taking 40 mg atorvastatin.
Her GP made a diagnosis of TIAs and prescribed a low-dose antiplatelet medication (75 mg aspirin). Four months later the patient reported visual disturbances consisting of brief episodes of blurry vision lasting a few minutes.
Days later she was found by a carer in bed with left-sided facial drooping and slurred speech. She was urgently admitted to our Stroke Unit; on examination she was found to have a left hemianopia, slurring dysarthria, left upper motor neuron facial weakness, pyramidal left arm and leg weakness and sensory disturbance to light touch in the left face, arm and leg.
Investigations
A non-contrast brain CT scan demonstrated a high attenuating (bright) intraparenchymal lesion in the right frontal lobe, measuring 3.8 cm in diameter. There was also associated right hemispheric sulcal effacement (figure 1). Subsequent imaging, including CT angiography and delayed imaging, showed no underlying structural lesion or vascular malformation. MRI also revealed widespread white matter changes (figure 2), and reduced signal change on susceptibility-weighted sequences indicating cortical superficial siderosis in several sulci of the convexities of both cerebral hemispheres (figure 3).
Figure 1.
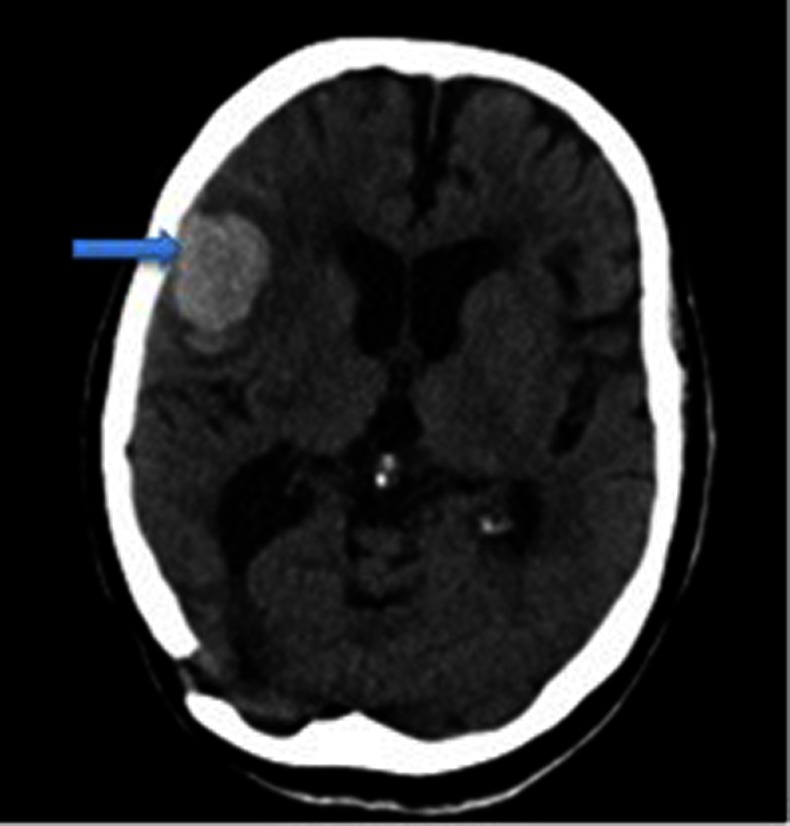
Non-contrast brain CT scan demonstrating a low attenuating intraparenchymal lesion of 3.8 cm diameter in the right frontal lobe (blue arrow).
Figure 2.
