Abstract
Therapeutic methods to reprogramme and destroy human solid tumour cells have not been developed. We show a proof-of-concept for the direct reprogramming therapy of human solid tumour cells. Furthermore, our study is the first to report on the development of a new treatment by using human-induced pluripotent stem cells technology.
Background
Therapeutic methods to reprogramme and destroy human solid tumour cells have not been developed. However, we have shown a novel therapeutic method for human hepatocellular carcinoma (HCC) by using human-induced pluripotent stem (iPS) cells in vitro.1
Case presentation
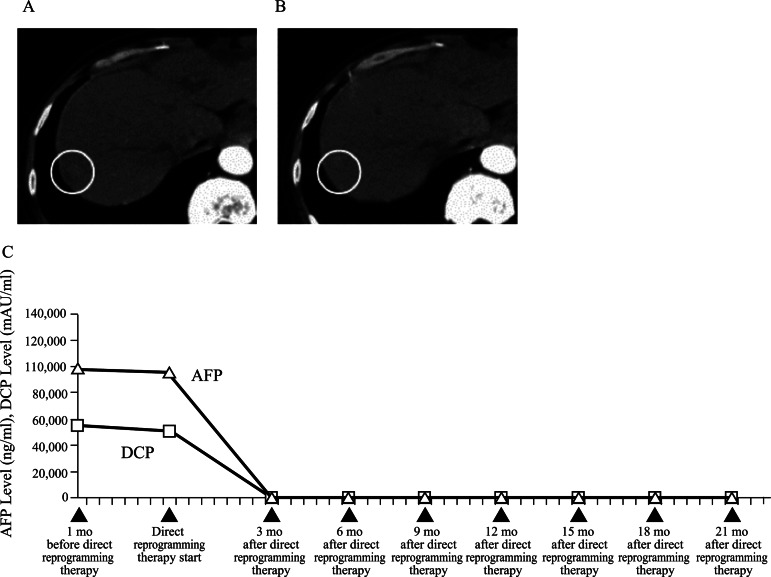
A 34-year-old man who had advanced HCC beyond Milan criteria (single tumour ≤5 cm in size or ≤3 tumours each ≤3 cm in size and no macrovascular invasion)2 3 and had experienced the recurrence of HCC in 12 months after liver transplantation was treated with sorafenib for 6 weeks at a dose of 400 mg twice daily. Diarrhoea, weight loss and hand–foot skin reaction as adverse reactions during sorafenib treatment were reported. However, he was turned out to be a non-responder of sorafenib (figure 1A). Furthermore, he was hepatitis C virus (HCV) infection-positive patient even after sorafenib treatment. The viral load was 6.20±0.73 (log10HCV RNA, mean±SD) in the patient. Then, alanine aminotransferase (ALT) level was 58.4±21.8 U/l (mean±SD), and aspartate transaminase (AST) level was 90.1±30.5 U/l (mean±SD) in the patient (normal range for each level in ALT and AST, 0–50 U/l).
Figure 1.
(A) Abdominal CT before the direct reprogramming therapy shows a tumour (circle). (B) CT after the direct reprogramming therapy shows the disappearance of HCC (circle). (C) The levels of two tumour markers (α-fetoprotein, des-γ-carboxyprothrombin.
Investigations
In vitro anticancer drug sensitivity testing was performed using the patient's HCC that had expressed aldo-keto reductase family 1 member B10 (AKR1B10) and retinoid X receptors. As the in vitro anticancer drug sensitivity testing, the liver cancer cells were incubated in a flask coated with collagen gel in a CO2 incubator at 37 °C for 24 h. Only the viable cells adhering to the collagen gel were collected and resuspended in the reconstituted type 1 collagen solution for a final density of 1×105 cells/ml. Three drops of the collagen–cell mixture were placed in each well of a six-well plate in a 60 mm dish, and the plates were allowed to reach 37 °C in a CO2 incubator for 1 h. The final concentration was approximately 3×104 cells/collagen gel droplet. Culture medium was added to each well, and the plate was incubated in a CO2 incubator at 37 °C overnight. The anticancer drugs (ie, 10 μM acyclic retinoid (ACR) alone, 10 μM tolrestat alone or 10 μM ACR plus 10 μM tolrestat1) were added to the cells for 2 days. As a result, his cancer cells were eliminated by ACR plus tolrestat as an AKR1B10 inhibitor1 6 days later in vitro.
Furthermore, the oxygen consumption (mean±SD, nmol min−1 mg−1 protein) in his AKR1B10-positive liver cancer cells was measured by a Clark-type oxygen microelectrode. Measurements were conducted 3 days after the administration of ACR alone, tolrestat alone or ACR plus tolrestat.1 As a result, although cancer cells preferentially utilise glycolytic pathways for energy generation while downregulating their aerobic respiratory activity as described by Warburg,4 oxygen consumption was significantly greater in the AKR1B10-positive liver cancer cells treated with ACR plus tolrestat1 than in the cells treated with either ACR alone or tolrestat alone (p<0.001, Mann-Whitney U test). The combined effects of ACR plus tolrestat1 may work efficiently in the direct reprogramming and destruction of human HCC cells.