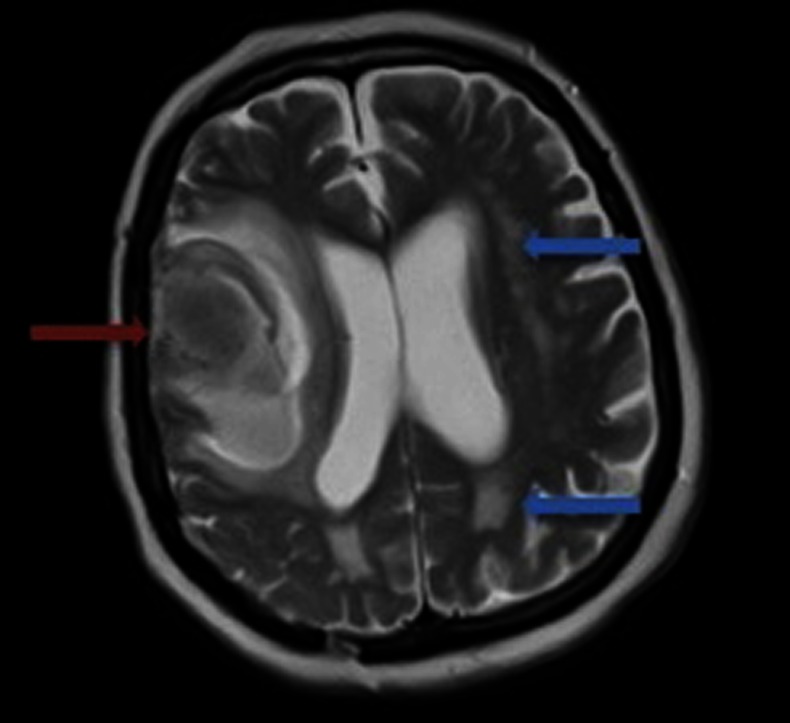
T2-weighted MRI demonstrating the right-sided intracerebral haemorrhage (red arrow) as well as white matter changes (leukoaraiosis, blue arrows).
Figure 3.
T2*-weighted gradient-recalled echo MRI demonstrating cortical superficial siderosis in several sulci contralateral to the intracerebral haematoma (white arrows). There is evidence of cortical superficial siderosis over the right frontal-parietal cortex, making this a likely cause of the patient's previous transient episodes of left-sided paraesthesias and limb jerking.
Differential diagnosis
The acute onset of focal neurological symptoms and signs in our patient pointed to a focal brain vascular insult, ischaemic or haemorrhagic in nature. The only reliable way to differentiate between these vascular events is with brain imaging; a non-contrast CT scan within the first 48 h is highly sensitive (>95%) for acute intracranial bleeding2 and rapidly confirmed the diagnosis of ICH in our case.
ICH has a world-wide incidence of 10–20 cases per 100 000 population, which increases with age.3 It is one of the most serious and catastrophic forms of stroke with a 3-month death rate of 34%4 in one prospective, multicentre study. The classical presentation is rapid onset of focal neurological deficit (eg, hemiparesis, visual or language disturbance), occasionally with vomiting or diminished consciousness suggesting raised intracranial pressure.
Spontaneous ICH, also termed primary or non-traumatic ICH, accounts for more than 75% of ICHs, while secondary causes account for the remainder. There a number of secondary causes that must be ruled out (box 1).5 Some form of cerebral angiographic imaging is indicated, for example, CT angiography, MR angiography or formal catheter angiography. If no underlying cause can be found an ICH is classified as ‘spontaneous’. Spontaneous ICH is caused by the rupture of small arteries in the brain, mainly affected by two cerebral small vessel diseases: hypertensive arteriopathy and CAA. These two processes have a different distribution in the brain and the likelihood of each underlying process differs according to the location of the ICH.6 Hypertensive arteriopathy has preference for the small deep lenticulostriate arteries, and is an important cause of spontaneous ICH in deep or infratentorial locations (eg, basal ganglia, thalamus and pons). By contrast, CAA is characterised by the progressive deposition of amyloid-β in the walls of neocortical and leptomeningeal small arteries, arteriole and capillaries. The distribution of these vessels typically leads to lobar (cortical-subcortical) ICH in patients with CAA; in particular, the occipital and temporal lobes are often affected, the frontal lobes probably less so. The cerebellum may be involved, but only vary rarely does CAA-related ICH occur in deep basal ganglia or brainstem structures.7
Box 1. Secondary causes of non-traumatic intracerebral haemorrhage.
Vascular: Arteriovenous malformations, cavernous haemangiomas, aneurysms
Haematological: Anticoagulants, antiplatelets, thrombolytic therapy, coagulation disorders, disseminated intravascular coagulation, venous thrombosis
Tumours: Primary or metastatic (especially from melanoma)
Drug abuse: Amphetamine, cocaine
Other: e.g. Moya moya disease, infective endocarditis, cerebral vasculitis, malaria.
As the vessels affected by CAA are too small to be easily seen in vivo, associated neuroimaging features in the brain parenchyma can act as surrogate markers for the disease. These include lobar cerebral microbleeds, white matter changes (also called leukoaraiosis), cortical superficial siderosis and acute convexity subarachnoid haemorrhage (cSAH).8 The ‘Boston criteria’ allow clinicians to estimate the probability of a CAA diagnosis in clinical practice.9 The most recent version of the Boston criteria have a sensitivity of 81% and specificity of 95% in a selected ICH patient population,10 although further validation of these criteria in other populations is required. Nevertheless, our patient fulfilled the following features for a diagnosis of ‘probable’ CAA, so on present evidence we concluded that it is highly likely that CAA is the cause of her ICH9:
Multiple (≥2) strictly lobar (cortical or cortical-subcortical) haemorrhage
Age ≥55 years
Absence of other causes of haemorrhage
(modified criteria) focal or disseminated cortical superficial siderosis10
Treatment and secondary prevention of spontaneous ICH
There are few proven effective acute treatments for ICH, but supportive care in a neuroscience intensive care environment with careful attention to homeostasis, intercurrent infection, venous thromboembolism and other complications is recommended in international guidelines.11
In addition to supportive treatment, our patient's antiplatelets were permanently stopped on admission. Antiplatelet or anticoagulant treatment should be withheld in the acute phase (first 48 h) after an ICH because of the early risk of haematoma expansion that may worsen if platelet aggregation or haemostasis is further impaired by medications.
Outcome and follow-up
Our patient was discharged from hospital and still attends follow-up clinic appointments. However, there is significant residual disability—from premorbidly being mobile with a stick and receiving limited help from relatives and carers, her Barthel Index is now 2/10.
Discussion
Symptomatic lobar ICH is the most common clinical presentation of CAA. However, cognitive impairment and dementia, rapidly progressive neurological and cognitive decline and transient focal neurological symptoms are also associated with CAA.7
ICH treatment recommendations
Many patients affected by CAA-related ICH are elderly and have important risk factors for occlusive ischaemic events (eg, atrial fibrillation) and an indication for antithrombotic agents beyond the acute phase. There are limited data to guide when or whether to restart antithrombotic drugs in this situation. Biffi et al undertook a prospective cohort study of spontaneous lobar ICH and found an association between aspirin use and ICH recurrence after adjusting for other potential ICH risk factors (HR 3.95, 95% CI 1.6 to 8.3; p<0.021). Patients with CAA seem to have a particularly high risk of anticoagulant-related ICH.12 Patients with lobar ICH suggestive of CAA have annual recurrent ICH risk of up to about 10%7 and anticoagulants appear to increase this risk by 7–10-fold, as well as increasing the clinical severity and death rate from of ICH.13 Current practice is that anticoagulation should generally be avoided in those with CAA-related ICH, unless the patient is at a extremely high risk of an ischaemic event, for example, with a mechanical heart valve.7 The use of antiplatelet drugs after ICH is currently decided on a case by case assessment of risks and benefits, but are used with caution where there is evidence of CAA; future randomised trials are needed to help address this question.
Neurological complications including seizures, raised intracranial pressure and hydrocephalus need prompt recognition and treatment. Rapid lowering of blood pressure (to <140 mm Hg systolic) in the first 6 h after ICH reduces haematoma expansion, which is itself associated with a predictor of worse outcome.7 14 A larger, definitive trial (INTERACT-2) will determine whether the effect of acute blood pressure lowering on early haematoma expansion also improves clinical outcome.
The role of neurosurgery for ICH, including CAA, remains limited. There is specific concern in CAA regarding the risk of damaging fragile cortical small vessels during surgery. However, small studies suggest that surgery in patients with CAA <75 years and no intraventricular extension is acceptably safe.15 The STICH II trial includes patients with small superficial lobar ICH, so may further elucidate the role of neurosurgery in CAA-related ICH.16
Blood pressure lowering has been convincingly shown to reduce the risk of ICH after stroke, even in cases where the ICH is attributed to CAA. The relative risk reduction for a 9/4 mm Hg reduction in the PROGRESS trial was reported to be 77%.17
Transient focal neurological episodes
Transient focal neurological episodes in the context of CAA (also known as ‘amyloid spells’) have been commonly described as recurrent, stereotyped episodes of spreading positive sensory symptoms (eg, paraesthesias), typically lasting a few minutes. However, they can also consist of seizure-like episodes (eg, limb-shaking), positive visual symptoms and negative symptoms (eg, focal weakness, or dysphasia typical of TIAs).1 18 19 Recent evidence shows that these episodes seem to be related to the haemorrhagic components of CAA, including lobar cerebral microbleeds, cSAH and cortical superficial siderosis, and predict a high early risk of symptomatic ICH (which may be amenable to prevention).1 20 Recognition of CAA as a cause of these attacks (rather than typical TIAs) is important, as the standard treatment for TIAs is antithrombotic drugs, which may aggravate CAA and cause serious symptomatic ICH.