Treatment
Considering the results of in vitro anticancer drug sensitivity testing, he was treated with ACR (600 mg per day) plus tolrestat (400 mg per day) for 48 weeks. Written informed consent was obtained before the study, which was approved by the institutional review board of our institute.
Outcome and follow-up
His HCC disappeared in 3 months of ACR plus tolrestat therapy (figure 1B) and serum α-fetoprotein (AFP) and des-γ-carboxyprothrombin (DCP)) levels were normalised (figure 1C). Furthermore, ALT and AST levels were improved (mean±SD: 39.3±20.5 U/l in ALT level, 46.5±20.3 U/l in AST level; normal range for each level in ALT and AST, 0–50 U/l) and the viral load for HCV decreased (mean±SD: 3.00±0.30, log10 HCV RNA) in the patient after ACR plus tolrestat therapy compared with after sorafenib treatment.
Furthermore, he survives 41 months recurrence-free of HCC after ACR plus tolrestat therapy. Only headache was observed as an adverse reaction during ACR plus tolrestat therapy.
Discussion
Patients undergoing liver transplantation for HCC within Milan criteria have an excellent outcome.2 3 Even after liver transplantation, however, the recurrence rate is higher and the prognosis is worse in patients with advanced HCC beyond Milan criteria, and the recurrence-free survival rates are 0.0% at 18 months in those patients.5 Though sorafenib is the only drug showing survival benefits in advanced HCC patients,6 the patient showed sorafenib resistance. Therefore, he was treated with ACR plus tolrestat therapy, and could get successful outcome. Considering our results for in vitro anticancer drug sensitivity testing and clinical results, his HCC cells with sorafenib resistance appeared to be directly reprogrammed to approximately normal hepatocytes and ACR plus tolrestat-induced apoptosis in 3 months. Therefore, we could show a proof-of-concept for the direct reprogramming therapy of human solid tumour cells in the current study. Furthermore, our study is the first report for the development of new treatment by using human iPS cells technology.
Moreover, he is HCV infection-positive. However, by the direct reprogramming therapy, the viral load of HCV decreased. Considering the viral load reduction of HCV under all-trans retinoic and monotherapy was observed,7 the phenomenon observed in our study may be explained as an effect of ACR.
In conclusion, ACR plus tolrestat therapy1 as the direct reprogramming therapy would warrant testing in the patients with advanced HCC that express AKR1B10 and retinoid X receptors, even if they have sorafenib resistance.
Learning points.
We show a proof-of-concept for the direct reprogramming therapy of human solid tumour cells.
Our study is the first to report on the development of new treatment by using human-induced pluripotent stem cells technology.
Acyclic retinoid plus tolrestat therapy as the direct reprogramming therapy would warrant testing in the patients with advanced HCC that express AKR1B10 and retinoid X receptors, even if they have sorafenib resistance.
Acknowledgments
We are grateful to many doctors (physicians, surgeons or laboratory technicians with PhD), and nurses in Boston, USA and Satoko Iioka, Midori Okabe, Tomoko Shiraishi, Akira Fujimoto.
Footnotes
Contributors: HM and JM: Conception and design, provision of study material, collection and/or assembly of data, data analysis and interpretation, manuscript writing and the final approval of manuscript.
Competing interests: None.
Patient consent: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Moriguchi H, Chung RT, Sato C. An identification of novel therapy for human hepatocellular carcinoma by using human induced pluripotent stem cells. Hepatology 2010;2013:1090–1 [DOI] [PubMed] [Google Scholar]
- 2.Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;2013:693–700 [DOI] [PubMed] [Google Scholar]
- 3.Mazzaferro V, Llovet JM, Miceli R, et al. Predicting survival after liver transplantation in patients with hepatocellular carcinoma beyond the Milan criteria: a retrospective, exploratory analysis. Lancet Oncol 2009;2013:35–43 [DOI] [PubMed] [Google Scholar]
- 4.Warburg O. On respiratory impairment in cancer cells. Science 1956;2013:269–70 [PubMed] [Google Scholar]
- 5.Teng CL, Hwang WL, Chen YJ, et al. Sorafenib for hepatocellular carcinoma patients beyond Milan criteria after orthotopic liver transplantation: a case control study. World J Surg Oncol 2012;2013:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;2013:378–90 [DOI] [PubMed] [Google Scholar]
- 7.Böcher WO, Wallasch C, Höhler T, et al. All-trans retinoic acid for treatment of chronic hepatitis C. Liver Int 2008;2013:347–54 [DOI] [PubMed] [Google Scholar]