Our patient had cortical superficial siderosis (ie, haemosiderin in the superficial layers of the cerebral cortex), which may follow repeated episodes of bleeding in the subarachnoid space. Cortical superficial siderosis is only well detected on T2*-GRE MRI (or other sequences sensitive to blood products) as a characteristic ‘gyriform’ pattern of hypointense signal,21 and is an important clue to the presence of CAA. One retrospective study found 47.4% (n=38) of patients with a clinical diagnosis of CAA had cortical superficial siderosis, compared with no controls (mean age 54 years).10 It should be noted that cortical superficial siderosis associated with CAA is distinct from the syndrome of CNS superficial siderosis. The former has a predilection for the cerebral convexities, as opposed to the brainstem and posterior fossa.22 Of note, there are several other rarer causes of intracranial bleeding that can give rise to cortical superficial siderosis and should be excluded (eg, reversible segmental cerebral vasoconstriction [Call-Fleming syndrome], cerebral venous thrombosis etc).
Given that the multiple areas of cortical superficial siderosis on MRI in our patient most likely preceded the acute ICH (no acute subarachnoid blood was seen on the acute CT scan), it is likely that her left-sided paraesthesias and limb jerking attacks she experienced prior to the ICH were caused by cortical superficial siderosis over the right frontal-parietal cortex.
It is of clinical importance to distinguish these types of episode from typical TIAs, since they could act as a clinical indicator of CAA; more importantly, if such attacks are misdiagnosed as TIAs and are treated with antithrombotic drugs the future risk of serious ICH may up to about 50% over the next 2 months.1 Advice we would offer to GP's is to be aware of these atypical TIA presentations—including recurrent stereotyped attacks, spreading gradual onset—or any known history of cerebral bleeding as a signal of possible CAA and warranting referral to a specialist for further imaging, before initiation of anticoagulation/antiplatelet treatment.23
The pathophysiology of transient focal neurological episodes in CAA remains unclear, but could involve seizure-like activity (perhaps related to small areas of bleeding: cerebral microbleeds, cortical superficial siderosis cSAH); or a direct effect of amyloid or bleeding on local cortical function; or spreading cortical depression.1 18 The response to antiepileptic drugs and their spreading nature in many of the case series and reports supports a seizure-like mechanism.
In summary, our case represents a common clinical scenario (transient focal neurological episodes and then acute stroke due to ICH), due to a common but under-recognised underlying cause (CAA).10 Emerging research into cerebral small vessel disease (including CAA) and its contribution to old-age morbidity is of increasing importance to doctors in training. ICH has generally received much less attention than ischaemic stroke, yet remains the most devastating type of stroke. Secondary prevention in ICH survivors (including careful blood pressure control) is extremely effective and important. Our case emphasises the important role of advanced vascular brain MRI including blood-sensitive sequence in the investigation of transient focal neurological episodes and ICH.
Learning points.
Cerebral amyloid angiopathy (CAA) is common in elderly populations and has a broad clinical and imaging spectrum, including transient focal neurological episodes.
The diagnosis of CAA during life is based on the Boston criteria.
Blood sensitive MRI sequences (T2*-GRE, SWI) are clinically useful in the investigation of elderly patients with transient focal neurological symptoms (including transient ischaemic attacks-like symptoms), and are essential to sensitively recognise CAA in vivo.
Prescription of antiplatelets or anticoagulation in patents with CAA-related transient focal neurological episodes could be potentially harmful and should be avoided in the absence of any compelling indication, since such episodes may herald a very high future risk of symptomatic ICH as illustrated by our case.
Footnotes
Contributors: All authors were involved in the conception and design. RH and AC wrote the initial draft and obtained the relevant imaging. DW edited the initial draft and provided expert advice.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Charidimou A, Peeters A, Fox Z, et al. Spectrum of transient focal neurological episodes in cerebral amyloid angiopathy: multicentre magnetic resonance imaging cohort study and meta-analysis. Stroke 2012;2013:2324–30 [DOI] [PubMed] [Google Scholar]
- 2.van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet 2007;2013:306–18 [DOI] [PubMed] [Google Scholar]
- 3.Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet 2009;2013:1632–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weimar C, Weber C, Wagner M, et al. Management patterns and health care use after intracerebral hemorrhage. a cost-of-illness study from a societal perspective in Germany. Cerebrovasc Dis 2003;2013:29–36 [DOI] [PubMed] [Google Scholar]
- 5.Clarke C. Neurology, A queen square textbook. London: Wiley-Blackwell, 2009 [Google Scholar]
- 6.Samarasekera N, Smith C, Al-Shahi Salman R. The association between cerebral amyloid angiopathy and intracerebral haemorrhage: systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 2012;2013:275–81 [DOI] [PubMed] [Google Scholar]
- 7.Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited: recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry 2012;2013:124–37 [DOI] [PubMed] [Google Scholar]
- 8.Wardlaw JM. Blood-brain barrier and cerebral small vessel disease. J Neurol Sci 2010;2013:66–71 [DOI] [PubMed] [Google Scholar]
- 9.Knudsen KA, Rosand J, Karluk D, et al. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology 2001;2013:537–9 [DOI] [PubMed] [Google Scholar]
- 10.Linn J, Halpin A, Demaerel P, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 2010;2013:1346–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furie KL, Kasner SE, Adams RJ, et al. Guidelines for the prevention of stroke in patients with stroke or transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011;2013:227–76 [DOI] [PubMed] [Google Scholar]
- 12.Rosand J, Hylek EM, O'Donnell HC, et al. Warfarin-associated hemorrhage and cerebral amyloid angiopathy: a genetic and pathologic study. Neurology 2000;2013:947–51 [DOI] [PubMed] [Google Scholar]
- 13.Izumihara A, Ishihara T, Iwamoto N, et al. Postoperative outcome of 37 patients with lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Stroke 1999;2013:29–33 [DOI] [PubMed] [Google Scholar]
- 14.Anderson CS, Huang Y, Wang JG, et al. Intensive blood pressure reduction in acute cerebral haemorrhage trial (INTERACT): a randomised pilot trial. Lancet Neurol 2008;2013:391–9 [DOI] [PubMed] [Google Scholar]
- 15.Hart RG, Boop BS, Anderson DC. Oral anticoagulants and intracranial hemorrhage. Facts and hypotheses. Stroke 1995;2013:1471–7 [DOI] [PubMed] [Google Scholar]
- 16.Mendelow AD, Gregson BA, Mitchell PM, et al. Surgical trial in lobar intracerebral haemorrhage (STICH II) protocol. Trials 2011;2013:124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arima H, Tzourio C, Anderson C, et al. Effects of perindopril-based lowering of blood pressure on intracerebral hemorrhage related to amyloid angiopathy: the PROGRESS trial. Stroke 2010;2013:394–6 [DOI] [PubMed] [Google Scholar]
- 18.Greenberg SM, Vonsattel JP, Stakes JW, et al. The clinical spectrum of cerebral amyloid angiopathy: presentations without lobar hemorrhage. Neurology 1993;2013:2073–9 [DOI] [PubMed] [Google Scholar]
- 19.Roch JA, Nighoghossian N, Hermier M, et al. Transient neurologic symptoms related to cerebral amyloid angiopathy: usefulness of T2*-weighted imaging. Cerebrovasc Dis 2005;2013:412–14 [DOI] [PubMed] [Google Scholar]
- 20.Raposo N, Viguier A, Cuvinciuc V, et al. Cortical subarachnoid haemorrhage in the elderly: a recurrent event probably related to cerebral amyloid angiopathy. Eur J Neurol 2011;2013:597–603 [DOI] [PubMed] [Google Scholar]
- 21.Linn J, Herms J, Dichgans M, et al. Subarachnoid hemosiderosis and superficial cortical hemosiderosis in cerebral amyloid angiopathy. AJNR Am J Neuroradiol 2008;2013:184–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar N, Cohen-Gadol AA, Wright RA, et al. Superficial siderosis. Neurology 2006;2013:1144–52 [DOI] [PubMed] [Google Scholar]
- 23.Charidimou A, Baron JC, Werring DJ. Transient focal neurological episodes, cerebral amyloid angiopathy, and intracerebral hemorrhage risk: looking beyond TIAs. Int J Stroke 2013;2013:105–8 [DOI] [PubMed] [Google Scholar]



